Abstract
Aim
Fingolimod, a sphingosine 1-phosphate receptor modulator, is the first oral disease modifying therapy approved for the treatment of relapsing multiple sclerosis. The aim of this double-blind, placebo-controlled study was to evaluate the effect of fingolimod on cerebral blood flow, platelet function and macular thickness in healthy volunteers.
Methods
The study included 88 healthy volunteers who received fingolimod 0.5 mg or 1.25 mg or matched placebo over a period of 4 weeks. Transcranial colour coded sonography was performed to measure mean blood flow velocities, the platelet function was measured by the PFA-100® assay using a collagen/epinephrine cartridge and macular thickness was measured using optical coherence tomography. An assessment of non-inferiority of fingolimod vs. placebo was performed against a reference value (20% of the overall baseline value).
Results
All 88 randomized participants completed the study. At day 28 compared with baseline value, for 0.5 mg, 1.25 mg and placebo treatments, the mean middle cerebral artery blood flow velocity decreased by 4, 1 and 3.7 cm s−1, respectively. The platelet function analyzer closure time increase was not significant (7.8, 7.5 and 10.4 s, respectively). The mean percentage change in the central foveal thickness from baseline for both eyes was below 3% for all groups. The safety profile of fingolimod in this study was found consistent with the previous reports.
Conclusions
In healthy volunteers, the changes seen with both fingolimod doses were found to be within normal variability, non-inferior and comparable with those observed with placebo for all the pharmacodynamic parameters assessed.
Keywords: cerebral blood flow, fingolimod, healthy volunteers, macular thickness, platelet function, sphingosine 1-phosphate
What is Already Known about this Subject
Fingolimod, a first-in-class sphingosine 1-phosphate (S1P) receptor modulator, targets multiple sclerosis by preventing the S1P-dependent autoreactive lymphocyte trafficking from lymphoid tissues to the inflammatory sites.
The effect of S1P receptor modulation on retino-cerebral vasculature and platelet function has not been reported in clinical studies.
What This Study Adds
The effect of steady-state fingolimod on cerebral blood flow and platelet function was similar to the effect of placebo in healthy volunteers.
The study provided additional information on the effects of S1P receptor modulation, particularly in relation to cerebrovascular reactivity and coagulation pathways and is of relevance to patients with autoimmune vascular disease conditions.
Introduction
Fingolimod (FTY720; Gilenya™, Novartis Pharma AG, Basel, Switzerland), leads a new class of therapeutic compounds, the sphingosine 1-phosphate (S1P) receptor modulators, and has been approved in more than 80 countries as a 0.5 mg once-daily oral therapy for relapsing multiple sclerosis (MS) 1–3. Fingolimod has demonstrated superior efficacy over the approved first line treatment interferon beta-1a (Avonex®) 4 and placebo 5 in terms of clinical and magnetic resonance imaging (MRI) outcome measures in the largest ever clinical development programme in relapsing MS.
S1P is a biologically active lysophospholipid which exerts its physiological effects via a set of five G-protein-coupled receptors (S1P1–5) 2. These receptors, ubiquitously distributed but displaying differential cell type expression, play an important role in the physiology of the cardiovascular, nervous and immune systems 6–8. S1P acts on endothelial cells to regulate barrier integrity, vascular permeability and tissue perfusion, and on platelets influencing activation, adhesion and aggregation, among several other functions 6–9.
Multiple sclerosis is a chronic, auto-immune, inflammatory and neurodegenerative disease of the central nervous system 1. MS lesions show evidence of vascular injury, such as thickening or hyalinization of vein walls, suggesting thrombosis of small veins and capillaries 10–14. There is evidence of a pro-coagulant state in MS and that platelets are significantly activated in MS patients 15,16. Patients with MS are at increased risk of thromboembolic events as compared with the age and gender matched general population 17. There are no clinical or preclinical data that suggest that fingolimod alters cerebrovascular reactivity or platelet function. The effect of S1P receptor modulation on cerebrovascular reactivity or platelet function has not been reported in clinical studies. Therefore, this study was undertaken to compare the pharmacodynamic effects of fingolimod (0.5 mg and 1.25 mg doses) and placebo on the retino-cerebral vasculature and platelet function in healthy volunteers.
Methods
Subjects
The study enrolled 88 healthy, male and female volunteers aged 18 to 50 years, weighing at least 50 kg with a body mass index ranging between 18 to 30 kg m−2. Study eligibility required volunteers to have normal platelet function and detectable and accurately measurable cerebral blood flow velocities of the middle cerebral artery (MCA), posterior cerebral artery (PCA) and basilar artery (BA) at the baseline visit. In addition, all eligible female volunteers could not be of childbearing potential and had to have negative pregnancy test results at screening and baseline, regardless of follicular stimulating hormone results and reported sterilization. Male volunteers were required to use two modes of contraception (spermicidal gel plus condom) for the entire duration of the study and refrain from fathering a child in the 3 months following the last dose.
Volunteers were ineligible if they were smokers, indulged in alcohol/drug abuse, had vaccination with live attenuated vaccine within 2 months before screening, had pulmonary symptoms, history of exercise-induced asthma, asthma or chronic obstructive pulmonary disorder, significant ECG abnormalities or prolonged QT interval syndrome, retinal abnormalities or other evidence of eye disease, suspicious skin lesions or skin cancer detected at screening. Intake of aspirin or other anticoagulant drugs within at least 2 weeks, prescription drugs within 4 weeks or over-the-counter drugs within 2 weeks before baseline were additional exclusion criteria. Clinical parameters determining exclusion were haemoglobin levels <12 g dl−1 at screening, a total white blood cell count outside the range of 4500–11 000 μl−1, lymphocyte count <600 mm3, platelets <100 000 μl−1, PR interval >220 ms, QRS interval >120 ms, abnormal liver function test and positive hepatitis B or C status. Volunteers with a history of significant illness within the 2 weeks prior the first dose, any surgical or medical condition that could significantly alter the pharmacokinetics, and blood (within 8 weeks) or plasma (within 7 days) donation before first dosing were not included.
