Abstract
Aim
Epilepsy is a complex disease necessitating continuous development of new therapeutic strategies to encounter drug-resistant cases. Among new adjuvant antiepileptic drugs, rufinamide is structurally distinct from other antiepileptic drugs. It is used to treat partial-onset seizures and seizures associated with Lennox-Gastaut syndrome (LGS) in adult and children. To date, there has been no attempt to evaluate systematically the risks of adverse events with rufinamide.
Methods
We performed a quantitative risk analysis of central nervous system (CNS) adverse events of rufinamide from all randomized, double-blind, add-on, placebo-controlled trials. The meta-analysis was undertaken with fixed effects models.
Results
Of the 886 publications reviewed, 99 papers were retrieved and five articles met the inclusion criteria. One thousand two hundred and fifty-two patients were included. Our study showed that exposure to rufinamide was associated with a significant increase in risk of somnolence [relative ratio (RR) 1.87; 95% confidence interval (CI) 1.33, 2.62; P = 0.0003], dizziness (RR 2.66; 95% CI 2.00, 3.55; P = 0.00001), fatigue (RR 2.14; 95% CI 1.57, 2.91; P = 0.01) and headache (RR 1.28; 95% CI 1.02, 1.59, P = 0.03). In addition, exposure to rufinamide was associated with higher treatment discontinuation rates as compared with placebo (RR 2.65; 95% CI 1.74, 4.03; P = 0.00001).
Conclusions
The risk of CNS adverse events appears to be increased in patients exposed to rufinamide as well as the treatment discontinuation rates. However, although statistical associations were significant, additional long term safety studies are required to confirm the clinical significance of these findings, as most reports described only mild and moderate adverse events.
Keywords: epilepsy, rufinamide, safety, central nervous system, children, adults
What is already known about this subject
Epilepsy is a complex disease necessitating continuous development of therapeutic strategies to encounter drug-resistant cases.
Among new adjuvant antiepileptic drugs, rufinamide is used to treat partial-onset seizures and seizures associated with Lennox-Gastaut syndrome (LGS) in adult and children.
To date, there has been no attempt to evaluate systematically the risks of adverse events with rufinamide.
What this study adds
This study systematically quantifies the risks of adverse central nervous system events based on rufinamide randomized, double-blind, add-on, placebo-controlled trials.
Introduction
Epilepsy is a complex disease necessitating continuous development of new therapeutic strategies to encounter drug-resistant cases. It is estimated that 30% of epileptic cases are refractory to the antiepileptic medications 1,2. In addition, epilepsy is more challenging in patients with Lennox–Gastaut syndrome (LGS) where more than 75% of seizures resist multiple antiepileptic drugs 3. Thus, efforts are currently devoted to discover adjuvant medications that could help in the management of epilepsy in these patients. Among the new adjuvant drugs, rufinamide is structurally distinct from other antiepileptic drugs 4. Rufinamide is used as an adjuvant anticonvulsant against partial-onset seizures and seizures associated with LGS in adults and children 5,6. Desirably, rufinamide has low plasma protein binding and is metabolized by hydrolysis without contribution of the cytochrome P450 system leading to uncommon drug interactions 7,8.
Moreover, several randomized controlled studies have confirmed the efficacy of rufinamide in the management of LGS and other drug-resistant epilepsies with limited effects on cognitive function 9. However, although the effectiveness of rufinamide has been confirmed 6, to date, there has been no attempt to evaluate systematically the risks of rufinamide-induced adverse effects. Usually, in clinical practice, decisions regarding the use of new adjuvant therapies to treat refractory epilepsy are quite complex and require careful weighing of different variables. Both safety and tolerability of rufinamide are considered among these determinants in defining the whole clinical effectiveness. Of interest, it has been reported that the incidence of drug-induced adverse events was higher in the rufinamide-treated group (around 5–10% higher than placebo) 10,11. Also, the drug was associated with higher withdrawal rates as compared with placebo. Of interest, the CNS adverse events were also reported among the most frequent reasons for treatment discontinuation during randomized clinical trials of rufinamide 10,11. Although some studies discussed tolerability of rufinamide 12, these studies were limited in their findings due to small sample size, few randomized trials and potential publication bias.
