Abstract
Pulmonary infections with non-tuberculous mycobacteria (NTM) affect a subset of older individuals (mostly women) with no known immunological defects. As NTMs are intracellular pathogens, it is important to establish whether NTM disease is associated with defective production of Th1 cytokines or poor responses by host macrophage/monocytes. We have shown that patients display vigorous production of interferon gamma (IFNγ) when CD4 T cells are stimulated with mycobacterial antigens. This implicated the macrophage response to IFNγ. Blood monocytes are poorly representative of lung macrophages, so monocyte-derived macrophages (MDMs) were created and then stimulated with lipomannan (a Toll-like receptor (TLR)2 agonist), lipopolysaccharide (LPS; a TLR4 agonist) or recombinant human IFNγ. MDMs from NTM patients, their offspring and healthy donors expressed similar amounts of IFNγR1, and cellular responses to IFNγ were similar, so there is no evidence of a genetic defect in this pathway. MDMs from NTM patients produced less interleukin-6 in response to LPS (P<0.01) than cells from controls, but other cytokine responses were normal. This warrants further study.
Keywords: interferon gamma, macrophages, monocytes, non-tuberculous mycobacteria, Toll-like receptors
Non-tuberculous mycobacteria (NTM) are ubiquitous in the environment and cause disease in patients with HIV/AIDS, children with genetically based susceptibility affecting Th1 responses and a small subset of older persons. Over the last decade, diagnoses of pulmonary disease due to NTM (particularly Mycobacterium avium and Mycobacterium intracellulare complex) have increased worldwide, predominantly in post-menopausal women, in whom disease typically presents as nodular bronchiectasis.1, 2, 3, 4 For example, a comparison of isolates obtained in 1999 and 2005, and data from the Queensland notification scheme showed the incidence of notified cases of clinically significant pulmonary disease rose from 2.2 (1999) to 3.2 (2005) per 100 000 population. The pattern of disease changed from predominantly cavitary disease in middle-aged men who smoke to fibronodular disease in elderly women. M. intracellulare is the main pathogen associated with the increase in isolates speciated in Queensland.5 NTM disease carries significant morbidity and requires an intensive antibiotic regimen.6, 7 However, few studies have addressed mechanisms behind susceptibility to NTM in adults with no known risk factors or immunological defects.
The outcome of mycobacterial infection is influenced by Th1 cytokines,8 so poor production of these cytokines in response to mycobacterial antigens is a candidate mechanism. This should be assessed in relation to population-based controls matched by age and geographic location (that is, exposure) with the patients. Two studies reported poor production of Th1 cytokines (including interferon gamma (IFNγ)) by T cells from NTM patients. However, Kwon et al.9 used cells stimulated with phytohemagglutinin rather than mycobacterial antigens and Vankayapati et al.10 restricted the control cohort to donors with a positive skin test response to mycobacteria. The age of these controls was not specified. Mycobacterial antigens and matched controls were used in a study by Kim et al.,11 which concluded that patients with pulmonary NTM infection are taller and leaner than control subjects, with high rates of scoliosis, pectus excavatum and mitral valve prolapse, but without recognized immune defects.
We also reported similar (or higher) frequencies of CD4+ T cells producing IFNγ (detected by intracellular flow cytometry) after stimulation with staphylococcal enterotoxin B, tuberculin or sensitin in patients with pulmonary M. avium and M. intracellulare infection compared with healthy controls. In addition, IFNγ levels in culture supernatants from NTM patients were not deficient.12 This suggests that susceptibility to pulmonary NTM disease is not associated with deficiencies in numbers of CD4+ T cells producing IFNγ or their production of IFNγ. The corollary is that there may be functional deficiencies in macrophages from patients with pulmonary NTM disease. Poor macrophage activation via the IFNγR could also promote NTM disease, reflecting the devastating effect of mutations affecting this receptor seen in children.13, 14
Ligation of Toll-like receptor (TLR)2 by lipomannan (LM) is important in the response to mycobacteria.15, 16 Ryu et al.17 reported reduced levels of TLR2 mRNA in peripheral blood monocytes after exposure to M. avium and M. intracellulare complex in NTM patients compared with healthy controls, but responses initiated via TLR2 were not investigated.
