Abstract
Aim
The aim of the study was to investigate whether human megakaryocytic cells have an adaptive response to aspirin treatment, leading to an enhancement of multidrug resistance protein-4 (MRP4) expression in circulating platelets responsible for a reduced aspirin action. We recently found that platelet MRP4 overexpression has a role in reducing aspirin action in patients after by-pass surgery. Aspirin enhances MRP4-mRNA levels in rat liver and drug administration transcriptionally regulates MRP4 gene expression through peroxisome proliferator-activated receptor-α (PPARα).
Methods
The effects induced by aspirin or PPARα agonist (WY14643) on MRP4 modulation were evaluated in vitro in a human megakaryoblastic DAMI cell line, in megakaryocytes (MKs) and in platelets obtained from human haematopoietic progenitor cell (HPC) cultures, and in vivo platelets obtained from aspirin treated healthy volunteers (HV).
Results
In DAMI cells, aspirin and WY14643 treatment induced a significant increase in MRP4 and PPARα expression. In human MKs grown in the presence of either aspirin or WY14643, MRP4 and PPARα-mRNA were higher than in control cultures and derived platelets showed an enhancement in MRP4 protein expression. The ability of aspirin to modulate MRP4 expression in MKs and to transfer it to platelets was also confirmed in vivo. In fact, we found the highest MRP4 mRNA and protein expression in platelets obtained from HV after 15 days' aspirin treatment.
Conclusions
The present study provides evidence, for the first time, that aspirin treatment affects the platelet protein pattern through MK genomic modulation. This work represents an innovative and attractive approach, useful both to identify patients less sensitive to aspirin and to improve pharmacological treatment in cardiovascular high-risk patients.
Keywords: aspirin, MRP4, platelets, PPARα
What is already known about this subject
Aspirin treatment reduces cardiovascular complication in high risk patients.
Less aspirin inhibition of platelet function, for a residual thromboxane formation, is independently associated with an increased risk of cardiovascular events.
Platelet multidrug resistance protein-4 (MRP4) overexpression is a new mechanism of suboptimal platelet inhibition by aspirin in patients who have undergone recent CABG surgery.
What this study adds
Aspirin induces changes in megakaryocytes gene expression leading to MRP4 protein up-regulation in human platelets.
The nuclear receptor, peroxisome proliferator-activated receptor-α (PPARα) is involved in aspirin dependent MRP4 overexpression.
Introduction
Exposure of eukaryotic cells to drugs can trigger modifications in the expression of mechanisms susceptible to favour their elimination. Most of them are often related to transient induction of the corresponding gene transcriptional regulation by nuclear receptors. Drug-induced overexpression of efflux transporters, multidrug resistance proteins (MRPs) are the most involved mechanism of drug resistance 1.
MRP4 is a member of the MRP/ABCC subfamily of ATP-binding cassette transporters, which are capable of pumping a wide variety of endogenous and xenobiotic organic anionic compounds out of the cell. Its induction reduces intracellular organic anion toxicity or cholestasis 2. In human cells MRP4 up-regulation, after long term exposure to nucleoside-based drugs, severely impaired the antiviral efficacy of PMEA (9-(2-phosphonylmethoxyethyl)adenine), azidothymidine and other nucleoside analogues enhancing drug efflux 3.
As first we demonstrated that aspirin is a target for MRP4 in human platelets 4 and very recently it was confirmed that both aspirin and its metabolite, salicylic acid, are substrates for mouse ABCC4 (MRP4) 5.
Aspirin, at low dosage, acts as an anti-platelet agent and it is able to reduce cardiovascular complications in high risk patients 6,7.
Some patients receiving aspirin therapy for secondary prevention do not respond appropriately to aspirin, a heterogeneous phenomenon which is known as ‘aspirin resistance’ 8. An incomplete suppression of thromboxane generation is independently associated with an increased risk of cardiovascular events 9,10 and residual platelet COX-1 function measured by serum thromboxane B2 correlates with subsequent major adverse cardiovascular events 11.
Suboptimal platelet inhibition by aspirin is particularly common in some diseases such as diabetes 12 and essential thrombocythemia 13 as well as in patients undergoing coronary artery bypass graft (CABG) surgery 14. In such patients we demonstrated that MRP4 is over-expressed and such up-regulation may be responsible for the reduced aspirin activity on COX-1 4. Eikelboom & Hankey suggested that platelet MRP4 overexpression is a new, hitherto unrecognized mechanism of ‘aspirin resistance’ that could explain suboptimal platelet inhibition by aspirin in patients with recent CABG surgery 15. It has been previously demonstrated that aspirin enhances MRP4 mRNA levels in rat liver 16. MRP4 up-regulation in platelets could be an adaptive response necessary to reduce intracellular organic anion toxicity.
Many transcription factor families participate in metabolic MRP4 regulation. In prostate cancer cells, MRP4 gene expression is transcriptionally regulated, at least in part, through androgen receptor activation 17; others showed that peroxisome proliferator-activated receptor-α (PPARα) regulates hepatic MRP4 expression in mouse, after in vivo perfluorooctanoic acid and perfluorodecanoic acid (PFDA) administration 18, while there is no significant correlation between mRNA expression of PPARγ and MRP4 genes 19.
PPARs are ligand activated transcription factors that heterodimerize with the retinoid X receptor and activate transcription binding to a specific DNA element, termed peroxisome proliferator response element (PPRE), in the regulatory region of a variety of genes encoding proteins that are involved in lipid and glucose metabolism and inflammatory response 20.
Human bone marrow megakaryocytes (MKs) express PPARα, as well as platelets, and its activation can decrease PDGF-BB expression, both in MKs and in platelets 21.
Aspirin might influence expression of PPARα related target genes. In fact, aspirin increases both PPARα mRNA and protein expression in macrophages 22, as well as the ABCA1 expression levels via a PPARα dependent mechanism 23.
Very recently, a set of platelet-enriched, co-expressed genes and proteins, named aspirin response signature (ARS), was identified. It was associated with platelet function in healthy volunteers (HV) and with death or myocardial infarction in cardiology patients only in those using aspirin 24. These data suggest that aspirin exposure may alter the genomic and proteomic content of circulating platelets.
The aim of our study is to verify whether aspirin affects megakaryocytic gene expression leading to MRP4 up-regulation in human platelets and whether this mechanism is dependent on PPARα activation. In order to reach our goal, we evaluated the aspirin effects on MRP4 gene modulation in a human megakaryoblastic DAMI cell line, MK progenitor cell cultures and in their derived platelets, and platelets obtained from in vivo aspirin treated HV.
Our results demonstrate, for the first time, the mechanism by which both in vitro and in vivo aspirin treatments influence MK gene expression leading to protein overexpression in human platelets.