Study design
This was a single centre, double-blind, randomized, placebo-controlled, multiple dose, parallel group study in healthy volunteers (Figure 1). The volunteers were randomly assigned in a ratio of 1:1:1 to take fingolimod 0.5 or 1.25 mg, or matching placebo, once daily by mouth. After a screening period (day −21 to day −2), and a baseline visit (day −1), there was a 4 week treatment period, an observational follow-up period of 2 weeks and finally an end-of-study visit. For the baseline assessments and loading dose phase (the first week of the treatment period) volunteers stayed at the study centre.
Figure 1.
Study design. BMI body mass index, EOS end of study, OCT optical coherence tomography, PF platelet function, R randomization, SA safety assessments, TCD transcranial Doppler
The study was conducted at Charité Research Organisation GmbH, Charitéplatz 1, D-10117 Berlin, Germany. The study protocol was approved by the local ethics committee and health authority and was conducted in accordance with Good Clinical Practice and the Declaration of Helsinki. All participants provided written informed consent. The trial was registered under the EudraCT registration number 2008-005461-65. The receptor nomenclature used in this report conforms to the British Journal of Pharmacology's The Concise Guide to PHARMACOLOGY 2013/14 18.
Interventions
During the loading phase, volunteers took escalating doses of fingolimod (Figure 1) over a 4 day period to reach pharmacokinetic steady-state. On day 4, the volunteers were allowed to leave the study centre if the results from the safety assessments were normal 6 h post-dose. After the initial loading phase, a once daily regimen was maintained for the remainder of the active treatment period to maintain steady-state. On weekly visits (days 7, 14, 21 and 28), volunteers took the medication at the study centre. On the remaining days, volunteers self-administered the allotted medication and we assessed compliance by counting pills counts at each weekly visit.
Outcome measures
The primary objectives of this study were to measure the effect of fingolimod on (i) mean blood flow velocity (Vm) in the MCA, (ii) PFA closure time of a collagen and epinephrine cartridge and (iii) central foveal thickness, in healthy volunteers. Secondary objectives were to measure the effect of fingolimod on (i) Vm in the PCA and BA and (ii) cerebrovascular reactivity of the MCA in response to hypercapnia and hypocapnia. In addition, we assessed effect of fingolimod on platelet aggregation in response to the inducers collagen, epinephrine, adenosine-5′-diphosphate (ADP) 19 and ristocetin and on plasma factors, von Willebrand factor (vWF), fibrinogen and d-dimers. The safety and tolerability of fingolimod dosing as compared with the matched placebo group was also evaluated.
Transcranial ultrasound
The volunteer lay in the supine position and we measured mean blood flow velocities in the target arteries using a transcranial colour coded sonography (TCCS) sector probe emitting sound waves at a frequency of 2 MHz (Powervision 6000, Toshiba, Japan). To identify the best side for measuring the Vm in the M1-MCA and P2-PCA segments of the respective arteries, both right and left temporal bone windows were assessed at baseline and the side with the best signal was used for subsequent assessments. For the BA, the Vm was measured using the Doppler signal from the proximal third of the BA using a trans-foraminal approach.
CO2vasomotor reactivity
Both M1-MCA segments were identified for measuring vasomotor reactivity (VMR). Two 2 MHz transcranial Doppler (TCD) probes of a multi-channel Doppler system (DWL X4, Compumedics, Germany) were fixed to the subject's head by means of a professional holding system (supplied by DWL, Germany) to ensure identical sample volume position during the whole procedure. The end-tidal expiratory CO2 (etCO2) was recorded using a capnometer. Initial measurements of etCO2 and mean MCA flow velocity were performed under normal breathing conditions (normocapnia). The next set of measurements were performed under steady-state mild hyperventilation conditions (hypocapnia) aided by a metronome at 20 breaths min−1. The third set of measurements were taken under hypercapnic condition, when subjects continuously inhaled mixture of 5% CO2 and 95% O2 (carbogen gas). After achieving a steady-state etCO2, the mean MCA flow velocity was recorded. VMR (percent VMR change per mmHg etCO2) was calculated for both MCAs under these three conditions.
Platelet function assays
Platelet function was measured by the PFA-100® assay (Platelet Function Analyzer-100, Siemens Healthcare; formerly Dade Behring) using a collagen/epinephrine cartridge. Platelet aggregation in platelet rich plasma was assessed using varying concentrations of the following inducers: ADP (5, 2.5 and 1.25 μmol) 19, epinephrine (20 and 10 μmol), collagen (4, 2, and 1 μg ml−1) and ristocetin (1.5 and 1 mg ml−1), to assess any effect of fingolimod on platelet function. The acute phase reactant vWF, which can affect platelet function, was measured in plasma. Fibrinogen and d-dimers, markers of inflammation and activation of coagulation, were also measured. The lower limit of quantification (LLOQ) values for vWF was 3% (STA-Liatest), for fibrinogen was 30 mg/dl and 0.22 mg l−1 for d-dimers (STA-Liatest).
For the PFA-100® assay 23 ml of whole blood was withdrawn, using syringe and needle or a butterfly, into Sarstedt tubes containing sodium citrate. Tubes were stored at room temperature and analyzed within 1 h of sampling for PFA and 3 h for aggregation. As the results of the PFA performed at the screening visit were to determine eligibility for inclusion in the study, these results were not included in the analysis.
Optical coherence tomography (OCT)
Central foveal thickness (CFT, mean thickness at the point of intersection of six radial scans) was measured through a dilated pupil using low intensity infrared laser light from the OCT 3 (Zeiss Stratus OCT 3.0, Carl Zeiss Ophthalmic Systems, Inc., Humphrey Division, Dublin). OCT reliably detects and measures small changes in macular thickness to an accuracy of ≤10 μm.