No studies to date have assessed macrophage function in NTM disease. As mature macrophages from the lung are difficult to source, we used monocyte-derived macrophages (MDMs) to find functional defects that could predispose to NTM. MDMs were cultured with LM, lipopolysaccharide (LPS) or recombinant human (rh) IFNγ to assess cytokine and chemokine production; specifically interleukin (IL)-6, IL-10, tumor necrosis factor-alpha (TNFα) and C-X-C motif chemokine 10 (CXCL10). Offspring of the patients were included as an additional control group, as their alignment with patients (rather than population controls) would suggest a genetic basis for any differences observed.
Results
TLR2 expression and IL-6 production by MDMs may be low in NTM patients
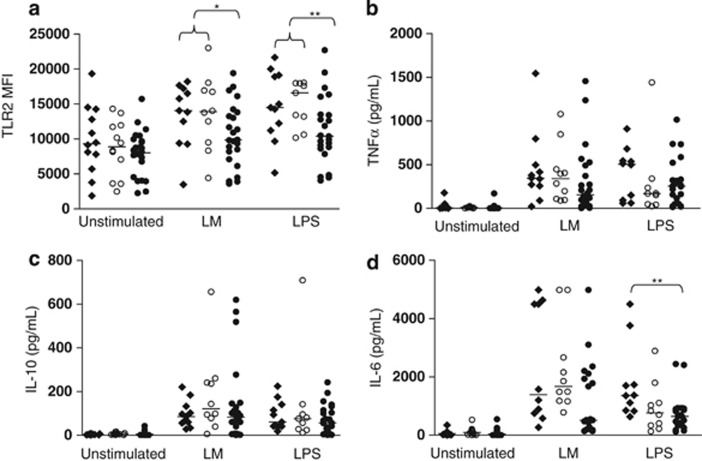
MDMs were cultured for 24 h with or without LM or LPS. Expression of TLR2 was assessed by flow cytometry, and levels of TNFα, IL-6 and IL-10 were assessed in supernatants. Compared with unstimulated cells, MDMs from all donors expressed more TLR2 after stimulation with LM or LPS (P=0.0006–0.024, Kruskal–Wallis test). The median level of TLR2 was marginally lower in NTM patients (Figure 1a). This difference became significant (P<0.05 and P<0.01 for LM and LPS, respectively) when MDMs from NTM patients were compared with MDMs from all healthy donors (offspring and healthy controls pooled).
Figure 1.
TLR2 and IL-6 responses are lower in NTM patients. TLR2 (a), TNFα (b), IL-10 (c) and IL-6 (d) responses assessed in MDMs from NTM patients (●), their offspring (○) and healthy population controls (⧫), with and without bacterial stimulation over 24 h. Horizontal lines represent median values. *P=0.05–0.01, patients versus offspring and population controls combined. **P<0.01, patients versus offspring and population controls combined (a) or patients versus population controls (d).
Levels of TNFα or IL-10 produced in response to LM or LPS were similar in NTM patients, offspring and controls (Figures 1b and c). However, cells from NTM patients produced less IL-6 than cells from controls in response to LPS (Mann–Whitney's test; P<0.01). IL-6 production in response to LPS appeared low in offspring as well as patients, but this did not reach statistical significance (P=0.063). IL-6 production in response to LM was lower in NTM patients than controls (P=0.047; Figure 1d).
In patients, levels of all three cytokines was directly correlated after stimulation with LM (r=0.57–0.74; P<0.001–0.0025, Spearman's correlation), so there is no evidence that IL-10 suppresses the pro-inflammatory response in NTM patients. LM-induced production of IL-10 and TNFα by cells from patients also correlated with their expression of TLR2 (r=0.51–59; P=0.002–0.01), providing a mechanism for low responses. Individual variation in these parameters did not correlate with treatment outcome (data not shown).