Methods
DAMI cultures
A human megakaryoblastic cell line (DAMI) was maintained in RPMI-1640 Media supplemented with 10% heat-inactivated fetal bovine serum, 20 mm L-glutamine, 100 units ml−1 of penicillin G sodium and 100 μg ml−1 streptomycin sulphate in a humidified atmosphere containing 5% CO2 at 37°C 25. The DAMI cell line was kept under control and its membrane phenotype was periodically monitored by fluorescence-activated cell sorting (FACS) analysis.
Cells were treated with either aspirin (50 μm−100 μm), salicylate (50 μm−100 μm) or the PPARα agonist (WY14643) (1 μm) (Sigma Chemicals Company, St Louis, MO, USA) for 48 h in a mock culture and an equivalent amount of DMSO, present in aspirin and WY14643 suspension, was added. At the end of the treatment, cells were processed for RNA and protein extraction.
RNA interference
Double strand interfering RNA (siRNA) targeting human PPARα and a control non-specific siRNA (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were transfected by means of the Lipofectamine RNAiMAX Reagent (Invitrogen, San Diego, CA). Twenty-four h after siRNA administration, cells were treated with aspirin (50 μm) or PPARα agonist (WY14643) (1 μm) (SIGMA Chemicals Company, St Louis, MO, USA). Forty-eight h after treatment, cells were processed for RNA and protein analysis.
Human haematopoietic progenitor cell (HPC) purification
Adult peripheral blood (PB) was obtained from 20−40-year-old healthy male donors after informed consent. Low density mononuclear cells (MNCs) (less than 1.077 g ml−1) were isolated by Ficoll-Hypaque density-gradient centrifugation and CD34+ HPCs were purified by using the MiniMACS isolation system (Milteny, Bergisch, Gladbach, Germany) according to the manufacturer's instructions. Purified cells were more than 90% CD34+, as evaluated by fluorescence-activated cell sorting (FACS) analysis.
MK unilineage cultures
Purified HPCs (1 × 105 cells ml−1) were grown in fetal calf serum free (FCS−) unilineage MK liquid culture 26, in the presence of saturating doses of thrombopoietin (TPO) (100 ng ml−1) (Peprotech, Rocky Hill, NJ, USA) alone or in combination with aspirin (50 μm) or WY14643 (1 μm). Either aspirin or WY14643 treatment started from day 6 of culture and continued for the following 4 days. In a mock culture an equivalent amount of DMSO, present in aspirin and WY14643 suspension, was added. Cells were incubated in a fully humidified atmosphere of 5% CO2, 5% O2 and 90% N2 for 14 days. The cells were periodically counted and analyzed for viability, morphology, membrane phenotype and polyploidy through 6 to 14 days' culture. MKs were collected at different days of differentiation for mRNA analysis. Platelets, produced at the end of the culture, were isolated from supernatants by centrifugation at room temperature for 10 min at 120 g, washed with phosphate buffered saline (PBS) and pelleted at room temperature at 900 g 27.
Platelet preparation and isolation
Platelets were obtained from Caucasian HV (aged 25 to 54 years, seven males, three females), who did not take any drugs the previous month, at 1 day (T1), 7 days (T7) and 15 days (T15) after beginning aspirin treatment (300 mg day−1). Informed written consent was obtained from each HV.
Platelet preparation was performed as previously reported 28. Briefly citrated venous blood was collected from the donors. Blood samples were centrifuged at 200 g for 15 min and platelet-rich plasma (PRP) was collected. ACD (39 mM citric acid, 75 mM sodium citrate, 135 mM dextrose, pH 7.4) buffer was added to the PRP which was then centrifuged at 1000 g for 10 min to remove the plasma. The platelet pellet was resuspended with platelet-washing buffer, in the presence of EDTA (5 mm), and then it was filtered through a 5 μm syringe-adaptable filter to remove white blood cell contaminants 29.
The mature transcript for CXCL8, (interleukin-8), a leukocyte-specific gene product 30, was absent in platelet preparation after filtration showing that leukocyte contamination was not significantly detectable.
Flow cytometry analysis of cell surface antigens
The phenotype of DAMI cells and differentiating HPCs was analyzed by using the following monoclonal antibodies (MoAbs) directly conjugated with either fluorescein isothiocyanate (FITC) or PE: anti-CD34, anti-CD42b, anti-CD61, anti-CD62 (Becton Dickinson) and anti CD41a (Serotec). Cells were incubated for 45 min at 4°C in the presence of proper amounts of specific MoAbs. After three washes with cold PBS, cells were resuspended in 1% formaldehyde and analyzed for fluorescence on a FACS SCAN flow cytometer (Becton Dickinson, Mountain View, CA, USA).
Protein extraction and Western blot
To analyze the MRP4 protein, cells were washed twice with cold PBS, collected and centrifuged at 400 g for 10 min. Platelets were washed twice with platelet-washing buffer, collected and centrifuged at 7000 g for 3 min.
Platelets and cell pellets were then resuspended in lysis buffer (RIPA buffer: 10 mm Tris-HCl pH 7.6, 160 mm NaCl, 1 mm EGTA, 1% deoxyxholic acid, 1% Triton, 0.1% SDS) incubated in ice for 30 min and centrifuged at 16 000 g for 30 min. The supernatant was then collected.
Protein extracts (30 μg) were incubated at 37°C for 30 min and separated on 4–12% SDS-PAGE gel, blotted onto PVDF membrane (GE Healthcare, Milano, Italy), and probed with rat anti-MRP4 (Alexis, Plymouth Meeting, Pennsylvania) and mouse anti-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) monoclonal antibodies. Immunoreactive bands were visualized by enhanced chemoluminescence (PerkinElmer, Waltham, MA, USA).
The densitometric analysis was performed with the National Institutes of Health ImageG analyzer programme.
RNA preparation and real-time quantitative PCR analysis
Total RNA from human cell lines, MKs and human platelets was extracted using TRIzol reagent (Invitrogen, San Diego, CA, USA).
For mRNA detection 1 μg of total RNA was transcribed using the GeneAmp Gold RNA PCR Reagent Kit pAW109 (Applied Biosystems, Warrington, UK) according to the manufacturer's instructions.
The analysis of gene expression was carried out with Q-RT-PCR using TaqMan® Master Mix and TaqMan®Assay Reagents (Applied Biosystems, Warrington, UK).
The programme of amplification, monitored using ABI Prism 7900 Sequencer Detector (Applied Biosystems, Foster City, CA, USA), was as follows: 50°C for 2 min, 95°C for 10 min, 95°C for 15 s and 60°C for 1 min, the latter two temperatures were repeated for 40 cycles.
All amplification reactions were performed in duplicate using 25 ng of cDNA.
Changes in MRP4, PPARα, CXCL8, actin, GAPDH and CD42B mRNA amounts were quantified by using the ΔΔCt method for relative quantification of gene expression using SDS software version 2.3 (Applied Biosystems Warrington, UK).