The assessment schedule for the above pharmacodynamic parameters is described in Figure 1. All subjects who completed the study and had evaluable parameters were included in the pharmacodynamic analysis. Results at baseline were compared with results at week 4. In addition, the data obtained with the placebo and fingolimod treatments were compared at week 4.
Pharmacokinetic assessments
In order to quantify drug exposure and subject compliance during the loading dose regimen and the ambulatory treatment phase, pre-dose blood samples were collected on days 2, 3, 4, 7, 14, 21 and 28. Fingolimod and fingolimod-phosphate (fingolimod-P) concentrations were measured in whole blood using a validated LC-MS/MS method with a LLOQ of 0.08 and 0.1 ng ml−1, respectively. The steady-state blood concentrations of fingolimod and fingolimod-P were measured throughout the study.
Safety and tolerability assessments
These included vital signs and body measurements, systolic and diastolic blood pressure and pulse rate, ECG evaluation, haematology, blood chemistry and urine analysis. Any adverse events (AEs) or serious adverse events (SAEs) that occurred were recorded. The safety population comprised those who had received at least one dose of the study drug.
Randomization and blinding
Participants were randomly assigned to one of the three groups using a computer-generated block randomization list. To maintain blinding, volunteers in the placebo group received a respective number of fingolimod-matched placebo capsules. The investigators were blinded to the lymphocyte count since knowledge of the lymphocyte-lowering effect of fingolimod could unblind the study.
Statistical methods
A sample size of approximately 87 subjects (29 in each of the three treatment groups) was planned for this study. This sample size had a power of at least 93% to reject the hypothesis that the difference between the means of the fingolimod and placebo groups is ≥20%, assuming the same SD in each treatment group, at a one-sided significance level of 2.5%. This sample size ensured an overall power of at least 80% to reject a mean difference of 20% or more between the treatment groups, for all primary endpoints simultaneously, assuming they are independent of each other.
Descriptive statistics and graphical representations were used to summarize the mean differences from placebo and the two fingolimod treatment groups at the end of week 4. Descriptive statistics were also used to summarize treatment effects within group over time, including the change from pre-dose values. A linear model including fixed effects for treatment group and baseline values was used to determine the one-sided 97.5% confidence intervals. Linear regression analysis was performed to measure VMR. An assessment of non-inferiority vs. placebo was performed using a reference value that was 20% of the overall average baseline value. The treatment was considered non-inferior to placebo if the one-sided 97.5% confidence intervals for the mean difference vs. placebo for all primary endpoints fell within this 20% limit.
Results
Patient disposition
A total of 88 subjects were enrolled and randomized. All subjects completed the study and were analyzed for the outcome measures. Demographics and baseline characteristics were balanced across the three study groups (Table 1).
Table 1.
Demographics and baseline characteristics
| Demographics | Fingolimod 0.5 mg, | Fingolimod 1.25 mg, | Placebo | |
|---|---|---|---|---|
| n = 30 | n = 29 | n = 29 | ||
| Age (years) mean ± SD | 37.3 ± 9.24 | 38 ± 10.92 | 38.6 ± 7.37 | |
| Gender, n (%) | Male | 27 (90) | 24 (82.8) | 26 (89.7) |
| Female | 3 (10) | 5 (17.2) | 3 (10.3) | |
| Race, n (%) | Caucasian | 30 (100) | 28 (96.6) | 29 (100) |
| Hispanics | 0 | 1 (3.4) | 0 | |
| Weight (kg), mean ± SD | 79 ± 11.32 | 78.9 ± 11.03 | 81.1 ± 8.95 | |
| BMI (kg m−2), mean ± SD | 24.4 ± 2.75 | 24.9 ± 2.90 | 25 ± 2.43 | |
BMI, body mass index; SD, standard deviation.
Primary outcome measures
Mean blood flow velocity in the MCA
The Vm for the placebo group was 56 cm s−1 at baseline and 52 cm s−1 at day 28. For the fingolimod 0.5 mg and 1.25 mg groups, the Vm was 58 cm s−1 and 56 cm s−1 at baseline and 54 cm s−1 and 55 cm s−1 at day 28. Figure 2 presents the change in Vm from baseline at day 28.
Figure 2.
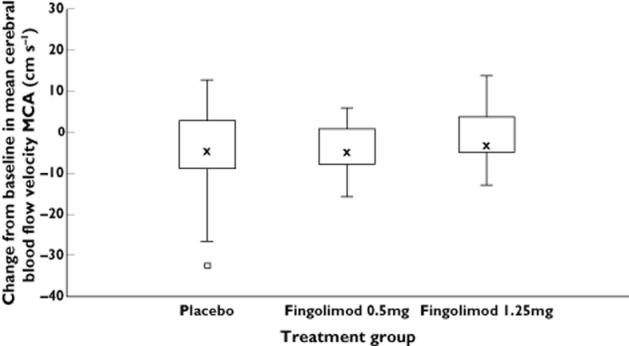
Change from baseline in mean cerebral blood flow velocity: Middle cerebral artery at day 28. The median for each treatment is represented by cross, the upper (lower) edge of the box represents the 75th (25th) percentile. A whisker is drawn from the upper (lower) edge of the box to the largest (smallest) value within 1.5 × interquartile range above (below) the edge of the box, values outside the whiskers are identified with a square. MCA middle cerebral artery
Platelet function (PFA)
The mean platelet closure time in the placebo group was 115.3 s at baseline and 125.7 s at day 28. For the 0.5 mg group, the mean closure time at baseline was 113.3 s and 121.1 s at day 28. Mean closure time for the 1.25 mg group was 110.4 s at baseline and 117.9 s at day 28. For all three groups, platelet closure time increased by 7–10% between baseline and day 28. Changes from baseline in the mean platelet closure time are shown in Figure 3.