NTM disease is not associated with impaired MDM responses to IFNγ
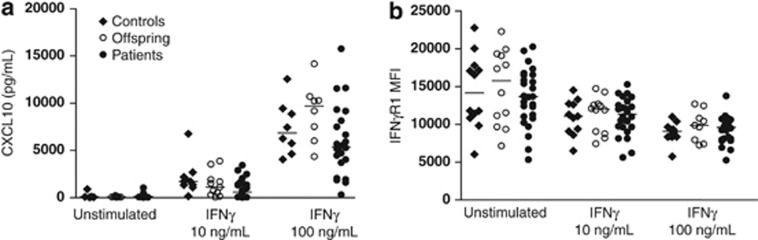
MDMs were cultured for 24 h in the presence of media alone or two concentrations of rhIFNγ, and CXCL10 was measured by enzyme-linked immunosorbent assay. Macrophages from NTM patients, offspring and controls produced similar levels of CXCL10 across all culture conditions (P=0.057–0.49; Figure 2a). Stimulation of MDMs with 10 ng ml−1 rhIFNγ reduced expression of IFNγR1 in cells from NTM patients (Wilcoxon's matched pair test, P<0.0001), offspring (P=0.0024) and healthy controls (P=0.0029), but created no differences between groups (P=0.65–0.94; Figure 2b).
Figure 2.
Macrophages from NTM patients display normal expression of IFNγR1 and CXC10 responses to IFNγ. Levels of CXCL10 (a) and expression of IFNγR1 (b) in MDM cultures after 24 h stimulation with rhIFNγ. Horizontal lines represent median values.
Blood monocytes from NTM patients and healthy controls display a similar phenotype and capacity for cytokine production
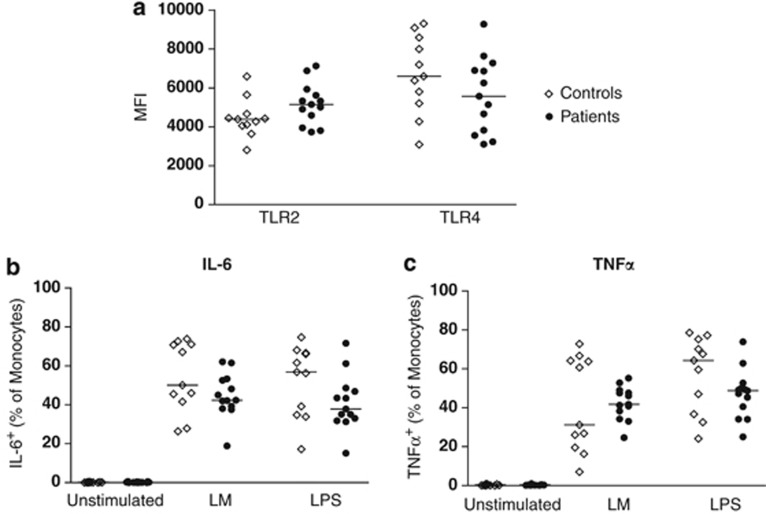
Uncultured peripheral blood mononuclear cells (PBMCs) from a subset of donors (n=10–12) were used to assess expression of TLR2 and TLR4 on CD14+ monocytes. PBMCs were then stimulated for 6 h with LPS or LM to assess production of IL-6 and TNFα. Monocytes from patients and controls did not differ significantly in their expression of TLR2 or TLR4 (Figure 3a), or in production of IL-6 or TNFα (Figures 3b and c). Although the median level of IL-6 production may be slightly lower in NTM patients in response to LPS, the trend did not reach statistical significance (P=0.118; Mann–Whitney's test).
Figure 3.
NTM disease is not associated with reduced responses by blood monocytes. Expression of TLR2 and TLR4 on unstimulated monocytes (a), and percentages of monocytes expressing IL-6 (b) and TNFα (c) after 6 h stimulation. Horizontal lines represent median values.