Therefore, as additional randomized placebo-controlled studies have been published, the overall sample size has increased and allows a more precise estimation of potential risks of rufinamide's adverse effects. The objective of the present study was to perform quantitative analysis of adverse CNS events of rufinamide including all randomized, double-blind, add-on, placebo-controlled trials.
Methods
Search strategy
We carried out literature searches of MEDLINE, EMBASE, Web of Science and the Cochrane Central Register for Controlled Trials database from their inception until 30 March 2014. The terms rufinamide and epilepsy were used in the systematic search with no language restrictions. We also searched for additional articles through review of the reference lists of published reviews.
Inclusion and exclusion criteria
Clinical trials were selected based on the following inclusion criteria: randomized controlled trials, double-blinded with placebo, conducted in patients with drug-resistant partial or generalized epilepsies, with the experimental drug or placebo added to a traditional antiepileptic drug therapy, in either adults or children. The primary outcome of interest in this meta-analysis was risks of CNS adverse events associated with exposure to rufinamide as defined by the authors of the original studies. We excluded reviews, case reports, editorials, and studies without placebo controls.
Statistical analyses
We calculated the relative risks of the different adverse CNS events by the ratio of their occurrence in the active vs. the placebo groups. Using a fixed effect model, Mantel–Haenszel analysis was utilized to calculate the risk ratios (RR) with 95% confidence interval (CI). The fixed effects model was used because the test for heterogeneity was negative. To assess for publication bias, we visually inspected the funnel plot of the study estimates on the log scale for relative ratios against their standard error. We also conducted sensitivity analyses to determine the influence of any individual study by excluding each study one by one and recalculating the pooled effect. To detect heterogeneity of studies and consistency of evidence, we used the χ2 and I2 tests. The benchmarks for I2 are 25%, 50%, and 75% representing low, moderate and high degrees of heterogeneity, respectively. All analyses were performed using Review Manager 5 13.
Results
The literature search resulted in 886 publications (Figure 1). After screening of titles and abstracts, 99 full articles were reviewed. Thereafter, 93 publications were excluded based on a carful review of the full texts, which did not include relevant information or were not controlled trials as defined in our inclusion criteria. Six studies met the inclusion criteria 5,10,11,14–16; however, after a careful analysis, we excluded one study by Kluger et al. 16 since it represents findings of the same group of subjects in Glauser et al.'s paper [5] with additional results from an open label follow-up, open label extension study. The remaining papers were five studies 5,10,11,14,15, which were included in the meta-analysis. In these clinical trials, the doses were titrated weekly based on weight, to a maximum dose of 45 mg kg−1 daily. Thereafter, the target dose was maintained throughout the trial.
Figure 1.
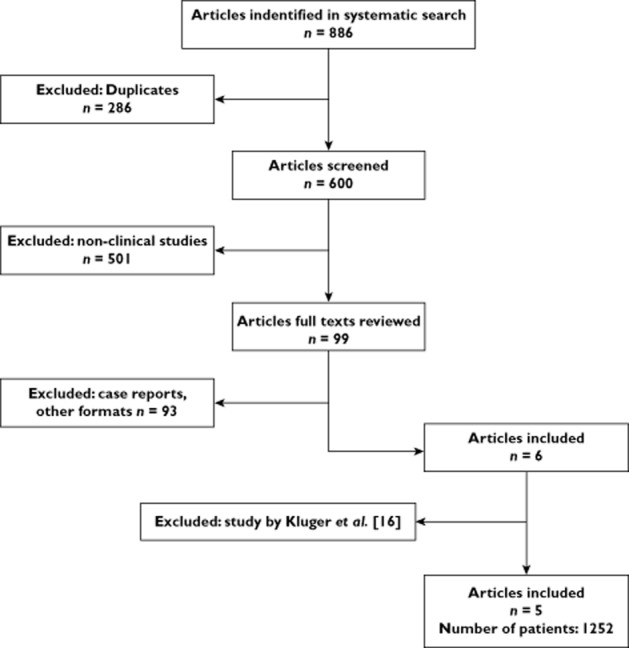
Study flow
A total of 1252 patients were included in the five studies accepted by us, varying from 25 to 262 patients per study (Table 1). In Glauser et al.'s study [5], there was a random assignment of patients into blocks of four for each centre 5. From Elger et al.'s paper [14], we included only two dosing groups (800 and 1600 mg/daily) in order to maintain consistency of dosage with the other studies 14. Also, taking into consideration patients' withdrawal from the trials, some minor differences were observed in patients' numbers within the rufinamide and placebo groups in some studies. The five studies included children and adults (age 4 to 80 years). However, the published data did not separate adverse events into children vs. adults.