Immunofluorescence
For immunofluorescence, DAMI cells and platelets were fixed with 4% paraformaldehyde in PBS for 30 min, washed in 0.1 m glycine for 20 min and permeabilized in 0.1% Triton X-100 for an additional 5 min. In order to investigate the subcellular localizations of MRP4, the cells were incubated with anti-MRP4 (Alexis) polyclonal followed by rodamine-anti rabbit (Alexis) or Alexa 488-anti-rabbit (Invitrogen, San Diego, CA, USA). The nuclei were stained with 4,6-diamido-2-phenylindole (DAPI, Sigma-Aldrich, Milan, Italy). Images at 40× and 60× were taken with an AxioCam with a Zeiss Apotome Axio Observer Z1 inverted microscope (Carl Zeiss, Thornwood, New York, USA).
Statistics
Data are presented as mean ± SD. The level of significance was determined by an unpaired, two-tailed Student's t-test (KaleidaGraph software 3.6). Results were considered statistically significant if a P value of less than 0.05 was reached.
Results
MRP4 expression in aspirin treated DAMI cells
The effect of aspirin on MRP4 expression was initially investigated in a human megakaryoblastic cell line (DAMI) that permanently showed the phenotype CD61+, CD41+, CD42+ as evaluated by FACS analysis (data not shown).
DAMI cells treated with aspirin (50−100 m for 48 h) showed higher MRP4 mRNA levels compared with mock culture (Figure 1A upper graph).
Figure 1.
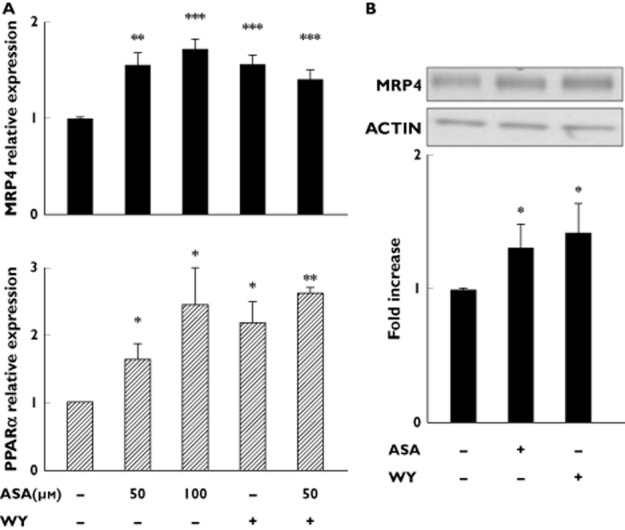
Aspirin stimulates endogenous MRP4 mRNA and protein expression in DAMI cells. (A) Q-RT-PCR analysis of endogenous MRP4 and PPARα expression 48 h after treatment with either 50 μm or 100 μm aspirin (ASA) and/or 1 μm PPARα agonist, WY14643 (WY). Data were normalized with the mean of the fold induction of GAPDH, ACTB and CD42B expression and reported as mean ± SD of three experiments (*P < 0.05, **P < 0.01, ***P < 0.001; t-test). Statistical analysis for the comparison WY14643 vs. WY14643+ASA 50 μm is not significant (NS). (B) Representative Western blot, of four performed, of endogenous MRP4 expression 48 h after treatment with either 50 μm aspirin (ASA) or 1 μm PPARα agonist (WY). Densitometric analysis is reported as mean ± SD of increasing fold, compared to control. (n = 4; *P < 0.05; t-test)
It was also demonstrated that aspirin in vitro treatment was associated with increases of PPARα expression 22. Therefore, we investigated whether aspirin treatment affected the expression of nuclear receptor PPARα also in the megakaryocytic lineage. As expected, aspirin treatment (50–100 μm for 48 h) increased PPARα mRNA expression in DAMI cells (Figure 1A lower graph), thus suggesting that PPARα regulates MRP4 expression.
To explore further whether PPARα was responsible for the MRP4 expression increase, DAMI cells were treated with the selective PPARα agonist, WY14643 (1 μm for 48 h) 31. The results obtained show that WY14643 cell treatment caused an increase of both MRP4 (fold increase 1.49) and PPARα (fold increase 2.2) mRNA expression as compared with untreated cells. Both increases were similar to those obtained in aspirin-treated cells (Figure 1A). To exclude that aspirin could act by different mechanisms from PPARα on MRP4 up-regulation, we performed experiments in which DAMI cells were treated with aspirin and WY14643 in combination. The results obtained suggest that there is not any synergistic effect between aspirin and WY14643. In fact the MRP4 and PPARα mRNA levels in aspirin plus WY14643 (fold increase 1.45 and 2.62 respectively) treated cells are similar to those obtained after treatment with WY14643 alone (Figure 1A).
MRP4 protein expression in aspirin treated cells was studied using two different experimental approaches, Western blot and immunofluorescence analysis.
Western blot analysis revealed an increase of MRP4 protein expression in cells treated with either aspirin (50 μm for 48 h) or WY14643 (1 μm for 48 h) (Figure 1B upper figure). Densitometry analysis showed a statistically significant higher induction of MRP4 protein expression, that is enhanced more than 30% in comparison with untreated cells (Figure 1B lower graph).
In the same experimental conditions, immunofluorescence analysis confirmed a consistent MRP4 staining increase in cells treated with either aspirin or WY14643 in comparison with mock cells, that showed only a punctate labelling pattern (Figure 2A).
Figure 2.
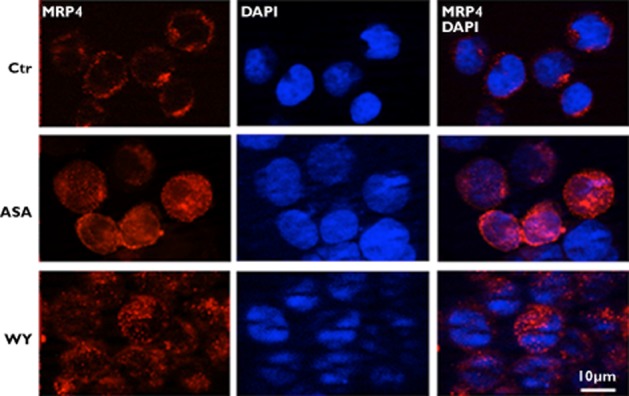
Aspirin stimulates endogenous MRP4 protein expression in DAMI cells. Immunofluorescence analysis of MRP4 (red) in DAMI cells, in untreated (Ctr) or treated, for 48 h, with either 50 μm aspirin (ASA) or 1 μm PPARα agonist, WY14643 (WY). Nuclei are counterstained with DAPI (blue). All data shown are representative of three independent experiments
More importantly, we measured MRP4 and PPARα expression after discontinuation of the drug and we observed a significant decrease of MRP4 and PPARα expression in in vitro studies 2 days after suspension of aspirin treatment (WO 2 days). This effect occured because, 2 days after suspension, about 80% of DAMI cells analyzed were grown in the absence of aspirin. Decrease in MRP4 (Figure 3A) and PPARα (Figure 3B) expression became more evident after 5 and 7 days (WO 5 and WO 7 days).