Figure 3.
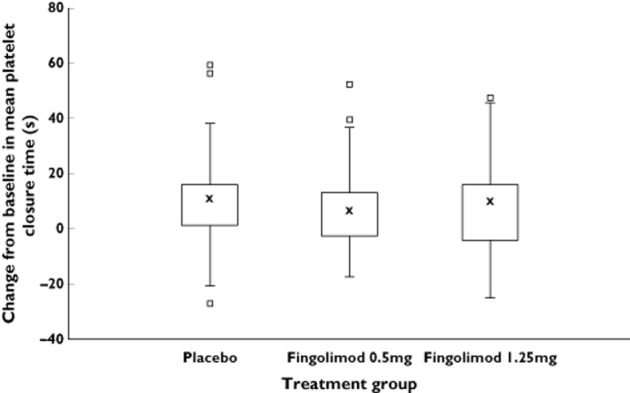
Change from baseline in platelet adhesion: Platelet closure time in response to collagen/epinephrine (PFA100®) at day 28. The median for each treatment is represented by cross, the upper (lower) edge of the box represents the 75th (25th) percentile. A whisker is drawn from the upper (lower) edge of the box to the largest (smallest) value within 1.5 × interquartile range above (below) the edge of the box; values outside the whiskers are identified with a square
Macular thickness
In the placebo group, the mean foveal thickness did not change from baseline to day 28 (178.9 μm right eye and 177.1 μm left eye to 179.9 μm right eye and 177.4 μm left eye). For the fingolimod 0.5 mg group, the values were 173.7 μm right eye and 169.4 μm left eye at baseline and 172.1 μm right eye and 172.4 μm left eye after 28 days of dosing. In the fingolimod 1.25 mg group, the values at baseline were 175.5 μm right eye and 173.0 μm left eye and at day 28 were 178.2 μm right eye and 177.4 μm left eye. The mean percentage change from baseline in CFT was always ≤3% (Figure 4A, B).
Figure 4.
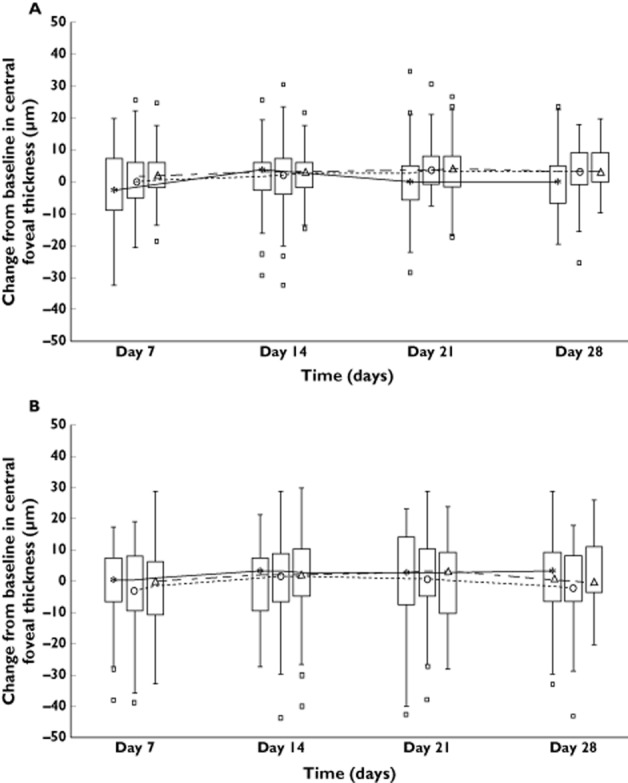
Change from baseline in central foveal thickness in (A) the left eye and in (B) the right eye. The median of each time point is joined by a line. The upper (lower) edge of the box represents the 75th (25th) percentile; a whisker is drawn from the upper (lower) edge of the box to the largest (smallest) value within 1.5 x interquartile range above (below) the edge of the box; values outside the whiskers are identified with a square.  , placebo;
, placebo;  , fingolimod 0.5 mg;
, fingolimod 0.5 mg;  , fingolimod 1.25 mg
, fingolimod 1.25 mg
The summary of statistical analysis for the primary variables is shown in Table 2. For all the primary variables assessed, the two-sided 95% confidence intervals for the mean difference vs. placebo fell within the pre-specified limit of non-inferiority.
Table 2.
Summary of statistical analysis of the change from placebo in primary endpoints at day 28
| Treatment | Statistics | Mean blood flow velocity MCA (cm s−1) | Platelet closure time (s) | Central foveal thickness right eye (μm) | Central foveal thickness left eye (μm) |
|---|---|---|---|---|---|
| Reference value* | 11.32 | 22.60 | 35.21 | 34.62 | |
| Fingolimod 0.5 mg | n | 30 | 30 | 30 | 30 |
| LS mean | 53.23 | 120.94 | 174.00 | 176.29 | |
| Difference (95% CI) | 0.58 (−2.83, 3.99) | −3.03 (−11.99, 5.94) | −3.58 (−10.28, 3.11) | 3.04 (−1.66, 7.75) | |
| Conclusion | Non-inferior | Non-inferior | Non-inferior | Non-inferior | |
| Fingolimod 1.25 mg | n | 29 | 29 | 29 | 29 |
| LS mean | 55.25 | 119.75 | 178.66 | 177.53 | |
| Difference (95% CI) | 2.60 (−0.83, 6.03) | −4.22 (−13.31, 4.88) | 1.08 (−5.65, 7.81) | 4.29 (−0.41, 8.99) | |
| Conclusion | Non-inferior | Non-inferior | Non-inferior | Non-inferior |
Reference value: 20% of mean baseline value. CI, confidence interval; LS mean, least square mean; MCA, middle cerebral artery.
