Abstract
Aims
The aims were to describe emtricitabine (FTC) pharmacokinetics in a large population of pregnant women during the different trimesters of pregnancy, and to explain FTC pharmacokinetic variability during pregnancy.
Methods
FTC plasma concentrations were measured in 103 non-pregnant and 83 pregnant women, including women in the different trimesters of pregnancy and on the day of delivery. A total of 457 plasma concentrations were available for analysis. A population pharmacokinetic model was developed with Monolix 4.1.3.
Results
FTC pharmacokinetics was best described by a two compartment model. The effect of creatinine clearance on apparent elimination clearance (CL/F) was significant. CL/F in pregnant women was significantly higher compared with non-pregnant women (geometric mean 24.1 vs 20.5 l h−1, P < 0.001), reflecting a modified renal function. FTC daily exposures (AUC) during pregnancy were lower than AUC in non-pregnant women, regardless of the trimester of pregnancy. FTC AUC geometric means were 8.38 mg l−1 h in the second trimester of pregnancy, 8.16 mg l−1 h in the third trimester of pregnancy, 8.30 mg l−1 h on the day of delivery and 9.77 mg l−1 h in non-pregnant women. FTC concentrations 24 h after administration were lower in pregnant women compared with non-pregnant women (0.054 vs. 0.079 mg l−1, P < 0.001) but still above the inhibitory concentration 50%.
Conclusions
FTC CL/F was increased by 18% during pregnancy, reflecting a modified renal function with 50% increase in estimated glomerular filtration rate. However, the impact of this modified renal function on FTC pharmacokinetics was not sufficiently large to consider dose adjustments during pregnancy.
Keywords: emtricitabine, population pharmacokinetics, pregnancy
What is Already Known about this Subject
Emtricitabine (FTC) pharmacokinetics has been described in pregnant women in the third trimester of pregnancy and at delivery.
Higher elimination clearance and lower FTC exposure have been reported during the third trimester of pregnancy compared with the post-partum period.
No FTC dosing adjustments are required during the third trimester of pregnancy.
What this Study Adds
This is the first population pharmacokinetic model of FTC during pregnancy, including data from women in the second trimester of pregnancy.
The FTC pharmacokinetic variability during pregnancy has been explained by a modified renal function during the gestational period, as indicated by an increase in estimated glomerular filtration rate.
No FTC dosing adjustments are required during the second trimester of pregnancy.
Introduction
Emtricitabine (FTC) is a once daily potent nucleoside reverse transcriptase inhibitor (NRTI) approved for the treatment of human immunodeficiency virus (HIV) infection in adult patients 1. The pharmacokinetics of a 200 mg FTC dose has been well described in healthy volunteers 2–4 and in HIV-infected patients 5–7.
According to the previous World Health Organization (WHO) recommendations 8,9, combined treatment containing FTC can be continued during the pregnancy of previously treated HIV-infected non-pregnant women. Combined treatment containing FTC can also be initiated in pregnant women needing a treatment for their own health or as a prophylaxis option for prevention of mother-to-child-transmission (PMTCT) 8,9.
Pregnancy and labour are associated with physiological changes (i.e. reduced intestinal motility, larger plasma volume, induced hepatic enzymes or increased glomerular filtration rate) which could lead to variations in pharmacokinetics 10. The effects of pregnancy on FTC pharmacokinetics have been explored. Two studies have described the pharmacokinetics of a single dose of FTC at labour (400 mg for Hirt et al. 11, 600 mg for Flynn et al. 12) and have reported higher FTC elimination clearance on the day of delivery. Two other studies have described FTC pharmacokinetics during the third trimester of pregnancy 13,14 and have found higher FTC elimination clearance (25.0 vs. 20.6 l h−1 14, 21 vs. 15 l.h−1 13) and lower FTC exposure (8.0 vs. 9.7 mg l−1 h 14, 9.56 vs. 13.0 mg l−1 h 13) compared with the post-partum period. However, these studies had some limitations. A small number of pregnant women were included (n = 8 to 38), no data during the first and second trimester of pregnancy were available and the higher elimination clearance during pregnancy has not been explained, despite the fact that a higher glomerular filtration rate has been suspected.