Figure 3.
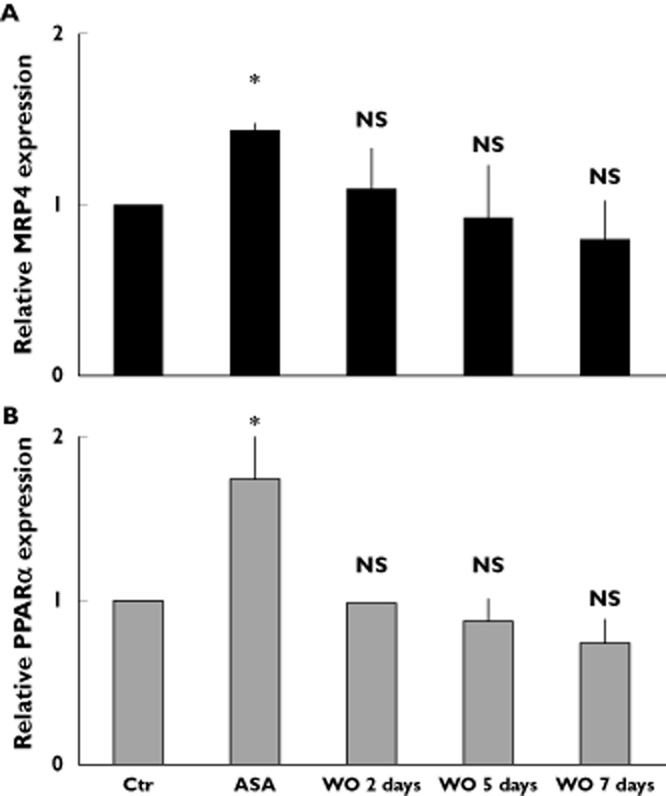
MRP4 expression after aspirin suspension in DAMI cells. Q-RT-PCR analysis of endogenous MRP4 and PPARα expression in DAMI cells, in either untreated (Ctr) or treated, for 48 h, with 50 μm aspirin (ASA) or after 2, 5 and 7 days of aspirin suspension (wash out, WO). Data were normalized with the mean of the fold induction of GAPDH, ACTB and CD42B expression and reported as mean ± SD of three experiments (NS = not significant, *P < 0.05; t-test)
In order to demonstrate whether aspirin dependent gene expression may be due to ‘salicylate moiety-associated’ effect, we also performed experiments with salicylate. Such experiments showed that salicylate treatment did not increase mRNA and protein MRP4 expression levels (data not shown).
To confirm further the involvement of PPARα in MRP4 up-regulation, DAMI cells were transfected with PPARα specific siRNA (PPAR-si). Cells transfected with PPAR-si did not show any significant increase of PPARα mRNA and protein expression after aspirin and WY14643 treatment while cells transfected with control non-specific siRNA (CTR-si) showed the same aspirin and WY14643 induced expression changes observed in untrasfected cells (Figure 4A and B). Similarly, no increase of aspirin and WY14643 dependent MRP4 expression was detected in cells treated with PPAR-si, unlike those found in CTR-si transfected cells (Figure 4C).
Figure 4.
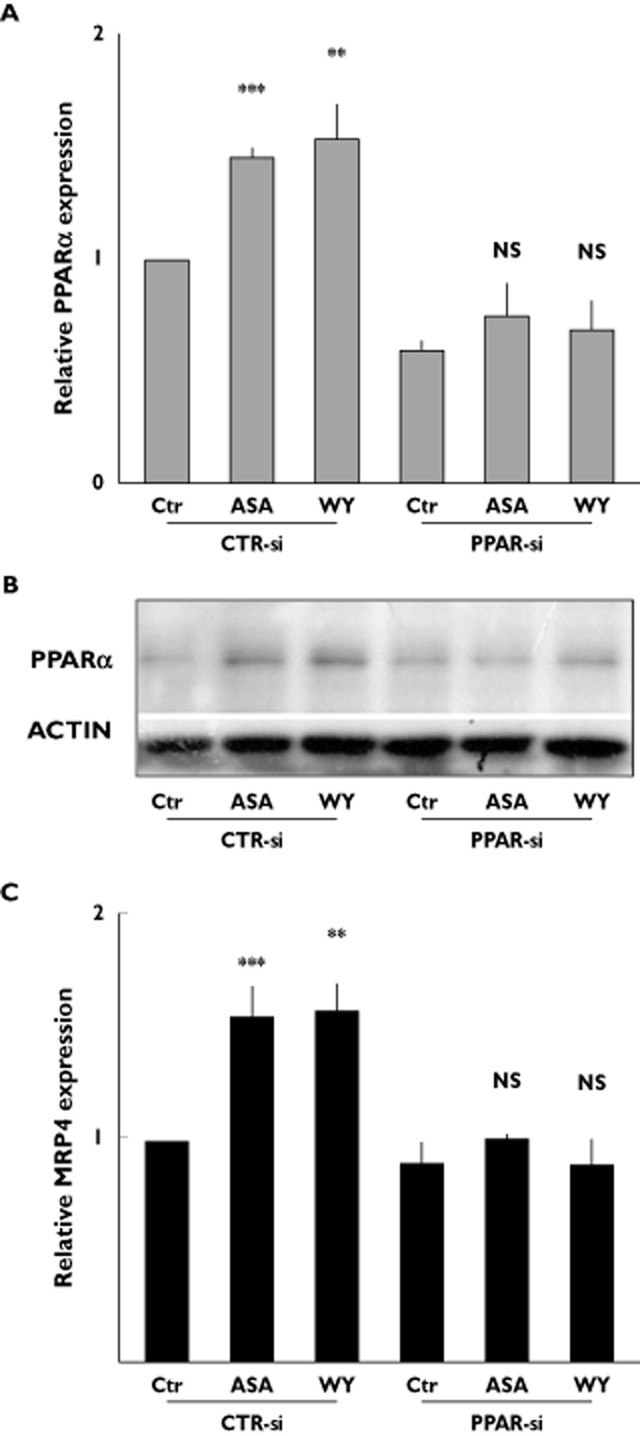
Aspirin stimulates endogenous MRP4 via a PPARα dependent mechanism in DAMI cells. Control non- specific siRNA (CTRsi) and PPARα specific siRNA (PPARsi) transfected DAMI cells were either untreated (Ctr) or treated, for 48 h, with either 50 μm aspirin (ASA) or 1 μm PPARα agonist, WY14643 (WY). (A) Q-RT-PCR analysis of endogenous PPARα expression. Data were normalized with the mean of the fold induction of GAPDH, ACTB and CD42B expression and reported as mean ± SD of three experiments (NS = not significant, **P < 0.01, ***P < 0.001; t-test). (B) Endogenous PPARα protein expression. Representative Western blot, out of three performed. (C) Q-RT-PCR analysis of endogenous MRP4 expression; Data were normalized with ACTB expression and reported as mean ± SD of three experiments (NS = not significant, **P < 0.01, ***P < 0.001; t-test)
Overall these data strongly support the hypothesis that aspirin exerts a PPARα-mediated activity on MRP4 expression.
