Abstract
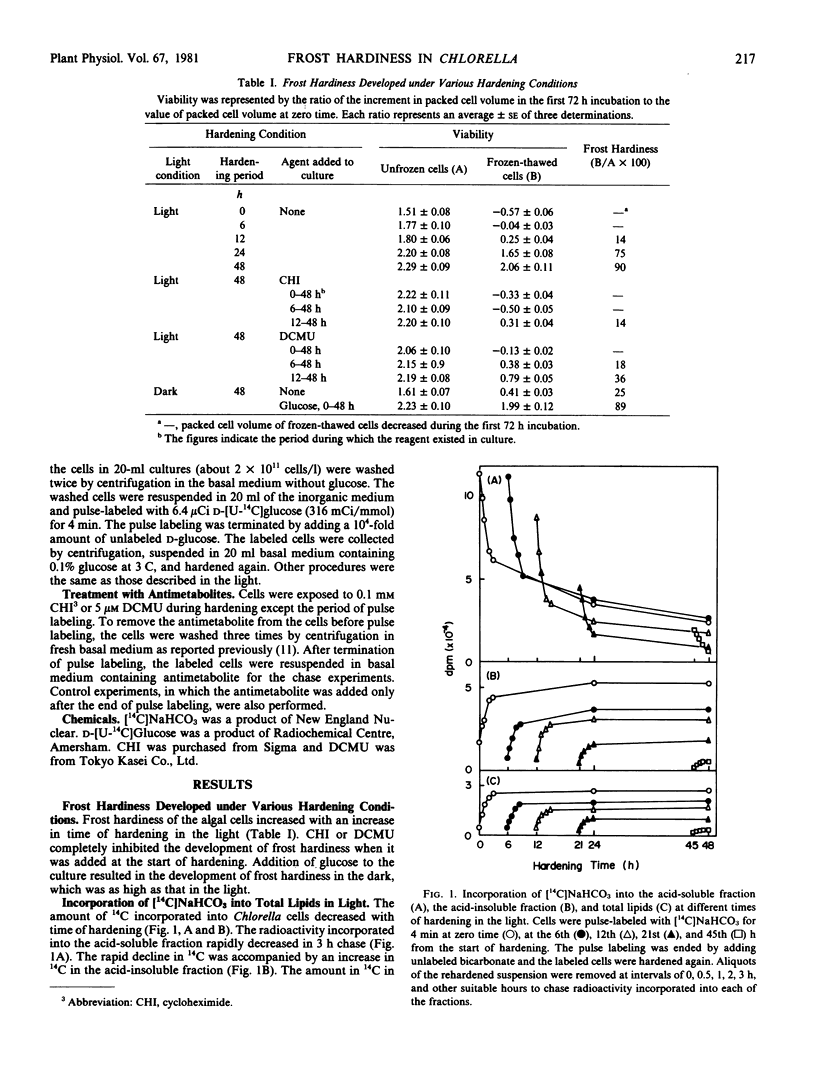
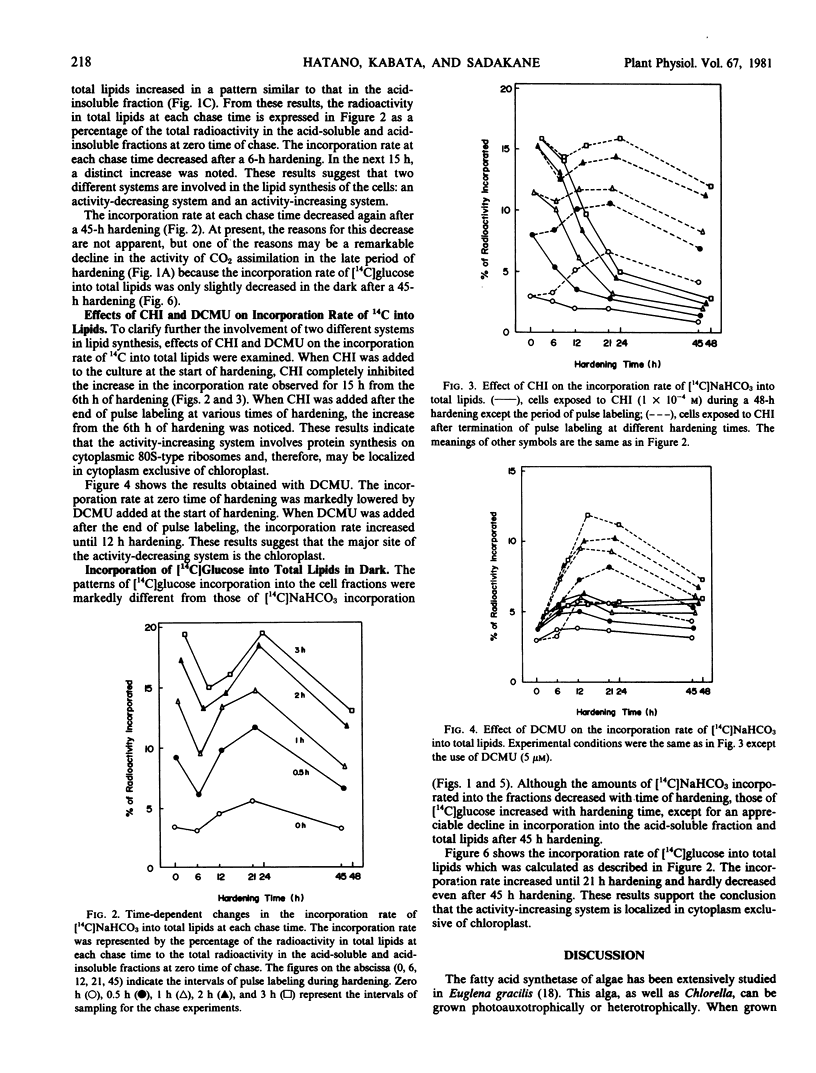
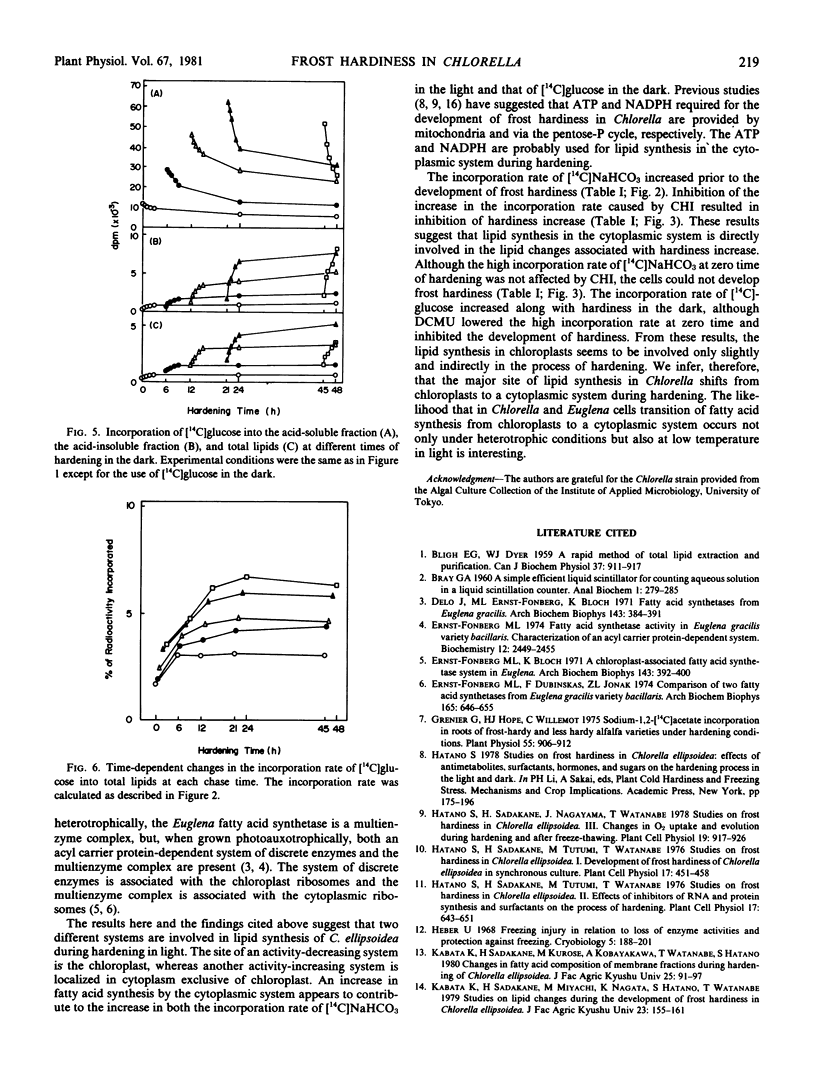
Chlorella ellipsoidea Gerneck (IAM C-27) was synchronously grown and cells at an intermediate stage in the ripening phase of the cell cycle were hardened at 3 C for 48 hours. At various times of hardening, the cells were pulse-labeled for 4 minutes with [14C]NaHCO3 in the light or with [14C]glucose in the dark, and the incorporation rate of 14C into total lipids was determined. A high incorporation rate of [14C]NaHCO3 at zero time of hardening decreased after 6 hours. In the next 15 hours, a distinct increase was noted. This increase occurred prior to the development of frost hardiness. Cycloheximide completely inhibited both the increase and the development, and 3-(3,4-dichlorophenyl)-1,1-dimethylurea remarkably lowered the high incorporation rate at zero time. The incorporation rate of [14C]glucose increased along with hardiness in the dark. These results suggest that the major site of lipid synthesis shifts from chloroplasts to a cytoplasmic system during hardening of Chlorella.