Table 1.
Characteristics of included studies
| Study characteristic | Rufinamide | Placebo | |||||
|---|---|---|---|---|---|---|---|
| Study number | Authors | Year | Multicentre | Randomized | Reported adverse events | Patients treated | Patients treated |
| 1 | Palhagen et al. [15] | 2001 | 9 | 1:1 ratio | Fatigue, headache and dizziness | 25 | 25 |
| 2 | Glauser et al. [5] | 2008 | 36 | Blocks of four, centre level | Somnolence | 74 | 64 |
| 3 | Elger et al. [14] | 2010 | Yes* | 1:1 ratio | Fatigue, headache, dizziness and somnolence | 262 | 133 |
| 4 | Biton et al. [11] | 2011 | 65 | 1:1 ratio | Fatigue, headache, dizziness and somnolence | 176 | 180 |
| 5 | Brodie et al. [10] | 2009 | 48 | 1:1 ratio | Fatigue, headache, dizziness and somnolence | 156 | 157 |
Number of centres is not mentioned in the original article.
Safety of rufinamide
In the following sections, the meta-analyses of specific adverse CNS events are described.
Somnolence
Ninety-seven cases of somnolence were reported out of 668 patients treated with rufinamide, as compared with 45 of 534 patients treated with placebo, yielding a RR 1.87 (95% CI 1.33 to 2.62) (Table 2, Figure 2). There was no heterogeneity among the studies (χ2 = 0.61; d.f. = 3; P = 0.89; I2 = 0%).
Table 2.
Exposure to rufinamide and risk of CNS adverse events: meta-analysis of somnolence
| Study characteristics | Rufinamide | Placebo | Risk ratio | Weight | ||||
|---|---|---|---|---|---|---|---|---|
| Study number | Authors | Year | Events | Total | Events | Total | M-H, Fixed, 95% CI | % |
| 1 | Biton et al. [11] | 2011 | 22 | 176 | 13 | 180 | 1.73 [0.90, 3.33] | 27.3 |
| 2 | Brodie et al. [10] | 2009 | 32 | 156 | 19 | 157 | 1.70 [1.01, 2.86] | 40.3 |
| 3 | Elger et al. [14] | 2010 | 25 | 262 | 5 | 133 | 2.54 [0.99, 6.48] | 14.1 |
| 4 | Glauser et al. [5] | 2008 | 18 | 74 | 8 | 64 | 1.95 [0.91, 4.17] | 18.3 |
| Total events | 97 | 668 | 45 | 534 | 1.87 [1.33, 2.62] | 100 | ||
Heterogeneity: χ = 0.61, d.f. = 3 (P = 0.89); I2 = 0%. Test for overall effect: Z = 3.64 (P = 0.0003).
Figure 2.
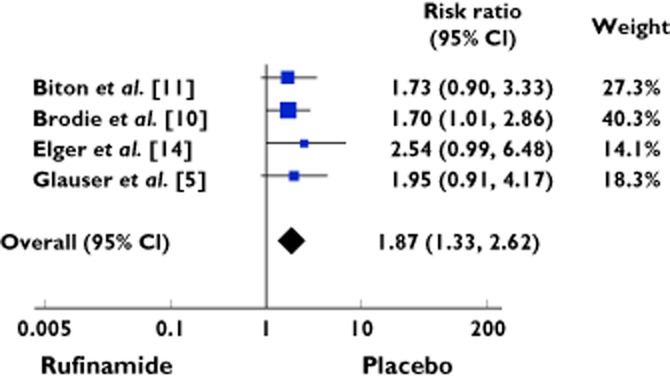
Forest plot showing the risk ratio with 95% confidence interval for somnolence in treatment vs. placebo
Dizziness
One hundred and fifty-eight cases presented with dizziness out of 619 patients treated with rufinamide as compared with 50 of 495 patients treated with placebo. The RR was 2.66 (95% CI 2.00 to 3.55) (Table 3, Figure 3). The studies were homogenous (χ2 = 3.34; d.f. = 3; P = 0.34; I2 = 10%).