Current 2013 WHO guidelines recommend starting antiretroviral therapy earlier in all adults (CD4 cell count of 500 cells mm−3 or less), including non-pregnant women who may continue their treatment while pregnant 15,16. Furthermore, antiretroviral therapy in pregnant women should be initiated regardless of CD4 cell count or WHO clinical stage 15,16 and a once daily fixed dose combination of tenofovir disoproxil fumarate (TDF) + lamivudine or FTC + efavirenz is now recommended both for PMTCT and for the own health of pregnant women 16. Thus, with an earlier start of treatment and one strongly recommended therapeutic option during pregnancy, the use of FTC during the gestational period will increase in the next few years. As knowledge about FTC pharmacokinetic changes during pregnancy is limited and as only data about late pregnancy is available, the aims of our work were (i) to describe FTC pharmacokinetics in a large population of pregnant women during the different trimesters of pregnancy by a population approach and (ii) to explain the pharmacokinetic variability during pregnancy.
Methods
Patients and treatment
The population included non-pregnant women, pregnant women and women on the day of delivery receiving FTC for the treatment of HIV infection or for PMTCT. Our population was based on two combined populations, women followed for therapeutic drug monitoring (TDM) and women at delivery from a previously published clinical study from ANRS 11. Patients from the TDM group were followed at Cochin Hospital and received an oral 200 mg FTC dose chronically. For these patients, plasma concentrations were monitored on a routine basis. Ethics committee approval and patients' consent are not required in France to use therapeutic drug monitoring data retrospectively. Women at labour from the TEmAA ANRS 12109 study received an oral 400 mg FTC dose at the start of labour and plasma concentrations were measured at 1, 2, 3, 5, 8, 12 and 24 h after administration. The TEmAA ANRS 12109 study protocol was approved by each country's health or medicine regulatory authorities, by the national ethics committees of Ivory Coast and Cambodia and by the University of the Witwatersrand Health Research Ethics Committee in South Africa. The women from the TEmAA ANRS 12109 study signed informed consent forms. For each woman, the time elapsed between FTC intake and sampling times, body weight (BW), age, serum creatinine (Scr) and associated antiretroviral drugs were recorded. The gestational age and trimester of pregnancy were also recorded for each pregnant woman. Creatinine clearance (CLcr) was calculated using the Cockcroft & Gault formula 17.
Analytical method
The FTC assay was performed according to a previously published method of liquid chromatography coupled to tandem mass spectrometry 18. The limit of quantification (LOQ) was 0.01 mg l−1, the intra-assay precision was 3.6% and the inter-assay precision was 7.9%.
Population pharmacokinetic modelling strategy
Data were analyzed with a non-linear mixed-effect modelling approach, using the Monolix software program version 4.1.3 (available at http://www.lixoft.eu) 19. Parameters were estimated by computing the maximum likelihood estimator of the parameters, using the stochastic approximation expectation maximization (SAEM) algorithm combined with a Markov Chain Monte Carlo (MCMC) procedure. The number of MCMC chains was fixed to five for all estimations. Several structural models for FTC pharmacokinetics were investigated, i.e. one, two or three compartments with linear absorption and elimination. Models with fixed values for the absorption rate constant (ka) or with a ka equal to the distribution rate constant (α) were also considered. Several error models (proportional, additive, mixed) were investigated to describe the residual variability (ε). One residual error model for both studies and one residual error model for each study were investigated. Inter-individual variabilities (IIV or η) were assumed to be exponential. The effect of each patient covariate on pharmacokinetic parameters was tested according to the following equations:
-
Continuous covariates
The continuous covariates (CO) such as age, BW, Scr, BW/Scr and CLcr were tested with the following equation, using apparent elimination clearance (CL/F) for example:
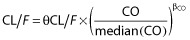 , where θCL/F is the typical value of apparent elimination clearance for a subject with the median covariate value and βCO is the estimated influential factor for the continuous covariate.
, where θCL/F is the typical value of apparent elimination clearance for a subject with the median covariate value and βCO is the estimated influential factor for the continuous covariate. -
Binary covariates
The binary covariates such as associated antiretroviral drugs were tested according to the equation
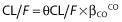 , and βCO is the estimated influential factor for the binary covariate.