Secondary outcome measures
A mean difference of <10% was observed in mean blood flow velocities and the CO2 reactivity from baseline to day 28 for all three treatment groups. The summary of statistical analysis for non-inferiority in these variables is shown in Table 3.
Table 3.
Summary of statistical analysis of the change from placebo in cerebral blood flow velocity in PCA, BA and MCA in response to hypercapnia at day 28
| Parameters | Statistics | Fingolimod 0.5 mg | Fingolimod 1.25 mg | |
|---|---|---|---|---|
| n = 30 | n = 29 | |||
| Reference value* | ||||
|
PCA (cm s−1) |
7.24 | n | 30 | 29 |
| LS mean | 34.39 | 37.11 | ||
| Difference (95% CI) | −1.09 (−3.69, 1.5) | 1.62 (−0.99, 4.24) | ||
| Conclusion | Non-inferior | Non-inferior | ||
|
BA (cm s−1) |
6.84 | n | 30 | 29 |
| LS mean | 33.01 | 33.66 | ||
| Difference (95% CI) | −0.49 (−2.87, 1.89) | 0.15 (−2.25, 2.55) | ||
| Conclusion | Non-inferior | Non-inferior | ||
|
MCA CO2 reactivity index (%mmHg) |
1.00 | n | 30 | 29 |
| LS mean | 4.95 | 5.05 | ||
| Difference (95% CI) | −0.04 (−0.16, 0.08) | 0.06 (−0.06, 0.18) | ||
| Conclusion | Non-inferior | Non-inferior |
Reference value: 20% of mean baseline value. CI, confidence interval; LS mean, least square mean; MCA, middle cerebral artery; PCA, posterior cerebral artery; BA, basilar artery.
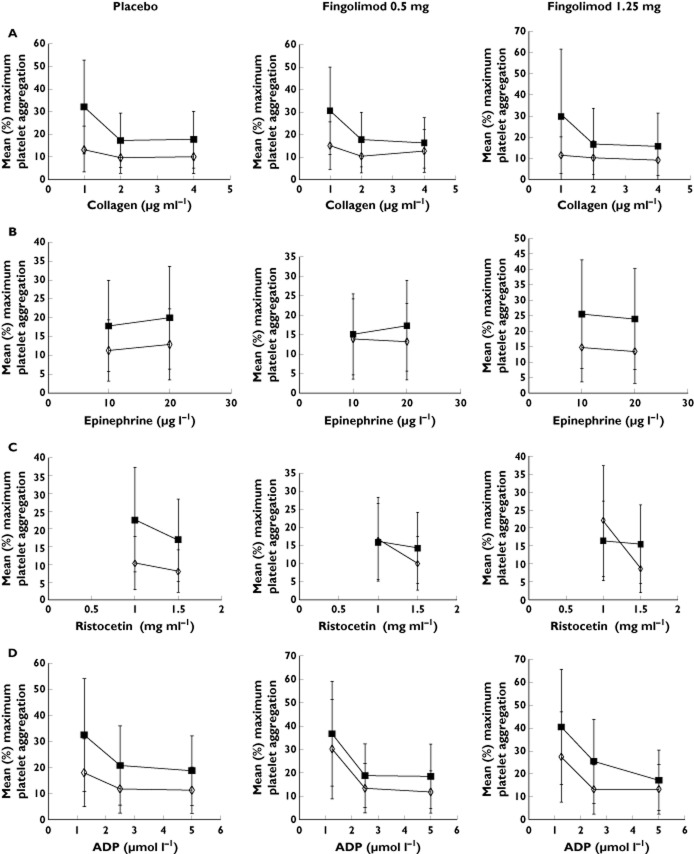
The change in mean maximum platelet aggregation in response to the various concentrations of aggregation inducers is shown in Figure 5 (A−D). The patterns of response in both fingolimod treatment groups were similar to those in the placebo group (Table 4). With the exception of the lower concentration of 1.25 μmol l−1 of ADP, all other measurements demonstrated non-inferiority to placebo. Figure 6 (A−C) shows the change from baseline to day 28 for all the acute phase reactants. The change in these parameters was <10% at the end of dosing (day 28), except for the d-dimers in the higher dose group of 1.25 mg where a mean increase of 19% was observed. The mean differences in the parameters assessed were statistically non-inferior to placebo.
Figure 5.
Change in mean maximum platelet aggregation from baseline to day 28 in response to various concentrations of (A) collagen, (B) epinephrine, (C) Ristocetin and (D) ADP. ADP, adenosine-5′-diphosphate. ◊, baseline;  , day 28
, day 28
Table 4.
Summary of analysis of the change from placebo in platelet aggregation (%) at day 28 in response to different concentrations of inducers
| Treatment | Statistics | ADP | Epinephrine | Collagen | Ristocetin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.25 μmol l−1 | 2.5 μmol l−1 | 5.0 μmol l−1 | 10 μmol l−1 | 20 μmol l−1 | 1 μg ml−1 | 2 μg ml−1 | 4 μg ml−1 | 1 mg ml−1 | 1.5 mg ml−1 | ||
| Reference value* | 14.30 | 15.89 | 15.78 | 14.71 | 14.89 | 14.48 | 14.85 | 15.10 | 14.13 | 14.97 | |
|
Fingolimod 0.5 mg n = 30 |
LS mean | 61.10 | 72.30 | 74.67 | 68.98 | 67.80 | 64.69 | 69.33 | 69.13 | 64.94 | 68.82 |
| Difference (95% CI) | −5.46 | −1.27 | 3.29 | 0.46 | −1.18 | 0.16 | −0.08 | −3.51 | 0.16 | −0.23 | |
| (−17.40, 6.49) | (−9.44, 6.90) | (−3.47, 10.05) | (−6.57, 7.49) | (−7.74, 5.39) | (−10.02, 10.34) | (−6.14, 5.97) | (−9.63, 2.62) | (−6.11, 6.43) | (−5.71, 5.25) | ||
| Conclusion | Inferior | Non–inferior | Non–inferior | Non–inferior | Non–inferior | Non–inferior | Non–inferior | Non–inferior | Non–inferior | Non–inferior | |
|
Fingolimod 1.25 mg n = 29 |
LS mean | 61.92 | 71.29 | 75.59 | 68.26 | 67.24 | 65.89 | 70.31 | 72.13 | 67.52 | 69.93 |
|
Difference (95% CI) |
−4.63 | −2.28 | 4.21 | −0.27 | −1.74 | 1.37 | 0.90 | −0.51 | 2.74 | 0.88 | |
| (−16.67, 7.41) | (−10.60, 6.03) | (−2.66, 11.08) | (−7.39, 6.85) | (−8.41, 4.94) | (−8.91, 11.64) | (−5.26, 7.06) | (−6.83, 5.81) | (−3.56, 9.03) | (−4.73, 6.49) | ||
| Conclusion | Inferior | Non-inferior | Non-inferior | Non-inferior | Non-inferior | Non-inferior | Non-inferior | Non-inferior | Non-inferior | Non-inferior | |
Reference value: 20% of mean baseline value. CI, confidence interval; LS mean, least square mean.