MDMs from NTM patients exhibit a normal capacity for phagocytosis and production of intracellular inflammatory mediators
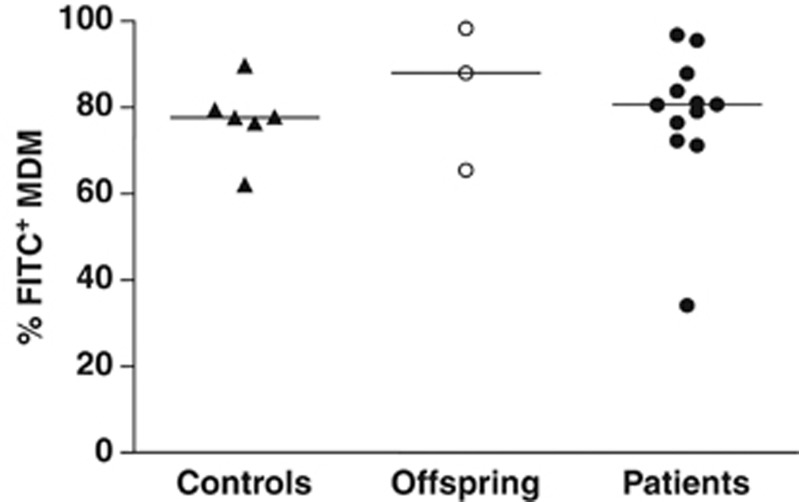
To determine whether an impairment in phagocytosis was associated with NTM disease, MDMs from healthy controls, offspring and patients were cultured for 2 h with rabbit IgG fluorescein isothiocyanate (FITC)-conjugated latex beads. There was no significant difference in the phagocytic capacities of MDMs across subject groups (Figure 4). Intracellular levels of COX-1, COX-2 and inducible nitric oxide synthase were measured by flow cytometry following stimulation with LM or LPS. Levels were not deficient in MDMs from NTM patients, compared with offspring or healthy controls (data not shown).
Figure 4.
MDMs from NTM patients exhibit a normal capacity for phagocytosis. The proportions of phagocytic MDMs (FITC+ cells) determined by co-culture with rabbit IgG FITC-conjugated latex beads for 2 h was similar between healthy controls (n=6), offspring (n=3) and NTM patients (n=12). Horizontal lines represent median values.
Discussion
The potential for defects in the functional capacity of macrophages to contribute to susceptibility to pulmonary NTM disease has not been addressed previously. Here we evaluated MDMs as a model for mature macrophages, such as are found in the lung. We included adult offspring of NTM patients as a healthy population sharing genetic factors with the patients.
A balance between TNFα and IL-10 production by macrophages is important in the regulation of inflammation. Excessive TNFα production may promote tissue damage, whereas IL-10 may suppress immune responses and subsequent clearance of bacteria.18 Here there were positive correlations between levels of IL-10, IL-6 and TNFα induced by LM, so the inflammatory response is not dominated by either TNFα or IL-10 in MDMs from NTM patients. It should be considered that many patients had been on effective therapy for over 6 months at the time of study. This may influence bacterial load and host immune responses, but an intrinsic (genetically determined) defect in macrophage activation would still be apparent.
Ryu et al.17 reported poor induction of TLR2 mRNA in blood monocytes (purified by adherence) from NTM patients, 4 h after stimulation with live M. avium. This correlated with poor production of TNFα and IL-12.17 It may be important that the cohort (n=17) included 9 patients infected with Mycobacterium abscessus; this generates a distinct and severe clinical presentation. Here induced expression of TLR2 was also low in MDM patients, but induction of IL-10 and TNFα was not depressed, so there is no evidence of a defect in signaling following ligation of TLR2. However, patients produced low levels of IL-6 in response to LPS, suggesting a subtle difference in the induction of this cytokine. This warrants further investigation, as IL-6 is involved in the induction of IL-17, and we have previously demonstrated poor production of IL-17 by CD4 T cells from patients drawn from the same cohort.12
It is also important to evaluate phagocytosis and intracellular killing in the setting of pulmonary NTM disease. Here the intracellular protein levels of COX-1, COX-2 and inducible nitric oxide synthase were not deficient in MDMs from NTM patients following stimulation with LM or LPS. Moreover, MDMs from NTM patients were not deficient in their capacity to take up fluorescently labeled beads (Figure 4), but assays using live mycobacteria are planned.
Expression of IFNγR1 was not deficient on MDMs from NTM patients following unstimulated culture or following stimulation by rhIFNγ (Figure 2). Decreased expression of IFNγR1 was seen with increasing concentrations of rhIFNγ, suggesting intact negative feedback in both NTM patients and healthy donors. This effect is paralleled by a significant increase in CXCL10 production by MDMs with increasing concentrations of rhIFNγ similarly evident across all groups (confirmed by Wilcoxon's matched pairs tests, P<0.0001–0.013). These results suggest that pulmonary NTM disease is not associated with deficient macrophage responses to IFNγ in terms of an ability to bind IFNγ (through IFNγR1) or become activated by rhIFNγ as evidenced by production of CXCL10. In accordance with this finding, plasma levels of CXCL10 were elevated in NTM patients from this cohort.19
Offsprings are genetically similar to the patients and may share a past or current exposure to the same NTM. Differences between patients and offsprings may be due to the disease process or may reflect genetic differences. Overall, there were no differences in MDM responses between patients and their offspring. However, offsprings were younger than the patients and some may develop NTM infection later in life. Healthy age-matched relatives of the patients would be ideal to study, but most patients had no siblings living locally.