Table 3.
Exposure to rufinamide and risk of CNS adverse events: meta-analysis of dizziness
| Study characteristics | Rufinamide | Placebo | Risk ratio | Weight | ||||
|---|---|---|---|---|---|---|---|---|
| Study No. | Authors | Year | Events | Total | Events | Total | M-H, Fixed, 95% CI | % |
| 1 | Biton et al. [11] | 2011 | 47 | 176 | 15 | 180 | 3.20 [1.86, 5.51] | 27.2 |
| 2 | Brodie et al. [10] | 2009 | 66 | 156 | 22 | 157 | 3.02 [1.97, 4.63] | 40.2 |
| 3 | Elger et al. [14] | 2010 | 43 | 262 | 13 | 133 | 1.68 [0.94, 3.01] | 31.6 |
| 4 | Palhagen et al. [15] | 2001 | 2 | 25 | 0 | 25 | 5.00 [0.25, 99.16] | 0.9 |
| Total events | 158 | 619 | 50 | 495 | 2.66 [2.00, 3.55] | 100 | ||
Heterogeneity: χ = 3.34, d.f. = 3 (P = 0.34); I2 = 10%. Test for overall effect: Z = 6.67 (P < 0.00001).
Figure 3.
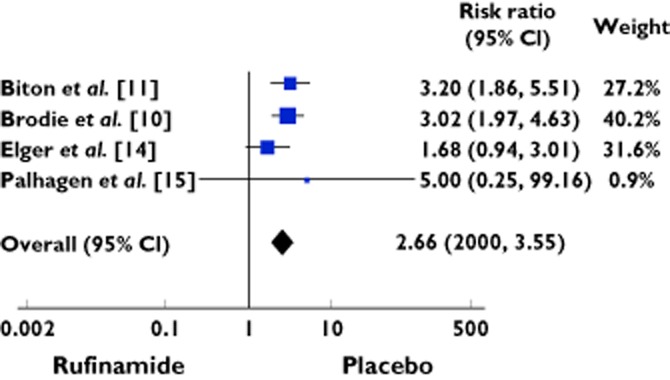
Forest plot showing the risk ratio with 95% confidence interval for dizziness in treatment vs. placebo
Fatigue/lethargy
Eighty-eight cases experienced fatigue/lethargy out of 619 patients treated with rufinamide as compared to 38 of 495 patients treated with placebo, yielding RR of 2.14 (95% CI 1.57 to 2.91) (Table 4, Figure 4). There was no heterogeneity among the studies (χ2 = 2.28; d.f. = 3; P = 0.52; I2 = 0%).
Table 4.
Exposure to rufinamide and risk of CNS adverse events: meta-analysis of fatigue/lethargy
| Study characteristics | Rufinamide | Placebo | Risk ratio | Weight | ||||
|---|---|---|---|---|---|---|---|---|
| Studynumber | Authors | Year | Events | Total | Events | Total | M-H, Fixed, 95% CI | % |
| 1 | Biton et al. [11] | 2011 | 4 | 176 | 3 | 180 | 1.36 [0.31, 6.01] | 6.6 |
| 2 | Brodie et al. [10] | 2009 | 25 | 156 | 13 | 157 | 1.94 [1.03, 3.64] | 28.9 |
| 3 | Elger et al. [14] | 2010 | 54 | 262 | 21 | 133 | 1.31 [0.83, 2.07] | 62.2 |
| 4 | Palhagen et al. [15] | 2001 | 5 | 25 | 1 | 25 | 5.00 [0.63, 39.79] | 2.2 |
| Total events | 88 | 619 | 38 | 495 | 1.57 [1.11, 2.24] | 100 | ||
Heterogeneity: χ = 2.28, d.f. = 3 (P = 0.52); I2 = 0%. Test for overall effect: Z = 2.53 (P = 0.01).
Figure 4.