Figure 6.
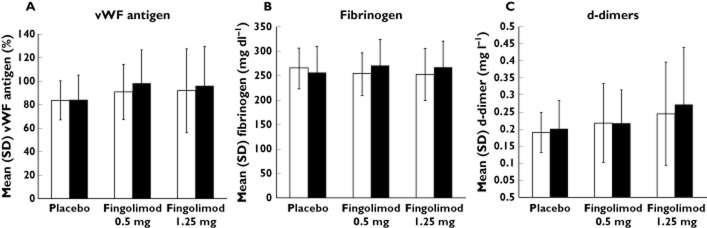
Acute phase reaction at baseline and day 28. (A) von Willebrand factor (%), (B) fibrinogen (mg dl−1) and (C) d-dimers (mg l−1). v. WF von Willebrand factor.  , baseline;
, baseline;  , day 28
, day 28
Pharmacokinetics assessments
In all but one subject, the pre-dose blood concentrations of fingolimod and fingolimod-P were greater than the LLOQ from day 2. The increase was observed up to day 7 and then remained constant thereafter.
Safety
A total of 47 subjects (53.4%) experienced at least one AE. The most frequent (≥5%) AEs are listed in Table 5. The most common AEs were headache, nasopharyngitis, oropharyngeal pain, back pain and diarrhoea, and these occurred across all treatment groups. All AEs were mild to moderate. There were no SAEs and no discontinuations due to adverse events.
Table 5.
Incidence of adverse events (at least 5% incidence in any group)
| Adverse event | Fingolimod 0.5 mg | Fingolimod 1.25 mg | Placebo |
|---|---|---|---|
| n = 30 | n = 29 | n = 29 | |
| n (%) | n (%) | n (%) | |
| Patients with at least one adverse event | 15 (50.0) | 17 (58.6) | 15 (51.7) |
| Preferred term | |||
| Headache | 1 (3.3) | 3 (10.3) | 7 (24.1) |
| Nasopharyngitis | 4 (13.3) | 1 (3.4) | 5 (17.2) |
| Oropharyngeal pain | 1 (3.3) | 3 (10.3) | 0 (0) |
| Back pain | 0 (0) | 2 (6.9) | 1 (3.4) |
| Diarrhoea | 2 (6.7) | 1 (3.4) | 0 (0) |
A considerable decrease in the mean leucocyte count was observed in both fingolimod groups, which did not return to baseline value within the 2 weeks follow-up period (at day 42). The drop in leucocyte count was largely due to a decrease lymphocytes and to a lesser extent, a drop in neutrophils. Liver transaminases were elevated only in the fingolimod groups. Alanine aminotransferase (ALT) was the predominant enzyme affected, followed by aspartate aminotransferase, with gamma-glutamyl transferase the least affected. Five subjects had ALT elevations 3–5-fold above the baseline value (fingolimod 0.5 mg: n = 1; 1.25 mg: n = 4). Other safety parameters were comparable between all three groups. Blood pressure was stable throughout the study. The mean pulse rate decreased during the loading phase and was lowest on day 2, but normalized by the study end. No deaths occurred in this study.
Discussion
The role of endogenous sphingolipid, S1P, and its G-protein-coupled receptors in the immune system, maintenance of vascular homeostasis and in preservation of endothelial permeability barrier functions is increasingly evident 20. S1P receptors represent a new drug target class and our understanding of the pharmacodynamic effects of S1P receptor modulation is evolving. Fingolimod is the first-in-class S1P receptor modulator which has been evaluated as a treatment for autoimmune diseases. Previously the drug has been studied for the treatment of renal transplant rejection, though the doses studied were 5–10 times higher than the presently approved 0.5 mg dose for the treatment of MS. In this study we attempted to evaluate the pharmacodynamic effects of steady-state, clinical and supra-therapeutic dosing of fingolimod for 1 month in healthy volunteers and to assess its impact on the vascular system.
Statistical analysis of the study results revealed non-inferiority of both doses of fingolimod to placebo for all primary comparisons, blood flow and platelet function assessments. In other words, at the level of detection provided by this study, both fingolimod and placebo treatments were indistinguishable from each other. Platelet aggregation induced by ADP 1.25 μmol l−1, a secondary comparison, was the only parameter for which the two-sided 95% confidence interval for the mean treatment difference vs. placebo slightly crossed the pre-specified limit of non-inferiority. This study was not powered to assess non-inferiority for the secondary comparisons and so it was not unexpected that for one of the multiple pharmacodynamic secondary endpoints the non-inferiority criteria was not met by chance alone.
The parameters we assessed were chosen to capture three primary features of the brain and systemic vasculature: blood flow, platelet function and vascular integrity. The three dynamic measures used to interrogate these features were, respectively, transcranial ultrasound, PFA100® and OCT.