At this point, it is feasible that MDMs may not usefully model lung macrophages from pulmonary NTM patients. For example, human blood monocytes, MDMs and alveolar macrophages differ in their patterns of TLR expression.20 Macrophages may be functionally polarized, with M1 or M2 phenotypes distinguished by gene profiling. M1 macrophages are pro-inflammatory, whereas M2 macrophages are important in resolving chronic infections and are weakly microbicidal. Genotypic and phenotypic indicators of an M2 profile characterize lepromatous lesions, whereas macrophages from tuberculoid granulomas possess an M1 phenotype.21 This warrants study in pulmonary NTM disease. Culture conditions may bias the polarization of MDMs, limiting their capacity to reveal differences relevant in vivo.22, 23
In summary, pulmonary NTM disease was not associated with deficient responses of MDMs to IFNγ. However, subtle deficiencies in TLR pathways may limit IL-6 production and perhaps IL-17 responses. Further research should utilize alveolar macrophages.
Methods
Study population and sample collection
PBMCs were collected from 26 patients with pulmonary NTM disease, attending outpatient clinics at Royal Perth Hospital (WA, Australia) between March 2007 and June 2010 (23 females, 3 males; median (range)=66 (54–82) years old). Pulmonary NTM disease was diagnosed using guidelines of the American Thoracic Society.6 All patients had disease due to M. intracellulare or M. avium. Exclusion criteria included cystic fibrosis, current smoking, HIV infection, alcohol excess or use of immunosuppressive medications; this evaluation was made by the patients' treating physician. At the time of study, 21 patients had been on treatment for >6 months (with 13 responding, 5 not responding and 3 patients partially responding to treatment) and 5 patients were treatment-naive. PBMCs were also collected from 12 adult offsprings (3 females, 9 males; 43 (27–66) years old) and from 12 healthy population controls (6 males, 6 females; 66 (54–82) years old), none of who reported a history of mycobacterial disease. Offsprings were significantly younger than patients and controls (P<0.0001, unpaired t-test). The study was approved by the Ethics Committee of Royal Perth Hospital, and informed written consent was obtained from all participants.
PBMC isolation and culture
PBMCs were isolated by Ficoll density centrifugation and cryopreserved in fetal calf serum containing 10% dimethyl sulfoxide. Once thawed, PBMCs were resuspended at 1 × 106 cells per ml in culture medium (RPMI containing 10% fetal calf serum, 1% penicillin/streptomycin solution and 2 mℳℒ-glutamine) and cultured for 6 h at 37 °C in polypropylene tubes containing media alone, 10 μg ml−1 LM (InvivoGen, San Diego, CA, USA) or 10 ng ml−1 LPS (Sigma, St Louis, MO, USA). Brefeldin A (BD Biosciences, San Diego, CA, USA) was added with the antigens to allow detection of cytokine production.
Monocyte isolation, generation of MDMs and culture with stimulants
Monocytes were isolated from PBMCs using EasySep magnetic separation (StemCell Technologies, Melbourne, VIC, Australia) to >90% purity based on expression of CD14. Enriched monocytes were cultured for 5 days in Teflon vials at 1 × 106 cells per ml in culture medium supplemented with 25 ng ml−1 macrophage colony-stimulating factor (ISOkine; ORF Genetics, Kopavogur, Iceland), with a half media change on day 3. A mature macrophage phenotype was confirmed by upregulation of CD80, HLA-DR and 25F9. MDMs were cultured at 105 cells per ml for 24 h at 37 °C in Immunosorp tubes (Nunc, Roskilde, Denmark) in media alone or with 10 μg ml−1 LM (InvivoGen), 10 ng ml−1 LPS (Sigma) or 10 or 100 ng ml−1 rhIFNγ (ISOkine; ORF Genetics). Supernatants were collected after 24 h and stored at −80 °C.