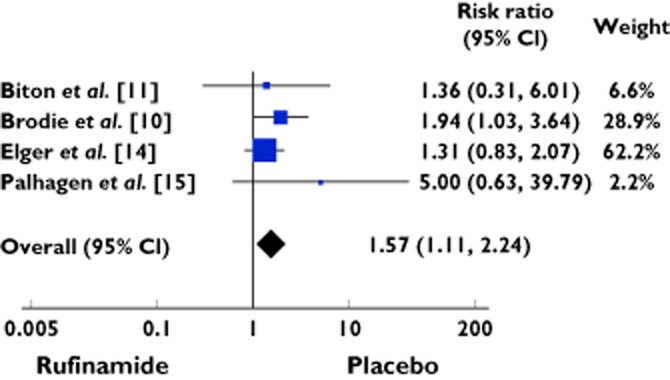
Forest plot showing the risk ratio with 95% confidence interval for fatigue/lethergy in treatment vs. placebo
Headache
One hundred and sixty cases exhibited headache out of 619 patients treated with rufinamide, vs. 98 of 495 patients treated with placebo. The RR was 1.28 (95% CI 1.02 to 1.59) (Table 5, Figure 5). The studies were homogenous (χ2 = 3.29; d.f. = 3; P = 0.35; I2 = 9%).
Table 5.
Exposure to rufinamide and risk of CNS adverse events: meta-analysis of headache
| Study characteristics | Rufinamide | Placebo | Risk ratio | Weight | ||||
|---|---|---|---|---|---|---|---|---|
| Study number | Authors | Year | Events | Total | Events | Total | M-H, Fixed, 95% CI | % |
| 1 | Biton et al. [11] | 2011 | 29 | 176 | 23 | 180 | 1.29 [0.78, 2.14] | 21 |
| 2 | Brodie et al. [10] | 2009 | 59 | 156 | 38 | 157 | 1.56 [1.11, 2.20] | 35 |
| 3 | Elger et al. [14] | 2010 | 69 | 262 | 32 | 133 | 1.09 [0.76, 1.57] | 39.3 |
| 4 | Palhagen et al. [15] | 2001 | 3 | 25 | 5 | 25 | 0.60 [0.16, 2.25] | 4.6 |
| Total events | 160 | 619 | 98 | 495 | 1.28 [1.02, 1.59] | 100 | ||
Heterogeneity: χ = 3.29, d.f. = 3 (P = 0.35); I2 = 9%. Test for overall effect: Z = 2.18 (P = 0.03).
Figure 5.
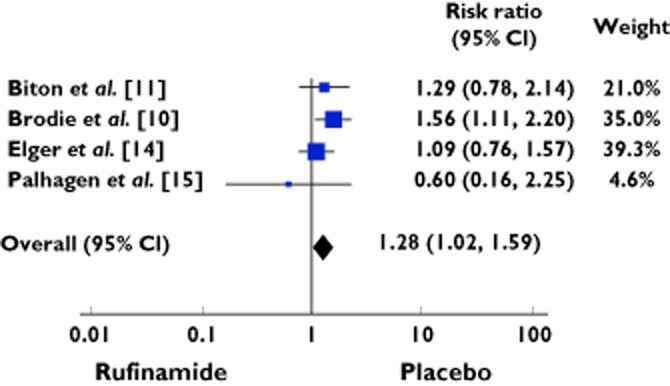
Forest plot showing the risk ratio with 95% confidence interval for headache in treatment vs. placebo
Overall treatment withdrawal rates due to overall adverse events
Eighty-five subjects receiving rufinamide were withdrawn from the studies due to overall adverse effects out of 693 as compared with 25 out of 559 patients receiving placebo [RR was 2.65 (95% CI 1.74 to 4.03)] (Table 6, Figure 6). The data was homogeneous (χ2 = 3.85; d.f. = 4; P = 0.43; I2 = 0%).
Table 6.
Exposure to rufinamide and treatment withdrawal due to overall adverse events: Meta-analysis
| Study characteristics | Rufinamide | Placebo | Risk ratio | Weight | ||||
|---|---|---|---|---|---|---|---|---|
| Study No. | Authors | Year | Events | Total | Events | Total | M-H, Fixed, 95% CI | % |
| 1 | Biton et al. [11] | 2011 | 27 | 176 | 11 | 180 | 2.51 [1.29, 4.90] | 37.7 |
| 2 | Brodie et al. [10] | 2009 | 21 | 156 | 5 | 157 | 4.23 [1.64, 10.93] | 17.3 |
| 3 | Elger et al. [14] | 2010 | 29 | 262 | 9 | 133 | 1.64 [0.80, 3.35] | 41.4 |
| 4 | Glauser et al. [5] | 2008 | 6 | 74 | 0 | 64 | 11.27 [0.65, 196.18] | 1.9 |
| 5 | Palhagen et al. [15] | 2001 | 2 | 25 | 0 | 25 | 5.00 [0.25, 99.16] | 1.7 |
| Total events | 85 | 693 | 25 | 559 | 2.65 [1.74, 4.03] | 100 | ||
Heterogeneity: χ = 3.85, d.f. = 4 (P = 0.43); I2 = 0%. Test for overall effect: Z = 4.57 (P < 0.00001).