MRP4 and PPARα expression in culture of aspirin-treated MKs
Analysis of MRP4 expression in DAMI cells suggested that aspirin could also affect its expression in MKs. Therefore, to corroborate our studies, we analyzed the effects of aspirin on MRP4 expression in differentiating human MK progenitor cells.
In our previous studies we optimized a serum-free liquid suspension culture for gradual HPC differentiation and maturation along the MK lineage, giving rise to a virtually pure MK population (97–99% of the cells were CD61+/CD41+) as well as to a pro-platelet and platelet formation, as previously reported 26,27. Either aspirin (50 μm) or WY14643 (1 μm) were added at day 6 of differentiative MK culture (when most cells are MK precursors) for 4 days. MKs were collected during the terminal days of differentiation to evaluate both MRP4 and PPARα mRNA expression while platelets, obtained at the end of in vitro MK maturation, were recovered and analyzed for MRP4 protein expression.
The results demonstrated that in mature MKs both aspirin and WY14643 increased the mRNA expression of MRP4 (1.7 and 1.8 fold increase, respectively) and PPARα (1.8 and 2.6 fold increase, respectively) as compared with mock culture (Figure 5A). In derived platelets, obtained at the end of the MK culture, treated with either aspirin or WY14643, protein analysis showed an increased MRP4 expression (1.6 fold for aspirin and 1.5 fold for WY14643) as compared with mock culture, suggesting that the effect of MK aspirin and WY14643 treatment persists in platelet progeny (Figure 5B).
Figure 5.
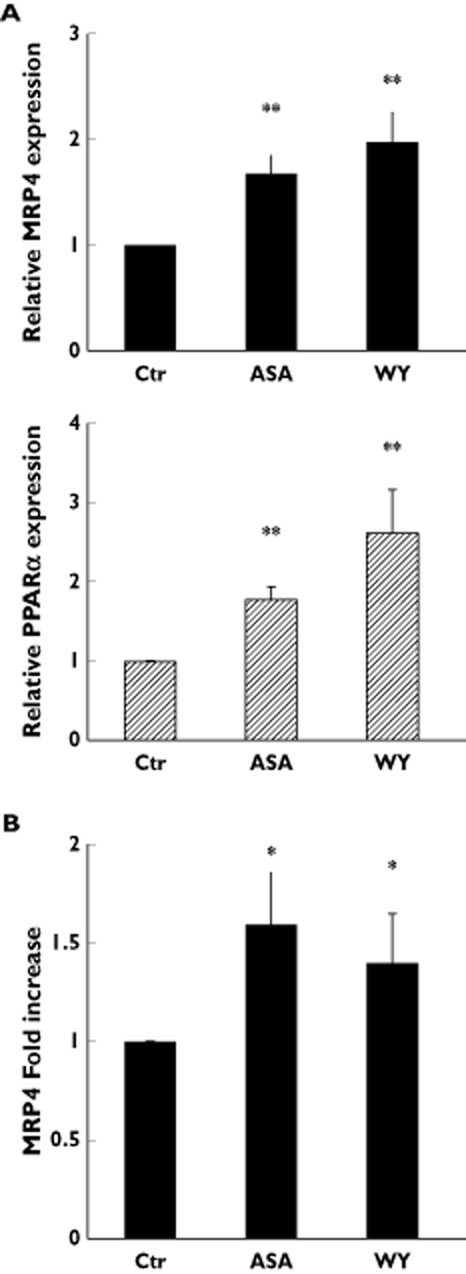
Aspirin stimulates endogenous MRP4 expression in megakaryocytes. (A) Q-RT-PCR analysis of endogenous MRP4 and PPARα expression in MKs, derived from HPC grown, in mock culture (Ctr), and grown the presence of either 50 μm aspirin (ASA) or 1 μm PPARα agonist, WY14643 (WY). Data were normalized with GAPDH expression and reported as mean ± SD of three experiments **P < 0.01; t-test). (B) Densitometric analysis of MRP4 protein expression in platelets derived from culture grown MKs in mock culture (Ctr) and in the presence of either 50 μm aspirin (ASA) or 1 μm PPARα agonist, WY14643 (WY). Data, normalized with actin expression, were reported as mean of fold increase ± SD, compared with Ctr (n = 3; *P < 0.05; t-test)
MRP4 expression in platelets obtained from healthy volunteers under aspirin treatment
To investigate whether aspirin could regulate MRP4 expression even after in vivo administration, we explored platelet MRP4 mRNA and protein expression in 10 HV after 1, 7 and 15 days' aspirin treatment (300 mg day−1).
The results revealed that platelet expression of MRP4 mRNA in HV, increased slightly after 7 days' aspirin treatment and significantly after 15 days' treatment (approximately 30%), as compared with those found in platelets obtained after 1 day's treatment (P = 0.08) (Figure 6A).
Figure 6.
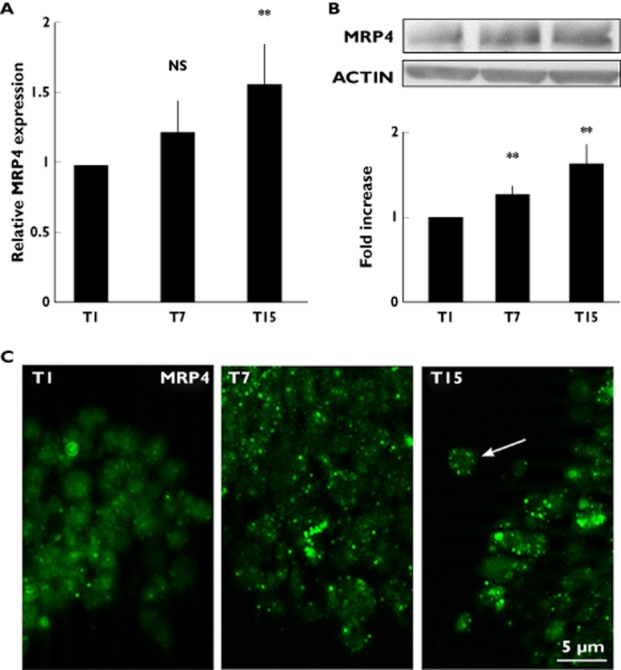
MRP4 expression in healthy volunteer (HV) platelets after aspirin treatment. (A) Q-RT-PCR analysis, MRP4 mRNA level expression in platelets obtained from HV after 1 (T1), 7 (T7) and 15 (T15) days' aspirin treatment (300 mg day−1). Data were normalized with the mean of the fold induction of GAPDH, ACTB and CD42B expression and reported as mean ± SD of 10 experiments using platelets obtained from different HV (NS = not significant, **P < 0.02). (B) Western blot analysis of MRP4 protein expression in platelets obtained from HV treated with aspirin for 1 (T1), 7 (T7) and 15 (T15) days. Densitometric analysis is reported as fold increase, compared with T1. Results are representative of 10 experiments (**P < 0.02; t-test). (C) Immunofluorescence microscopy of platelets obtained from HV treated with aspirin for 1 (T1), 7 (T7) and 15 (T15) days. Platelets were incubated with antibodies against MRP4 (green fluorescence). Representative experiment out of five performed using platelets obtained from different HV. The arrow points to MRP4 plasma membrane localization
The Western blot analysis showed a parallel behaviour of the mRNA expression, with a small increase of MRP4 protein expression after 7 days' aspirin treatment that became more evident after 15 days (Figure 6B).