Flow cytometry
PBMCs and MDMs were washed with 1% bovine serum albumin in phosphate-buffered saline and then stained for either surface markers alone (15 min, room temperature in the dark) or for surface and intracellular markers using Cytofix/Cytoperm reagents (BD Biosciences). The following conjugated monoclonal antibodies were used: CD14-PECy7 (clone M5E2) from BioLegend (San Diego, CA, USA); CD16-APC-H7 (3G8), CD80-PE (L307.4), HLA-DR-Alexa647 (G46-6), IL-6 FITC (AS12), TNFα PE (MAb11) and TLR2 Alexa647 (11G7) from BD Biosciences; and TLR4-PE (HTA125) from eBioscience (San Diego, CA, USA). Viability was confirmed using DAPI (4',6-diamidino-2-phenylindole; Invitrogen, Grand Island, NY, USA) in which cells were stained for surface markers alone, and a Fixable Aqua LIVE/DEAD stain (Invitrogen) in which cells were also stained for intracellular markers. Data were acquired using a FACSCanto II cytometer (BD Biosciences) immediately after staining. A minimum of 10 000 MDM events and 100 000 lymphocyte and monocyte events were recorded and analyzed using FlowJo software version 7.6.1 (Tree Star, Ashland, OR, USA). Following exclusion of non-viable cells, doublets were excluded using forward-scatter height and area parameters.
Quantification of cytokines and chemokines in MDM culture supernatants
Levels of IL-6, IL-10 and TNFα were determined in undiluted supernatants using Cytometric Bead Array Flex Sets (BD Biosciences) and a FACSArray Bioanalyser (Software v1.0.3, BD Biosciences). Data were analyzed using FCAP Array Software v1.0.1 (Soft Flow, Pécs, Hungary). An enzyme-linked immunosorbent assay kit (BD Biosciences) was used to quantify CXCL10. The lower limit of detection was 5 pg ml−1 for cytometric bead array and 15 pg ml−1 for enzyme-linked immunosorbent assay.
Phagocytosis assay
The phagocytic capacity of MDMs was assessed using the Phagocytosis Assay Kit (Cayman Chemical, Ann Arbor, MI, USA) as per the manufacturer's instructions. Briefly, 5 × 104 MDMs were co-cultured with latex beads–rabbit IgG–FITC solution or media alone for 2 h at 37 °C in Immunosorp tubes. The MDMs were then washed and stained for CD14-PECy7 (clone M5E2; BioLegend) and DAPI (Invitrogen). Data were acquired using a FACSCanto II cytometer (BD Biosciences). The proportion of phagocytic (FITC+) MDM was determined by comparison with the untreated sample. Only viable cells (DAPI−) were included in the analysis.
Statistical analysis
Analyses were performed using Prism 5 (GraphPad Software, La Jolla, CA, USA) using non-parametric statistics. Kruskal–Wallis with Dunn's multiple comparison tests were used to compare results between pulmonary NTM patients, offspring and healthy controls. Mann–Whitney's tests were used to compare groups of >6 subjects. Wilcoxon's matched pairs tests were used to assess the effects of culture stimulants. Spearman's correlations for non-parametric data were used. P-values less than 0.05 were considered to be statistically significant.
Acknowledgments
We thank Shona Hendry for her assistance with patient medical records, Dr Justin Waring for recruiting patients, and all participants who donated samples. Financial support came from the Medical Research Foundation, Royal Perth Hospital.
The authors declare no conflict of interest.