Figure 6.
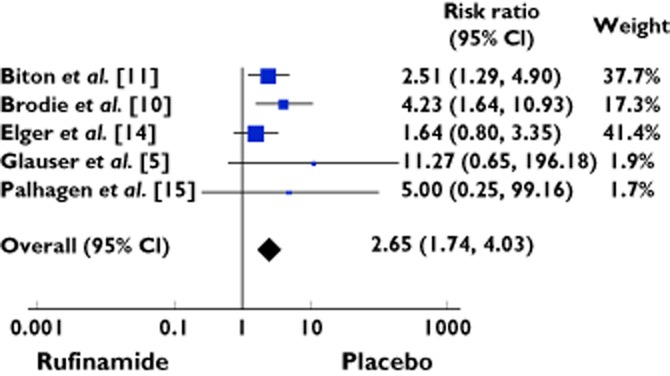
Forest plot showing the risk ratio with 95% confidence interval for treatment withdrawal in treatment vs. placebo
Influential studies and sensitivity analysis
Potential publication bias was assessed and ruled out by the symmetry of the funnel plot and sensitivity analysis. In the meta-analysis of somnolence, the studies by Biton et al. 11 and Brodie et al. 10 accounted for most of the relative weight of the analysis for exposure to rufinamide (27.3% and 40.3%, respectively). In the meta-analysis for dizziness, the studies by Elger et al. 14 and Brodie et al. 10 accounted for most of the relative weight of the analysis for exposure to rufinamide (31.6% and 40.2%, respectively), as was the case in the meta-analyses of fatigue and lethargy (62.2% and 28.9%, respectively) and headache (39.3% and 35%, respectively). Finally, in the meta-analysis of treatment discontinuation rate, the studies by Biton et al. 11 and Elger et al. 14 accounted for most of the relative weight of the analysis for exposure to rufinamide (37.7% and 41.4%, respectively).
In the sensitivity analyses, we excluded the studies one by one in order to recalculate the pooled risk. With the exception of Brodie et al.'s study 10, the relative ratios remained significant when each study was excluded in the meta-analysis of somnolence, dizziness, fatigue/lethargy, headache and treatment discontinuation rate. However, in the meta-analysis of fatigue/lethargy, when we excluded Brodie et al.'s study 10, the RR was 1.43, which was not significant (P = 0.10). Similarly, when we excluded Brodie et al.'s study 10, the RR of headache was 1.12, which was not significant (P = 0.43).
Estimates of absolute risk difference and number needed to harm
The absolute risks for the exposure groups were determined for rufinamide-induced CNS adverse events and treatment discontinuation rates. Our analysis revealed an absolute risk of 0.07, 0.16, 0.06, 0.06 and 0.07 for somnolence, dizziness, fatigue, headache and the treatment discontinuation rates, respectively. Accordingly, the number needed to harm was 14, 6, 16, 16 and 14 patients for somnolence, dizziness, fatigue, headache and the treatment discontinuation rates, respectively.
Discussion
During management of refractory epilepsy, rufinamide confers desirable benefits such as fewer drug-drug interactions, a lower cognitive adverse event profile and correlation of plasma concentrations with clinical efficacy 4,7,8. However, several randomized placebo-controlled studies have revealed mild to moderate risks of CNS and gastrointestinal adverse events. These adverse events were reported previously with an onset during the titration period and continuing throughout the maintenance period. Among different adverse events, rufinamide has specifically been associated with adverse CNS events including somnolence, dizziness, fatigue, and headache, which probably can explain the higher treatment discontinuation rates. To the best of our knowledge, the current study is the first meta-analysis to examine the association between exposure to rufinamide and risks of adverse CNS events. Our analysis revealed that exposure to the drug is associated with 2–3 fold increase above those reported with placebo. These data are important when rufinamide is added as adjuvant antiepileptic drug with a special focus on increased CNS adverse effects.