Immunofluorescence analysis clearly confirmed these results, revealing that at 7 and 15 days' aspirin treatment MRP4 was more detectable, being predominantly localized in discrete spots, often distributed towards the periphery of the platelets (Figure 6C).
All together, our data consistently support the view that, also in vivo, aspirin treatment induces MRP4 overexpression in human platelets.
Discussion
In this study we investigated the effect of aspirin treatment on MK gene expression leading to the up-regulation of the MRP4 protein in human platelets.
The modulation of the MRP4 mRNA and protein expression was evaluated after aspirin treatment in i) a DAMI megakaryoblastic cell line, ii) human cultured MKs and derived platelets and iii) platelets obtained from in vivo aspirin treated HVs.
In DAMI cells, aspirin treatment induced a statistically significant increase of MRP4 and PPARα expression. In addition, as with aspirin, MRP4 overexpression was induced by a PPARα agonist and the same expression levels were also obtained by addition of both aspirin and a PPARα agonist to the cells. All this suggests that the effect is not synergistic. All these results lead us to ascribe the ability of aspirin in enhancing MRP4 expression to the activation of the nuclear receptor PPARα.
Our hypothesis is also supported by siRNA experiments. In fact any MRP4 up-regulation was not observed after aspirin or PPARα specific agonist treatment in PPARα siRNA-transfected DAMI cells.
Such a relationship between PPARα activation and MRP4 drug dependent up-regulation is similar to that reported in the mouse. In fact, after in vivo perfluorooctanoic acid and perfluorodecanoic acid administration, the liver mounts a compensatory hepatoprotective response via PPARα, leading to a marked increase in MRP4 expression 18.
The effect of aspirin on MRP4 expression is also observed in MK cultures as 4 days' in vitro aspirin treatment (starting 6 days' culture) induced an enhancement of MRP4 expression in MKs. Such protein up-regulation is also evident in platelets recovered at the end of the culture. As observed in DAMI cells, PPARα activation by its agonist enhances MRP4 expression in both MKs and their derived platelets. These data suggest that aspirin affects MRP4 expression in MKs, through PPARα activation, and such protein up-regulation can be transferred to the derived platelets.
The PPARα transcriptional effect on MK gene regulation, responsible for the changes in platelet protein expression, is not surprising. In fact, a previous study showed that PPARα activation in human bone marrow MKs induced a transcriptional change (suppression of the PDGF-BB protein) that can be transmitted to circulating platelets 21.
The ability of aspirin to modulate MRP4 expression in MKs and to transfer it to platelets was confirmed in our in vivo studies. In fact, we found the highest MRP4 mRNA amount in platelets obtained from HV at 15 days' treatment, when all the platelets analyzed derived from MKs under in vivo aspirin treatment, in line with their 10 days' half-life. In platelets obtained from HV after 7 days' treatment, the increase of MRP4 mRNA and protein expression was less evident than the one observed at 15 days' treatment, but higher in comparison with those shown in platelets obtained from the same HV after 1 day's treatment. The enhanced mRNA and protein-MRP4 expression in platelets, obtained from patients after 15 days' aspirin treatment, is in agreement with compelling evidence that MKs transfer their mRNA to platelets 32.
In addition, as already observed in platelets obtained from CABG patients 4 immunoflorescence analysis highlighted that MRP4 was particularly distributed along plasma membrane.
Our data provide further evidence that drug-dependent over-expression of multidrug transporters is a global adaptation of the cells to what seems to be a major toxic stress 1.
It was previously reported that aspirin was a substrate of either human or mouse MRP4 4,5, and a reduced aspirin action in CABG patient platelets was associated to MRP4 overexpression 4. As in our previous reports we observed a progressive reduction in platelet sensitivity to aspirin 33 and in this paper we can speculate that the limited drug capacity in reducing platelet function, observed in patients treated with aspirin long term, could be due to a drug dependent MRP4 up-regulation.
Fibrates are PPARα agonists and are frequently used as lipid lowing drugs in cardiology patients 34. Although our in vitro experiments did not show any synergistic effect between WY14643 (fibrate) 34 and aspirin in inducing MRP4 expression, we cannot exclude that in vivo treatment with fibrates in patients on aspirin could reduce aspirin action. To the best of our knowledge there are no control randomized studies to identify aspirin responsiveness in patients treated with fibrates.
In addition, as MRP4 acts as a negative regulator that limits the effect of platelet cyclic nucleotides 35, its up-regulation could reduce the endothelial effects on platelets important in limiting thrombus formation such as PGI2 and NO secretion.
Our studies are in line with a very recent paper published by Voora et al. 24 in which they point out that the biology of aspirin is complex and involves additional mechanisms besides inhibiting platelet COX-1, and some of these mechanisms underlie the risk for cardiovascular events. They identified a set of platelet-enriched genes and proteins, named aspirin response signature (ARS), that was associated with platelet function in HV only after aspirin administration, and with death or myocardial infarction in cardiology patients only in those using aspirin at the enrolment. For this reason they hypothesized that the genomic response to a pharmacological ‘challenge’ with aspirin could be due to aspirin exposure that altered the genomic and protein content of circulating platelets. Moreover, very recently Floyd et al. found an increased platelet expression of glycoprotein IIIa (GpIIIa) following aspirin treatment 36. From this point of view, our study finds a clinical implication and although we did not analyze the effect of aspirin on ARS gene expression or GpIIIa platelet expression, our data support both hypotheses and we can affirm that aspirin is able to alter the protein content of circulating platelets.
Clinicians currently need a readily available biomarker for identifying patients who do not respond adequately to aspirin and hence are at the risk of death or MI. Voora et al. 24 proposed novel biomarkers in the Whole-Blood RNA profiling, a well established diagnostic testing platform for cardiac allograft rejection and CAD diagnosis, approved by FDA and insurance coverage; Floyd et al. 36 identified glycoprotein IIIa expression as a novel candidate protein biomarkers of aspirin resistance. Our paper suggests that it could be important to verify the possibility to add a MRP4-mRNA assay into the PCR based platform.