, and βCO is the estimated influential factor for the binary covariate. -
Influence of pregnancy
The effect of pregnancy on FTC pharmacokinetics was investigated using several equations. Pregnancy and delivery were considered as binary covariates and were tested with the equation previously described (see (ii)). Gestational age (GA) was tested according to the following equations:
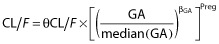 , where Preg = 1 for pregnant or parturient women and Preg = 0 otherwise, and βGA is the estimated influential factor of GA.
, where Preg = 1 for pregnant or parturient women and Preg = 0 otherwise, and βGA is the estimated influential factor of GA.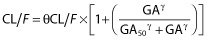 , where GA50 and γ are the estimated parameters of the Hill equation.
, where GA50 and γ are the estimated parameters of the Hill equation.The effect of gestational age was also tested as a categorical covariate by splitting women into the first, second, and third trimester of pregnancy according to their GA. The effect of GA by grouping two of these classes has also been investigated.
The objective function value (OFV) was used to test different hypotheses regarding the structural model, the structure of the variance-covariance matrix for IIV and residual variability models. The OFV was also used to test the covariate effect(s) on pharmacokinetic parameter(s). A covariate was finally retained in the model if its effect was biologically plausible, if the OFV was decreased by at least 3.84 and if it produced a reduction in the variability of the pharmacokinetic parameter (IIV).
Model evaluation and validation
For evaluation of the goodness of fit, the following graphs were performed: observed concentrations vs. population predictions, weighted residuals vs. time and weighted residuals vs. population predictions. Similar graphs using individual predictions were displayed.
To validate the final model, a visual predictive check (VPC) was performed. The final model was used to simulate 1000 FTC concentrations at each sampling time. The simulated and observed data stratified by dose were compared. The final model was also validated by the normalized prediction distribution errors metrics (NPDE) 20. Diagnostic graphics and distribution statistics were performed using RfN (link on http://wfn.sourceforge.net) from the R program.
FTC pharmacokinetic parameters in women
FTC concentration at time point 24 h (C24) and daily exposures (AUC) were derived from the estimated individual pharmacokinetic parameters. Geometric mean values were calculated and compared between non-pregnant women and pregnant women, as well as between the different trimesters of pregnancy.
Results
Demographic data
Data from 179 women (148 from Cochin Hospital and 31 from the TEmAA ANRS 12109 study) were available for FTC pharmacokinetic evaluation. Among these women, 103 were non-pregnant, 83 were pregnant (seven were in these two different groups on different occasions). Among the 83 pregnant women, one was in the first trimester of pregnancy, 26 were in the second trimester of pregnancy and 67 were in the third trimester of pregnancy (48 women on the day of delivery). Table 1 summarizes the patient characteristics. Among non-pregnant women, 94% were co-treated with TDF, 61% were co-treated with a protease inhibitor (PI) and 22% were co-treated with a non-nucleoside reverse transcriptase inhibitor (NNRTI). Among the pregnant women, 97% were co-treated with TDF, 57% were co-treated with a PI, 40% were co-treated with a NNRTI and 51% were co-treated with a NRTI.
Table 1.
Characteristics of the HIV-infected women
| Number of patients | Number of samples | Median (range) | |||||
|---|---|---|---|---|---|---|---|
| Age (years) | BW (kg) | Scr (μmol l−1) | CLcr (ml min−1) | GA (weeks) | |||
| Population | 179 | 457 | 35 (16–72) | 69 (37–130) | 60 (27–183) | 135 (35–335) | 37 (5–41) |
| Pregnant women | 83 | 307 | 31 (19–43) | 70 (47–106) | 49 (27–119) | 160 (70–335) | 37 (5–41) |
| Non-pregnant women | 103 | 150 | 40 (16–72) | 69 (37–130) | 66 (35–183) | 106 (35–247) | – |
BW, body weight; Scr, serum creatinine; CLcr, creatinine clearance; GA, gestational age.
Population pharmacokinetics
A total of 457 FTC plasma concentrations were available for the analysis. Among these concentrations, 307 samples were obtained in pregnant women (one sample in the first trimester of pregnancy, 29 in the second trimester, 22 in the third trimester and 255 on the day of delivery). Fourteen samples out of 457 were lower than the LOQ (3%), and were set to half of the LOQ 21. A two compartment model with first order absorption and elimination best described the data. The pharmacokinetic parameters of this model were the absorption rate constant (ka), the apparent elimination clearance (CL/F), the apparent central volume of distribution (Vc/F), the intercompartmental clearance (Q/F), and the peripheral volume of distribution (Vp/F) with F being the unknown bioavailability.