Of importance, it is necessary to acknowledge limitations within the current meta-analysis. While the meta-analytical techniques pool all the available data, limitations of the original articles are still exist as potential confounders and methodological limitations. Our study was based on five studies where the removal of studies that did account for the most weight was not associated with a change in statistical significance. The only exception was in meta-analysis of headache and fatigue where the removal of the study by Brodie et al. resulted in non-significant results 10. However, the lack of significance is probably secondary to reduced power. Moreover, existing randomized placebo-controlled studies did not separate adverse events into children vs. adults. Thus, it is possible that the rates of rufinomide's adverse CNS effects might be age-dependent. Early pharmacokinetic/pharmacodynamic analysis of rufinamide has suggested that the probability of adverse events is significantly affected by the patient's age with toxicity being more frequently encountered in adults than in children 17. Thus, further investigations are required to address this potential source of variability.
Another potential limitation of the current study might be attributed to differences in the methods of reporting drug-induced adverse events among different randomized studies with some adverse effects not being consistently reported in all trials including tremor, ataxia, nausea, vomiting and loss of appetite. Recently, there have been several case series and reports of weight loss that could be attributed to the nausea, vomiting and constipation associated with use of rufinamide 18,19. Furthermore, it is quite possible that the adverse events of rufinamide might be dose-dependent. Several studies have reported that the adverse events of this drug are more frequent during titration than during the maintenance phase. In pediatric trials, at a fixed titration dose of 45 mg kg−1 day−1, it has been shown that somnolence and headache were more common in the rufinamide-treated groups 5,20. In addition, several studies have suggested that drug adverse events occur more frequently at higher levels 21. However, to date, there are insufficient data to define a reference range for rufinamide 8. Therefore, further research is needed to determine the dose dependence of rufinamide toxicity.
In summary, rufinamide is associated with a 2–3 fold increased risk of adverse CNS events as compared with placebo. These effects were consistent among studies included in our meta-analysis and were rated as mild to moderate in severity. These data are important when rufinamide is added as an adjuvant antiepileptic drug with special focus on increased CNS adverse effects. Future randomized placebo-controlled studies should be designed to confirm the clinical significance of our findings and to compare the incidence of adverse CNS events in children vs. adults.
Competing Interests
Both authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work. AMSA was supported by an active scholarship from the Ministry of Higher Education and KSU. The sponsor had no role in the study design or data collection, analysis or interpretation.
We are grateful for the professional librarian help provided by Cheri Nickel at the library of the Hospital for Sick Children. We are thankful for the Deanship of Scientific Research, King Saud University (KSU), Riyadh, Saudi Arabia for supporting A.M.S.A. A.M.S.A. is clinical pharmacologist/toxicologist at KSU and active member of Motherisk, Division of Clinical Pharmacology and Toxicology, Hospital for Sick Children, University of Toronto.