In conclusion, the present study demonstrates that human megakaryocytic cells have an adaptive response to aspirin treatment, leading to an enhancement of MRP4 expression in circulating platelets and this represents an innovative and attractive approach with clinical implications in both identifying patients less sensitive to aspirin treatment and in improving aspirin treatment in high risk cardiovascular patients.
Competing Interests
All authors have read and approved the manuscript. The manuscript has not been published nor is being considered for publication elsewhere in whole or part in any language except as an abstract. All authors have completed the Unified Competing Interest form (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Author contributions
IM, RG and FMP conceived the experiments, performed the experiments, analyzed data and wrote the manuscript, LVL performed the experiments, wrote the manuscript and analyzed data, LF conceived the experiments and wrote the manuscript, AB, FB, CG and MG analyzed the data and VL and FR performed the experiments.
Financial support
This work was supported partially by a grant from the Italian Ministry of Education, University and Scientific Research (MIUR), in part from PRIN project 2009 to FMP and in part from ex 60%-Ateneo University 2009, 2010 to FMP. There is no relationships with industry.
References
- Vallet CM, Marquez B, Nhiri N, Anantharajah A, Mingeot-Leclercq MP, Tulkens PM, Lallemand JY, Jacquet E, Van Bambeke F. Modulation of the expression of ABC transporters in murine (J774) macrophages exposed to large concentrations of the fluoroquinolone antibiotic moxifloxacin. Toxicology. 2011;290:178–186. doi: 10.1016/j.tox.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Russel FG, Koenderink JB, Masereeuw R. Multidrug resistance protein 4 (MRP4/ABCC4): a versatile efflux transporter for drugs and signalling molecules. Trends Pharmacol Sci. 2008;29:200–207. doi: 10.1016/j.tips.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Schuetz JD, Connelly MC, Sun D, Paibir SG, Flynn PM, Srinivas RV, Kumar A, Fridland A. MRP4: a previously unidentified factor in resistance to nucleoside-based antiviral drugs. Nat Med. 1999;5:1048–1051. doi: 10.1038/12487. [DOI] [PubMed] [Google Scholar]
- Mattiello T, Guerriero R, Lotti LV, Trifiro E, Felli MP, Barbarulo A, Pucci B, Gazzaniga P, Gaudio C, Frati L, Pulcinelli FM. Aspirin extrusion from human platelets through multidrug resistance protein-4-mediated transport: evidence of a reduced drug action in patients after coronary artery bypass grafting. J Am Coll Cardiol. 2011;58:752–761. doi: 10.1016/j.jacc.2011.03.049. [DOI] [PubMed] [Google Scholar]
- Furmanski BD, Hu S, Fujita K, Li L, Gibson AA, Janke LJ, Williams RT, Schuetz JD, Sparreboom A, Baker SD. Contribution of ABCC4-mediated gastric transport to the absorption and efficacy of dasatinib. Clin Cancer Res. 2013;19:4359–4370. doi: 10.1158/1078-0432.CCR-13-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrono C, Coller B, Dalen JE, FitzGerald GA, Fuster V, Gent M, Hirsh J, Roth G. Platelet-active drugs: the relationships among dose, effectiveness, and side effects. Chest. 2001;119:39S–63S. doi: 10.1378/chest.119.1_suppl.39s. [DOI] [PubMed] [Google Scholar]
- Floyd CN, Ferro A. Mechanisms of aspirin resistance. Pharmacol Ther. 2014;141:69–78. doi: 10.1016/j.pharmthera.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Eikelboom JW, Hirsh J, Weitz JI, Johnston M, Yi Q, Yusuf S. Aspirin-resistant thromboxane biosynthesis and the risk of myocardial infarction, stroke, or cardiovascular death in patients at high risk for cardiovascular events. Circulation. 2002;105:1650–1655. doi: 10.1161/01.cir.0000013777.21160.07. [DOI] [PubMed] [Google Scholar]
- Eikelboom JW, Hankey GJ, Thom J, Bhatt DL, Steg PG, Montalescot G, Johnston SC, Steinhubl SR, Mak KH, Easton JD, Hamm C, Hu T, Fox KA, Topol EJ. Incomplete inhibition of thromboxane biosynthesis by acetylsalicylic acid: determinants and effect on cardiovascular risk. Circulation. 2008;118:1705–1712. doi: 10.1161/CIRCULATIONAHA.108.768283. [DOI] [PubMed] [Google Scholar]
- Frelinger AL, 3rd, Li Y, Linden MD, Barnard MR, Fox ML, Christie DJ, Furman MI, Michelson AD. Association of cyclooxygenase-1-dependent and -independent platelet function assays with adverse clinical outcomes in aspirin-treated patients presenting for cardiac catheterization. Circulation. 2009;120:2586–2596. doi: 10.1161/CIRCULATIONAHA.109.900589. [DOI] [PubMed] [Google Scholar]
- Pulcinelli FM, Biasucci LM, Riondino S, Giubilato S, Leo A, Di Renzo L, Trifiro E, Mattiello T, Pitocco D, Liuzzo G, Ghirlanda G, Crea F. COX-1 sensitivity and thromboxane A2 production in type 1 and type 2 diabetic patients under chronic aspirin treatment. Eur Heart J. 2009;30:1279–1286. doi: 10.1093/eurheartj/ehp097. [DOI] [PubMed] [Google Scholar]
- Pascale S, Petrucci G, Dragani A, Habib A, Zaccardi F, Pagliaccia F, Pocaterra D, Ragazzoni E, Rolandi G, Rocca B, Patrono C. Aspirin-insensitive thromboxane biosynthesis in essential thrombocythemia is explained by accelerated renewal of the drug target. Blood. 2012;119:3595–3603. doi: 10.1182/blood-2011-06-359224. [DOI] [PubMed] [Google Scholar]
- Zimmermann N, Kienzle P, Weber AA, Winter J, Gams E, Schror K, Hohlfeld T. Aspirin resistance after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2001;121:982–984. doi: 10.1067/mtc.2001.111416. [DOI] [PubMed] [Google Scholar]
- Eikelboom JW, Hankey GJ. Overexpression of the multidrug resistance protein-4 transporter in patients undergoing coronary artery bypass graft surgery a cause of aspirin resistance? J Am Coll Cardiol. 2011;58:762–764. doi: 10.1016/j.jacc.2011.04.025. [DOI] [PubMed] [Google Scholar]