Residual variability was best described by a proportional error model. Estimating a residual variability for each study resulted in a 12.4 point decrease in the OFV and improved the goodness of fit. Inter-individual variabilities were significant for ka and CL/F. The effects of pregnancy, GA (continuous and categorical by trimester of pregnancy), age, BW, Scr and CLcr on CL/F significantly decreased the OFV and the IIV on CL/F. The most significant decrease in OFV was obtained by adding the effect of CLcr on CL/F (−36.57 points). After the inclusion of CLcr on CL/F in the model, the addition of other covariates did not further improve the model. Thus, the final covariate model was 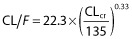 , with 22.3 l.h−1 the typical value of CL/F for a patient with a median CLcr value of 135 ml.min−1 and 0.33 the influential factor of CLcr on CL/F. Table 2 summarizes the final population pharmacokinetic estimates, including the relative standard errors.
, with 22.3 l.h−1 the typical value of CL/F for a patient with a median CLcr value of 135 ml.min−1 and 0.33 the influential factor of CLcr on CL/F. Table 2 summarizes the final population pharmacokinetic estimates, including the relative standard errors.
Table 2.
Population pharmacokinetic parameters of FTC from the final model
| Parameter | Estimate | RSE (%) |
|---|---|---|
| Structural model | ||
| ka (h−1) | 0.616 | 32 |
| CL/F (l h−1) | 22.3 | 3 |
| Vc/F (l) | 100 | 16 |
| Q/F (l h−1) | 5.89 | 22 |
| Vp/F (l) | 76.1 | 28 |
| βCLcr on CL/F | 0.33 | 16 |
| Statistical model | ||
| η ka | 0.503 | 36 |
| η CL/F | 0.151 | 14 |
| σ1 | 0.505 | 6 |
| σ2 | 0.373 | 6 |
RSE, relative standard error; ka, absorption rate constant; CL/F, apparent elimination clearance; Vc/F, apparent central volume of distribution; Q/F, apparent intercompartmental clearance; Vp/F, apparent peripheral volume of distribution; βCLcr on CL/F, influential factor of creatinine clearance on CL/F; η, interindividual variability; σ1, residual variability estimate for therapeutic drug monitoring study; σ2, residual variability estimate for TEmAA ANRS 12109 study.
Model evaluation and validation
As shown in Table 2, all the parameters were well estimated, with small relative standard errors (RSE). The visual predictive check of the final model (Figure 1) confirmed that the average prediction matched the observed concentrations and that variability was well estimated. The mean and variance of the NPDE metrics were not significantly different from 0 (P = 0.49, Wilcoxon signed rank test) and 1 (P = 0.901, Fisher variance test), respectively, and their distribution was not different from a normal one at the typical α = 0.01 level of significance (P = 0.031, Shapiro-Wilk test of normality). Overall, the global adjusted P value was 0.093.