References
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- French JA. Refractory epilepsy: clinical overview. Epilepsia. 2007;48:3–7. doi: 10.1111/j.1528-1167.2007.00992.x. [DOI] [PubMed] [Google Scholar]
- Kluger G, Bauer B. Role of rufinamide in the management of Lennox-Gastaut syndrome (childhood epileptic encephalopathy) Neuropsychiatr Dis Treat. 2007;3:3–11. doi: 10.2147/nedt.2007.3.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JR, Schultz RJ, Wilfong AA. Rufinamide for refractory epilepsy in a pediatric and young adult population. Epilepsy Res. 2011;93:87–89. doi: 10.1016/j.eplepsyres.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Glauser T, Kluger G, Sachdeo R, Krauss G, Perdomo C, Arroyo S. Rufinamide for generalized seizures associated with Lennox-Gastaut syndrome. Neurology. 2008;70:1950–1958. doi: 10.1212/01.wnl.0000303813.95800.0d. [DOI] [PubMed] [Google Scholar]
- Verrotti A, Loiacono G, Ballone E, Mattei PA, Chiarelli F, Curatolo P. Efficacy of rufinamide in drug-resistant epilepsy: a meta-analysis. Pediatr Neurol. 2011;44:347–349. doi: 10.1016/j.pediatrneurol.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Satoh T, Hosokawa M. Structure, function and regulation of carboxylesterases. Chem Biol Interact. 2006;162:195–211. doi: 10.1016/j.cbi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Perucca E, Cloyd J, Critchley D, Fuseau E. Rufinamide: clinical pharmacokinetics and concentration-response relationships in patients with epilepsy. Epilepsia. 2008;49:1123–1141. doi: 10.1111/j.1528-1167.2008.01665.x. [DOI] [PubMed] [Google Scholar]
- Aldenkamp AP, Alpherts WC. The effect of the new antiepileptic drug rufinamide on cognitive functions. Epilepsia. 2006;47:1153–1159. doi: 10.1111/j.1528-1167.2006.00589.x. [DOI] [PubMed] [Google Scholar]
- Brodie MJ, Rosenfeld WE, Vazquez B, Sachdeo R, Perdomo C, Mann A, Arroyo S. Rufinamide for the adjunctive treatment of partial seizures in adults and adolescents: a randomized placebo-controlled trial. Epilepsia. 2009;50:1899–1909. doi: 10.1111/j.1528-1167.2009.02160.x. [DOI] [PubMed] [Google Scholar]
- Biton V, Krauss G, Vasquez-Santana B, Bibbiani F, Mann A, Perdomo C, Narurkar M. A randomized, double-blind, placebo-controlled, parallel-group study of rufinamide as adjunctive therapy for refractory partial-onset seizures. Epilepsia. 2011;52:234–242. doi: 10.1111/j.1528-1167.2010.02729.x. [DOI] [PubMed] [Google Scholar]
- Wheless JW, Conry J, Krauss G, Mann A, LoPresti A, Narurkar M. Safety and tolerability of rufinamide in children with epilepsy: a pooled analysis of 7 clinical studies. J Child Neurol. 2009;24:1520–1525. doi: 10.1177/0883073809350508. [DOI] [PubMed] [Google Scholar]
- RevMan. Review Manager (RevMan) [Computer Program] Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2012. Version 52. [Google Scholar]
- Elger CE, Stefan H, Mann A, Narurkar M, Sun Y, Perdomo C. A 24-week multicenter, randomized, double-blind, parallel-group, dose-ranging study of rufinamide in adults and adolescents with inadequately controlled partial seizures. Epilepsy Res. 2010;88:255–263. doi: 10.1016/j.eplepsyres.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Palhagen S, Canger R, Henriksen O, van Parys JA, Riviere ME, Karolchyk MA. Rufinamide: a double-blind, placebo-controlled proof of principle trial in patients with epilepsy. Epilepsy Res. 2001;43:115–124. doi: 10.1016/s0920-1211(00)00185-6. [DOI] [PubMed] [Google Scholar]
- Kluger G, Glauser T, Krauss G, Seeruthun R, Perdomo C, Arroyo S. Adjunctive rufinamide in Lennox-Gastaut syndrome: a long-term, open-label extension study. Acta Neurol Scand. 2010;122:202–208. doi: 10.1111/j.1600-0404.2010.01334.x. [DOI] [PubMed] [Google Scholar]
- Eisai Data on File: Study E2080-A001-001. A Double-Blind, Placebo-Controlled, Ascending Multiple-Dose Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of Rufinamide in Healthy Subjects. Teaneck, NJ: Eisai Ltd; 2005. [Google Scholar]
- Mourand I, Crespel A, Gelisse P. Dramatic weight loss with rufinamide. Epilepsia. 2013;54:e5–8. doi: 10.1111/j.1528-1167.2012.03579.x. [DOI] [PubMed] [Google Scholar]
- Drake K, Labiner DM. Severe constipation associated with the use of rufinamide (Banzel) in an adolescent. Epilepsy Behav. 2010;18:132. doi: 10.1016/j.yebeh.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Wheless J, Conry J, Krauss G, Mann A, LoPresti-Solis A, Narurkar M. Safety and tolerability of rufinamide in pediatric patients: pooled data from the rufinamide clinical trials in epilepsy. Ann Neurol. 2009;66:S126. doi: 10.1177/0883073809350508. [DOI] [PubMed] [Google Scholar]
- Arroyo S. Rufinamide. Neurother. 2007;4:155–162. doi: 10.1016/j.nurt.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