References
- Billinger ME, Olivier KN, Viboud C, de Oca RM, Steiner C, Holland SM, et al. Nontuberculous mycobacteria-associated lung disease in hospitalized persons, United States, 1998–2005. Emerg Infect Dis. 2009;15:1562–1569. doi: 10.3201/eid1510.090196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley CL, Griffith DE. Pulmonary non-tuberculous mycobacterial infections. Int J Tuberc Lung Dis. 2010;14:665–671. [PubMed] [Google Scholar]
- Prevots DR, Shaw PA, Strickland D, Jackson LA, Raebel MA, Blosky MA, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. 2010;182:970–976. doi: 10.1164/rccm.201002-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ingen J, Hoefsloot W, Dekhuijzen PN, Boeree MJ, van Soolingen D. The changing pattern of clinical Mycobacterium avium isolation in the Netherlands. Int J Tuberc Lung Dis. 2010;14:1176–1180. [PubMed] [Google Scholar]
- Thomson RM. Changing epidemiology of pulmonary nontuberculous mycobacteria infections. Emerg Infect Dis. 2010;16:1576–1583. doi: 10.3201/eid1610.091201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- Thomson RM, Yew WW. When and how to treat pulmonary non-tuberculous mycobacterial diseases. Respirology. 2009;14:12–26. doi: 10.1111/j.1440-1843.2008.01408.x. [DOI] [PubMed] [Google Scholar]
- Young DB, Perkins MD, Duncan K, Barry CE. Confronting the scientific obstacles to global control of tuberculosis. J Clin Invest. 2008;118:1255–1265. doi: 10.1172/JCI34614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon YS, Kim EJ, Lee SH, Suh GY, Chung MP, Kim H, et al. Decreased cytokine production in patients with nontuberculous mycobacterial lung disease. Lung. 2007;185:337–341. doi: 10.1007/s00408-007-9040-z. [DOI] [PubMed] [Google Scholar]
- Vankayalapati R, Wizel B, Samten B, Griffith DE, Shams H, Galland MR, et al. Cytokine profiles in immunocompetent persons infected with Mycobacterium avium complex. JInfect Dis. 2001;183:478–484. doi: 10.1086/318087. [DOI] [PubMed] [Google Scholar]
- Kim RD, Greenberg DE, Ehrmantraut ME, Guide SV, Ding L, Shea Y, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med. 2008;178:1066–1074. doi: 10.1164/rccm.200805-686OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim A, Allison C, Price P, Waterer G. Susceptibility to pulmonary disease due to Mycobacterium avium-intracellulare complex may reflect low IL-17 and high IL-10 responses rather than Th1 deficiency. Clin Immunol. 2010;137:296–302. doi: 10.1016/j.clim.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Cottle LE, Sargur R, Egner W, Shackley F, Greig J. Susceptibility to mycobacterial infection in a young man with a hypoglossal nerve palsy: the hunt for an immunological defect. JRSM Short Rep. 2010;1:21. doi: 10.1258/shorts.2010.010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman SE, Picard C, Lammas D, Heyne K, van Dissel JT, Baretto R, et al. Clinical features of dominant and recessive interferon gamma receptor 1 deficiencies. Lancet. 2004;364:2113–2121. doi: 10.1016/S0140-6736(04)17552-1. [DOI] [PubMed] [Google Scholar]
- Drennan MB, Nicolle D, Quesniaux VJ, Jacobs M, Allie N, Mpagi J, et al. Toll-like receptor 2-deficient mice succumb to Mycobacterium tuberculosis infection. Am J Pathol. 2004;164:49–57. doi: 10.1016/S0002-9440(10)63095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng CG, Scanga CA, Collazo-Custodio CM, Cheever AW, Hieny S, Caspar P, et al. Mice lacking myeloid differentiation factor 88 display profound defects in host resistance and immune responses to Mycobacterium avium infection not exhibited by Toll-like receptor 2 (TLR2)- and TLR4-deficient animals. J Immunol. 2003;171:4758–4764. doi: 10.4049/jimmunol.171.9.4758. [DOI] [PubMed] [Google Scholar]
- Ryu YJ, Kim EJ, Lee SH, Kim SY, Suh GY, Chung MP, et al. Impaired expression of Toll-like receptor 2 in nontuberculous mycobacterial lung disease. Eur Respir J. 2007;30:736–742. doi: 10.1183/09031936.00039507. [DOI] [PubMed] [Google Scholar]
- Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- Lim A, Allison C, Tan DB, Oliver B, Price P, Waterer G. Immunological markers of lung disease due to non-tuberculous mycobacteria. Dis Markers. 2010;29:103–109. doi: 10.3233/DMA-2010-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez E, Nunez C, Sada E, Ellner JJ, Schwander SK, Torres M. Differential expression of Toll-like receptors on human alveolar macrophages and autologous peripheral monocytes. Respir Res. 2010;11:2. doi: 10.1186/1465-9921-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733–3739. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- Fleetwood AJ, Lawrence T, Hamilton JA, Cook AD. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J Immunol. 2007;178:5245–5252. doi: 10.4049/jimmunol.178.8.5245. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]