Figure 1.
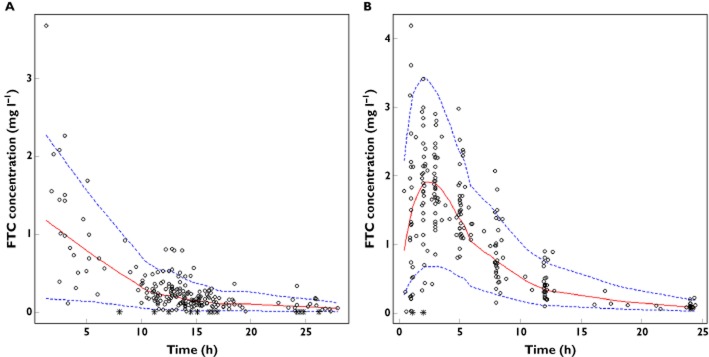
Validation of the final model by the visual predictive check (VPC) stratified by dose. Comparison between the 5th (dashed line), 50th (solid line) and 95th (dashed line) percentiles obtained from 1000 simulations and the observed data (circles) for FTC concentrations in (A) therapeutic drug monitoring study (200 mg dose) and (B) TEmAA ANRS 12109 study (400 mg dose). Asterisks denote data below the limit of quantification (LOQ)
FTC pharmacokinetic parameters in women
FTC apparent elimination clearance geometric mean [minimum–maximum] was 20.5 [13.5–28.1] l h−1 in non-pregnant women and was 24.1 [15.2–32.2] l h−1 in pregnant women (Figure 2). Figure 3 depicts individual CL/F estimates as a function of GA, showing the significant increase of CL/F in pregnant women compared with non-pregnant women (P < 0.001). As only one pregnant woman was in the first trimester of pregnancy, no results for this trimester could be presented. CL/F geometric means in pregnant women during the different trimesters of pregnancy were close (23.9 l h−1 for the second trimester of pregnancy, 24.5 l h−1 for the third trimester and 24.1 l h−1 on the day of delivery) (Figure 3). Table 3 summarizes AUC and C24 geometric mean values [minimum–maximum] obtained for a FTC 200 mg dose. These values are stratified between pregnant and non-pregnant women and between trimesters of pregnancy. FTC exposures decreased from 15% in pregnant women compared with non-pregnant women. This magnitude of decrease was similar whatever the trimester of pregnancy (14% decrease for the second trimester of pregnancy, 16.5% decrease for the third trimester of pregnancy and 15% decrease on the day of delivery). Table 4 compares AUC and C24 geometric mean values with previously published values during pregnancy 13,14. It also shows the percentage of pregnant women with an AUC higher than 7 mg l−1 h (previous target exposure chosen in pregnancy 13,14). To compare with previous results 14, the percentage of pregnant women with a C24 lower than the in vitro inhibitory concentration 50% (IC50 = 0.004 mg l−1) 6,22 was calculated.
Figure 2.
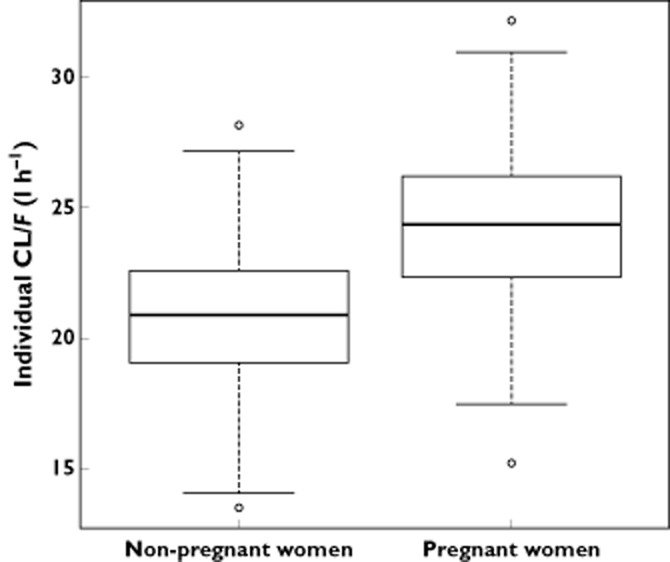
Comparison of individual CL/F estimates between non-pregnant and pregnant women. Boxes represent the median and interquartile range and whisker plots represent values within 1.5 times the interquartile range
Figure 3.
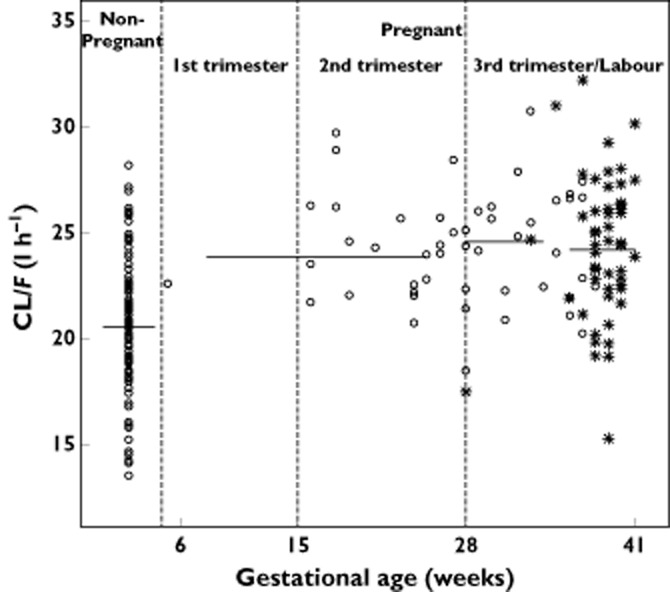
Individual FTC CL/F (l h−1) estimates as a function of gestational age (weeks). Solid lines represent the geometric mean value of CL/F, asterisks represent values of CL/F at labour
Table 3.