Blood flow velocity measurements and vasomotor reactivity as determined by TCCS and TCD are rigorous methods of detecting abnormal brain artery vascular function 21,22. The primary endpoint in this study was blood flow velocity in the MCA. For this measure, assuming that blood flow will remain constant for this main conduit vessel of the brain, a significant increase in the velocity of blood flow would be evidence of a reduced luminal cross section, potentially due to increased vessel tone. We could not detect a significant effect of steady-state fingolimod on vessel tone of the MCA or other cerebral vessels. In order to confirm these results further we also used the provocative interventions of hypo- and hypercapnia which allowed the calculation of the CO2 reactivity of the MCA. Again, there were no differences in the CO2 reactivity between the three treatment groups. There is a robust literature on the use of the PFA-100® device in measuring the effects of various drugs on platelet function 23,24. In this study, neither platelet function, as measured by the PFA-100, nor platelet aggregation assays using a range of stimulation methods, showed evidence of a fingolimod treatment effect on platelet function. In addition, none of the plasma factors assessed in this study was affected. Although a mean increase of 19% from baseline was observed for d-dimers, at both time points in the study, all individual subject d-dimer values were below the declared cut-off value (500 μg ml−1) typically used for the exclusion of deep vein thrombosis and pulmonary embolism 25. Finally, OCT is recognized as a very sensitive tool for the detection of early signs of macular oedema 26. Using this tool to interrogate possible changes in the hydrodynamics of the macula, we were not able to detect any significant changes in macular thickness with fingolimod treatment.
Steady-state dosing of fingolimod 0.5 and 1.25 mg for 1 month was well tolerated in healthy volunteers. The safety profile of fingolimod was consistent with previous reports 4,5,27–29. The observed asymptomatic reduction in heart rate, decrease in mean lymphocyte count, and mild increase in liver transaminases were expected and well-characterized pharmacologic effects of fingolimod 30.
The parallel design might be considered as a limitation for this type of study. While a crossover design would have been preferred, the parallel design was employed owing to the long half-life of 7–10 days of fingolimod. Baseline measurements and a placebo treatment group allowed the comparisons both within and between treatment groups. The escalating doses of fingolimod 0.5 and 1.25 mg used in this study ensured a steady-state blood concentration at the end of the loading phase. Healthy volunteers were chosen for this study as they would provide normative data on the potential pharmacodynamic effects of fingolimod. While the sample size was sufficient to allow accurate quantification of the dynamic effects, it does not rule out possible outlier or idiosyncratic effects which might occur infrequently. Another limitation would be the 1 month study duration, which might not capture potential long term effects, for example on macular thickness. Pivotal phase 3 studies with fingolimod have reported an approximately 0.4% incidence of cystoid macular oedema with the approved 0.5 mg dose with most cases diagnosed within 3–4 months of starting treatment with fingolimod 4,5.
A clinical implication of this study relates to patients who may be misdiagnosed as having MS and started on fingolimod treatment. For example, systemic diseases such as antiphospholipid antibody syndrome (APS) and systemic lupus erythematosus can present with symptomatology resembling that of MS. APS presenting with complex neurological manifestations and multifocal white matter lesions similar to MS lesions has been described 31. Results of our study suggest that fingolimod would not be expected to contribute untoward pharmacological changes that might increase the risk of cerebrovascular events (embolism, thrombosis and stroke) inherently associated with these autoimmune vascular disease conditions that may be misdiagnosed as MS.
In addition to providing additional understanding of the effects of S1P receptor modulation on the cerebrovascular system, we have shown that the effect of fingolimod 0.5 and 1.25 mg once daily in healthy volunteers was similar to the effect of placebo for all the pharmacodynamic parameters assessed in this study. Steady-state dosing with fingolimod in healthy volunteers had no effects beyond the normal variability of these parameters.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and FD, SS, UP and SZ declare no support from any organization for the submitted work. The authors MD, KM, OD, JV and RS were employed by Novartis during the conduct of the study and are still employed by Novartis outside the submitted work.
Ethical standard
This study has been approved by the local ethics committee and has been performed in accordance with the ethical standards.
The authors would like to acknowledge Uma Kundu (Medical communications, Novartis Healthcare Pvt. Ltd) for providing medical writing support, which encompassed preparation of a first draft, formatting, referencing, preparing tables and figures, incorporating the authors' revisions, and submission all under the direction of the authors.