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Delo J., Ernst-Fonberg M. L., Bloch K. Fatty acid synthetases from Euglena gracilis. Arch Biochem Biophys. 1971 Apr;143(2):384–391. doi: 10.1016/0003-9861(71)90225-6. [DOI] [PubMed] [Google Scholar]
- Ernst-Fonberg M. L., Bloch K. A chloroplast-associated fatty acid synthetase system in Euglena. Arch Biochem Biophys. 1971 Apr;143(2):392–400. doi: 10.1016/0003-9861(71)90226-8. [DOI] [PubMed] [Google Scholar]
- Ernst-Fonberg M. L., Dubinskas F., Jonak Z. L. Comparison of two fatty acid synthetases from Euglena gracilis variety bacillaris. Arch Biochem Biophys. 1974 Dec;165(2):646–655. doi: 10.1016/0003-9861(74)90293-8. [DOI] [PubMed] [Google Scholar]
- Ernst-Fonberg M. L. Fatty acid synthetase activity in Euglena gracilis variety bacillarius. Characterization of an acyl carrier protein dependent system. Biochemistry. 1973 Jun 19;12(13):2449–2455. doi: 10.1021/bi00737a013. [DOI] [PubMed] [Google Scholar]
- Grenier G., Hope H. J., Willemot C. Sodium-1,2-C Acetate Incorporation in Roots of Frost-hardy and Less Hardy Alfalfa Varieties under Hardening Conditions. Plant Physiol. 1975 May;55(5):906–912. doi: 10.1104/pp.55.5.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U. Freezing injury in relation to loss of enzyme activities and protection against freezing. Cryobiology. 1968 Nov-Dec;5(3):188–201. doi: 10.1016/s0011-2240(68)80163-4. [DOI] [PubMed] [Google Scholar]
- Steponkus P. L., Garber M. P., Myers S. P., Lineberger R. D. Effects of cold acclimation and freezing on structure and function of chloroplast thylakoids. Cryobiology. 1977 Jun;14(3):303–321. doi: 10.1016/0011-2240(77)90178-x. [DOI] [PubMed] [Google Scholar]
- Yoshida S. Changes in Microsomal Enzymes and Phospholipid during Dehardening in Stem Bark of Black Locust. Plant Physiol. 1976 May;57(5):710–715. doi: 10.1104/pp.57.5.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Sakai A. Phospholipid degradation in frozen plant cells associated with freezing injury. Plant Physiol. 1974 Mar;53(3):509–511. doi: 10.1104/pp.53.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]