Comparison of the C24 and AUC geometric mean values [minimum–maximum] between non-pregnant and pregnant women
| Non-pregnant women (n = 103) | Pregnant women (n = 83) | Pregnant women | |||
|---|---|---|---|---|---|
| Second trimester (n = 26) | Third trimester (n = 19) | At labour (n = 48) | |||
| AUC (mg l−1 h) | 9.77 [7.10–14.83] | 8.30 [6.22–13.15] | 8.38 [6.74–10.85] | 8.16 [6.51–9.89] | 8.30 [6.22–13.15] |
| C24 (mg l−1) | 0.079 [0.038–0.197] | 0.054 [0.026–0.116] | 0.055 [0.033–0.099] | 0.052 [0.030–0.081] | 0.047 [0.026–0.116] |
Table 4.
Comparison of the AUC and C24 geometric means (GM) values in pregnant women for the present study vs. other studies
Discussion
The pharmacokinetics of FTC in pregnant and non-pregnant women was satisfactorily described by a two compartment model with linear absorption and elimination, including an effect of CLcr on CL/F. The following observations support the model used: (i) the final model was validated by visual predictive check and (ii) the pharmacokinetic parameters such as AUC or CL/F in non-pregnant women were close to previously published values in adults 2,4,6. Two studies have described FTC pharmacokinetics during pregnancy 13,14 and have reported 20% and 34% increases in FTC apparent elimination clearance during the third trimester of pregnancy compared with post-partum. The 200 mg dose administered during the third trimester of pregnancy produced FTC exposures reduced by 18 and 25% compared with post-partum. Hirt et al. 11 and Flynn et al. 12 have reported FTC pharmacokinetics on the day of delivery, after a single dose of 400 mg or 600 mg, respectively. Despite a higher elimination clearance of FTC on the day of delivery, the 400 mg dose and the 600 mg dose produced higher AUC values than the ones in non-pregnant adults for a 200 mg dose. In our study, we have shown an 18% increase in CL/F and a 15% decrease in AUC between pregnant and non-pregnant women for a 200 mg dose. FTC pharmacokinetics were described in pregnant women with a GA from 5 to 41 weeks. Our results were consistent with previous studies focused on late pregnancy 13,14. For the first time, our study presents data on FTC pharmacokinetics during the second trimester of pregnancy. Geometric mean values were 23.9 l h−1 for CL/F, 8.38 mg l−1 h for AUC and 0.055 mg l−1 for C24. These pharmacokinetic parameters during the second trimester of pregnancy were close to the ones obtained during the third trimester of pregnancy and on the day of delivery. Thus, no differences in pharmacokinetic parameters with clinical relevance were found between the different trimesters of pregnancy.
FTC is mainly eliminated by renal excretion, by a combination of glomerular filtration and active tubular secretion, with 86% of an oral dose recovered in urine 5. A previous study has described the impact of an impaired renal function on FTC pharmacokinetics 7, suggesting that a modification in renal function could significantly alter FTC pharmacokinetics.
During pregnancy, effective renal plasma flow increases by 80% 10,23,24 and glomerular filtration rate increases by 50% 10,23–25. Previous studies on FTC pharmacokinetics have suspected that these physiological changes could explain the higher elimination clearance found during pregnancy. However, this hypothesis has never been demonstrated. In our study, we measured Scr for each woman and used the Cockcroft & Gault formula 17 to obtain CLcr estimating glomerular filtration rate and renal function. The effect of CLcr on CL/F was significant, with CL/F increasing according to CLcr. The median CLcr was estimated at 106 ml min−1 in non-pregnant women and at 160 ml min−1 in pregnant women, confirming the 50% increase in glomerular filtration rate previously described 10,23–25. These results confirmed that renal function, especially glomerular filtration rate, was the most important factor explaining the higher FTC elimination clearance observed during pregnancy. This higher FTC elimination clearance was observed for pregnant women, regardless of the trimester of pregnancy. Thus, even women in the second trimester of pregnancy had a modified FTC elimination clearance compared with non-pregnant women. This is in agreement with previous studies, suggesting that the increase in glomerular filtration rate begins early after conception and remains constant throughout pregnancy 24,26. One limitation of our study was the use of the Cockcroft & Gault formula to estimate glomerular filtration rate during pregnancy. As ethnicity was not available for all women, we could not estimate glomerular filtration rate by the MDRD formula. The Cockcroft & Gault formula might overestimate glomerular filtration rate because the increase in weight during pregnancy is due to an increase in water and fat mass but not in muscle mass 27. However, despite its limitation, estimated glomerular filtration rate during pregnancy with this formula was in agreement with previous studies using inulin clearance 23,24.