References
- Chun J, Hartung HP. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol. 2010;33:91–101. doi: 10.1097/WNF.0b013e3181cbf825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V. FTY720 (fingolimod) in multiple sclerosis: therapeutic effects in the immune and the central nervous system. Br J Pharmacol. 2009;158:1173–1182. doi: 10.1111/j.1476-5381.2009.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2010. FDA approves first oral drug to reduce MS relapses. Available at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm226755.htm. last accessed September 2013)
- Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, Pelletier J, Capra R, Gallo P, Izquierdo G, Tiel-Wilck K, deVera A, Jin J, Stites T, We S, Aradhye S, Kappos L for the TRANSFORMS Study Group. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- Kappos L, Radue EW, O'Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L, Burtin P for the FREEDOMS Study Group. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- Hla T. Physiological and pathological actions of sphingosine 1-phosphate. Semin Cell Dev Biol. 2004;15:513–520. doi: 10.1016/j.semcdb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Alewijnse AE, Peters SLM, Michel MC. Cardiovascular effects of sphingosine1-phosphate and other sphingomyelin metabolites. Br J Pharmacol. 2004;143:666–684. doi: 10.1038/sj.bjp.0705934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucke S, Levkau B. Endothelial functions of sphingosine1- phosphate. Cell Physiol Biochem. 2010;26:87–96. doi: 10.1159/000315109. [DOI] [PubMed] [Google Scholar]
- Igarashi J, Michel T. Sphingosine1-phosphate and modulation of vascular tone. Cardiovasc Res. 2009;82:212–220. doi: 10.1093/cvr/cvp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzuner N, Ozkan O, Cinar N. Cerebrovascular reactivity in multiple sclerosis patients. Mult Scler. 2007;13:737–741. doi: 10.1177/1352458506074645. [DOI] [PubMed] [Google Scholar]
- Adams CW, Poston RN, Buk SJ. Pathology, histochemistry and immunocytochemistry of lesions in acute multiple sclerosis. J Neurol Sci. 1989;92:291–306. doi: 10.1016/0022-510x(89)90144-5. [DOI] [PubMed] [Google Scholar]
- Kornek B, Lassmann H. Neuropathology of multiple sclerosis – new concepts. Brain Res Bull. 2003;61:321–326. doi: 10.1016/s0361-9230(03)00095-9. [DOI] [PubMed] [Google Scholar]
- Adams CW, Poston RN, Buk SJ, Sidhu YS, Vipond H. Inflammatory vasculitis in multiple sclerosis. J Neurol Sci. 1985;69:269–283. doi: 10.1016/0022-510x(85)90139-x. [DOI] [PubMed] [Google Scholar]
- Wakefield AJ, More LJ, Difford J, McLaughlin JE. Immunohistochemical study of vascular injury in acute multiple sclerosis. J Clin Pathol. 1994;47:129–133. doi: 10.1136/jcp.47.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheremata WA, Jy W, Horstman LL, Ahn YS, Alexander JS, Minagar A. Evidence of platelet activation in multiple sclerosis. J Neuroinflammation. 2008;5:27. doi: 10.1186/1742-2094-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright HP, Thompson RH, Zilkha KJ. Platelet adhesiveness in multiple sclerosis. Lancet. 1965;2:1109–1110. doi: 10.1016/s0140-6736(65)90069-3. [DOI] [PubMed] [Google Scholar]
- Peeters PJHL, Bazelier MT, Uitdehaag BMJ, Leufkens HGM, De bruin ML, De Vries F. The risk of venous thromboembolism in patients with multiple sclerosis: the Clinical Practice Research Datalink. J Thromb Haemost. 2014;12:444–451. doi: 10.1111/jth.12523. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, McGrath JC, Catterall WA, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14. Br J Pharmacol. 2013a;170:1449–1867. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born GV. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962;194:927–929. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Baumruker T. Pulmonary and vascular pharmacology of sphingosine 1-phosphate. Curr Opin Pharmacol. 2006;6:244–250. doi: 10.1016/j.coph.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Piechnik SK, Chiarelli PA, Jezzard P. Modelling vascular reactivity to investigate the basis of the relationship between cerebral blood volume and flow under CO2 manipulation. Neuroimage. 2008;39:107–118. doi: 10.1016/j.neuroimage.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Reinhard M, Waldkircher Z, Timmer J, Weiller C, Hetzel A. Cerebellar autoregulation dynamics in humans. J Cereb Blood Flow Metab. 2008;28:1605–1612. doi: 10.1038/jcbfm.2008.48. [DOI] [PubMed] [Google Scholar]
- Harrison P, Robinson M, Liesner R, Khair K, Cohen H, Mackie I, Machin S. The PFA-100: a potential rapid screening tool for the assessment of platelet dysfunction. Clin Lab Haematol. 2002;24:225–232. doi: 10.1046/j.1365-2257.2002.00451.x. [DOI] [PubMed] [Google Scholar]
- Franchini M. The platelet-function analyzer (PFA-100) for evaluating primary hemostasis. Hematology. 2005;10:177–181. doi: 10.1080/10245330400026097. [DOI] [PubMed] [Google Scholar]
- Vossen JA, Albrektson J, Sensarma A, Williams SC. Clinical usefulness of adjusted D-dimer cut-off values to exclude pulmonary embolism in a community hospital emergency department patient population. Acta Radiol. 2012;53:765–768. doi: 10.1258/ar.2012.120105. [DOI] [PubMed] [Google Scholar]
- Ouyang Y, Keane PA, Sadda SR, Walsh AC. Detection of cystoid macular oedema with three-dimensional optical coherence tomography versus fluorescein angiography. Invest Ophthalmol Vis Sci. 2010;51:5213–5218. doi: 10.1167/iovs.09-4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappos L, Antel J, Comi G, Montalban X, O'Connor P, Polman C, Haas T, Korn AA, Karlsson G. Radue E-W for the FTY720 D2201 Study Group. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355:1124–1140. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- O'Connor P, Comi G, Montalban X, Antel J, Radue EW, de Vera A, Pohlmann H, Kappos L for the FTY720 D2201 Study Group. Oral fingolimod (FTY720) in multiple sclerosis: Two-year results of a phase II extension study. Neurology. 2009;72:73–79. doi: 10.1212/01.wnl.0000338569.32367.3d. [DOI] [PubMed] [Google Scholar]
- Comi G, O'Connor P, Montalban X, Antel J, Radue E-W, Karlsson G, Pohlmann H, Aradhye S, Kappos L for the FTY720 D2201 Study Group. Phase II study of oral fingolimod (FTY720) in multiple sclerosis: 3-year results. Mult Scler. 2010;16:197–207. doi: 10.1177/1352458509357065. [DOI] [PubMed] [Google Scholar]
- Schmouder R, Aradhye S, O'Connor P, Kappos L. Pharmacodynamic effects of oral fingolimod (FTY720) Mult Scler. 2006;12:S101. [Google Scholar]
- Ferreira S, D'Cruz DP, Hughes GR. Multiple sclerosis, neuropsychiatric lupus and antiphospholipid syndrome: where do we stand? Rheumatology. 2005;44:434–442. doi: 10.1093/rheumatology/keh532. [DOI] [PubMed] [Google Scholar]