- Chen C, Klaassen CD. Rat multidrug resistance protein 4 (Mrp4, Abcc4): molecular cloning, organ distribution, postnatal renal expression, and chemical inducibility. Biochem Biophys Res Commun. 2004;317:46–53. doi: 10.1016/j.bbrc.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Cai C, Omwancha J, Hsieh CL, Shemshedini L. Androgen induces expression of the multidrug resistance protein gene MRP4 in prostate cancer cells. Prostate Cancer Prostatic Dis. 2007;10:39–45. doi: 10.1038/sj.pcan.4500912. [DOI] [PubMed] [Google Scholar]
- Maher JM, Aleksunes LM, Dieter MZ, Tanaka Y, Peters JM, Manautou JE, Klaassen CD. Nrf2- and PPAR alpha-mediated regulation of hepatic Mrp transporters after exposure to perfluorooctanoic acid and perfluorodecanoic acid. Toxicol Sci. 2008;106:319–328. doi: 10.1093/toxsci/kfn177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksunes LM, Yeager RL, Klaassen CD. Application of multivariate statistical procedures to identify transcription factors that correlate with MRP2, 3, and 4 mRNA in adult human livers. Xenobiotica. 2009;39:514–522. doi: 10.1080/00498250902952514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yessoufou A, Wahli W. Multifaceted roles of peroxisome proliferator-activated receptors (PPARs) at the cellular and whole organism levels. Swiss Med Wkly. 2010;140:w13071. doi: 10.4414/smw.2010.13071. [DOI] [PubMed] [Google Scholar]
- Hashizume S, Akaike M, Azuma H, Ishikawa K, Yoshida S, Sumitomo-Ueda Y, Yagi S, Ikeda Y, Iwase T, Aihara K, Abe M, Sata M, Matsumoto T. Activation of peroxisome proliferator-activated receptor alpha in megakaryocytes reduces platelet-derived growth factor-BB in platelets. J Atheroscler Thromb. 2011;18:138–147. doi: 10.5551/jat.5868. [DOI] [PubMed] [Google Scholar]
- Xue J, Hua YN, Xie ML, Gu ZL. Aspirin inhibits MMP-9 mRNA expression and release via the ΠΠAPαlpha/gamma and COX-2/mPGES-1-mediated pathways in macrophages derived from THP-1 cells. Biomed Pharmacother. 2010;64:118–123. doi: 10.1016/j.biopha.2009.04.033. [DOI] [PubMed] [Google Scholar]
- Wang YH, Chen YF, Chen SR, Chen X, Chen JW, Shen XY, Mou YG, Liu PQ. Aspirin increases apolipoprotein-A-I-mediated cholesterol efflux via enhancing expression of ATP-binding cassette transporter A1. Pharmacology. 2010;86:320–326. doi: 10.1159/000321727. [DOI] [PubMed] [Google Scholar]
- Voora D, Cyr D, Lucas J, Chi JT, Dungan J, McCaffrey TA, Katz R, Newby LK, Kraus WE, Becker RC, Ortel TL, Ginsburg GS. Aspirin exposure reveals novel genes associated with platelet function and cardiovascular events. J Am Coll Cardiol. 2013;62:1267–1276. doi: 10.1016/j.jacc.2013.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg SM, Rosenthal DS, Greeley TA, Tantravahi R, Handin RI. Characterization of a new megakaryocytic cell line: the Dami cell. Blood. 1988;72:1968–1977. [PubMed] [Google Scholar]
- Guerriero R, Testa U, Gabbianelli M, Mattia G, Montesoro E, Macioce G, Pace A, Ziegler B, Hassan HJ, Peschle C. Unilineage megakaryocytic proliferation and differentiation of purified hematopoietic progenitors in serum-free liquid culture. Blood. 1995;86:3725–3736. [PubMed] [Google Scholar]
- Guerriero R, Mattia G, Testa U, Chelucci C, Macioce G, Casella I, Samoggia P, Peschle C, Hassan HJ. Stromal cell-derived factor 1alpha increases polyploidization of megakaryocytes generated by human hematopoietic progenitor cells. Blood. 2001;97:2587–2595. doi: 10.1182/blood.v97.9.2587. [DOI] [PubMed] [Google Scholar]
- Pulcinelli FM, Gresele P, Bonuglia M, Gazzaniga PP. Evidence for separate effects of U73122 on phospholipase C and calcium channels in human platelets. Biochem Pharmacol. 1998;56:1481–1484. doi: 10.1016/s0006-2952(98)00146-4. [DOI] [PubMed] [Google Scholar]
- Freedman JE, Larson MG, Tanriverdi K, O'Donnell CJ, Morin K, Hakanson AS, Vasan RS, Johnson AD, Iafrati MD, Benjamin EJ. Relation of platelet and leukocyte inflammatory transcripts to body mass index in the Framingham heart study. Circulation. 2010;122:119–129. doi: 10.1161/CIRCULATIONAHA.109.928192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann SW, Yost CC, Denis MM, McIntyre TM, Weyrich AS, Zimmerman GA. Neutrophils alter the inflammatory milieu by signal-dependent translation of constitutive messenger RNAs. Proc Natl Acad Sci U S A. 2004;101:7076–7081. doi: 10.1073/pnas.0401901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawabe Y, Shimamoto C, Sakai A, Kuwabara H, Saad AH, Nakano T, Takitani K, Tamai H, Mori H, Marunaka Y, Nakahari T. Peroxisome proliferation activation receptor alpha modulation of Ca2+-regulated exocytosis via arachidonic acid in guinea-pig antral mucous cells. Exp Physiol. 2010;95:858–868. doi: 10.1113/expphysiol.2010.053603. [DOI] [PubMed] [Google Scholar]
- Rowley JW, Schwertz H, Weyrich AS. Platelet mRNA: the meaning behind the message. Curr Opin Hematol. 2012;19:385–391. doi: 10.1097/MOH.0b013e328357010e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulcinelli FM, Pignatelli P, Celestini A, Riondino S, Gazzaniga PP, Violi F. Inhibition of platelet aggregation by aspirin progressively decreases in long-term treated patients. J Am Coll Cardiol. 2004;43:979–984. doi: 10.1016/j.jacc.2003.08.062. [DOI] [PubMed] [Google Scholar]
- Vu-Dac N, Schoonjans K, Kosykh V, Dallongeville J, Fruchart JC, Staels B, Auwerx J. Fibrates increase human apolipoprotein A-II expression through activation of the peroxisome proliferator-activated receptor. J Clin Invest. 1995;96:741–750. doi: 10.1172/JCI118118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgognone A, Pulcinelli FM. Reduction of cAMP and cGMP inhibitory effects in human platelets by MRP4-mediated transport. Thromb Haemost. 2012;108:955–962. doi: 10.1160/TH12-04-0232. [DOI] [PubMed] [Google Scholar]
- Floyd CN, Goodman T, Becker S, Chen N, Mustafa A, Schofield E, Campbell J, Ward M, Sharma P, Ferro A. Increased platelet expression of glycoprotein IIIa following aspirin treatment in aspirin-resistant but not aspirin-sensitive subjects. Br J Clin Pharmacol. 2014;78:320–328. doi: 10.1111/bcp.12335. [DOI] [PMC free article] [PubMed] [Google Scholar]