A relationship between FTC daily exposure and anti-HIV activity has been previously described 6. The anti-HIV activity (reduction of viral load) increased with FTC exposure and reached a plateau for a FTC daily AUC of 10 mg l−1 h. This exposure corresponds to the typical exposure in adults receiving 200 mg FTC once a day 2–5. Thus, one reasonable goal to ensure FTC efficacy in pregnant women is to achieve an AUC value associated with a maximal anti-HIV activity in non-pregnant adults. Other studies in pregnancy have defined a target exposure above 7 mg l−1 h, considering that a 30% reduction from the typical AUC value may be of clinical relevance 13,14. In our study, 93% of pregnant women had a daily AUC lower than 10 mg l−1 h, suggesting that FTC maximal anti-HIV activity was not reached in the majority of pregnant women. However, the percentage of pregnant women with a daily AUC higher than 7 mg l−1 h (92%) was close to the one for non-pregnant women (100%). Furthermore, 100% of the C24 values were above the IC50 value. Thus, even if a 15% reduction in daily FTC exposure was observed between pregnant and non-pregnant women, this difference may not be sufficiently large to suspect a clinically relevant reduction in FTC efficacy in pregnant women. FTC dosing adjustments during pregnancy, including the second trimester of pregnancy, do not appear to be necessary for FTC.
In conclusion, our study presents for the first time FTC pharmacokinetic data during the second trimester of pregnancy. No differences were found between the pharmacokinetic parameters in the second trimester of pregnancy and in the third trimester of pregnancy. FTC apparent elimination clearance was increased by 18% during pregnancy, reflecting a modified renal function with a 50% increase in estimated glomerular filtration rate. FTC exposures in pregnant women were slightly lower (15%) than the ones in non-pregnant women. The magnitude of change in FTC exposures during pregnancy was not considered as clinically relevant, both during the second or third trimester of pregnancy. Thus no dosing adjustments for pregnant women were suggested. However, as very few data about FTC pharmacokinetics in the first trimester of pregnancy were available, further investigations on this period are needed.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
We thank the medical teams involved in the therapeutic drug monitoring of women in Cochin Hospital, France.
We acknowledge the French Agence Nationale de Recherche contre le VIH/SIDA et les hepatites virales (ANRS) for sponsoring the TEmAA trial, as well as the European and Developing Countries Clinical Trials Partnership (EDCTP) for their additional financial support. We greatly thank the local investigators and their staff at the Formations Sanitaires Urbaines de Youpougon and Abobo and the Centre Hospitalier Universitaire de Yopougon in Abidjan, Côte d'Ivoire, the Calmette Hospital and Pasteur Institute in Phnom Penh, Cambodia and the Perinatal HIV Research Unit and Lesedi Clinic in Soweto, South Africa. We also thank the women who agreed to participate in the trial and their infants. We acknowledge Gilead Sciences for providing the study drugs. DKE was an EDCTP senior fellow from 2005 to 2007.
The TEmAA trial group is constituted as follows. Co-investigators include Christine Rouzioux, Stéphane Blanche and Jean-Marc Treluyer, Marie-Laure Chaix and Elisabeth Rey (Paris, France), N'dri Yoman (Abidjan, Côte d'Ivoire), Kruy Leang Sim and Eric Nerrienet (Phnom Penh, Cambodia), and Glenda Gray and James McIntyre (Soweto, South Africa). The trial coordinator is Elise Arrivé (Bordeaux, France). Other members of the TEmAA ANRS 12109 study group (by location and alphabetical order) include the following: Déborah Hirt and Saik Urien (Paris, France); Gérard Allou, Clarisse Amani-Bosse, Divine Avit, Gédéon Bédikou, Kouakou Brou, Patrick Coffié, Patrice Fian, Eulalie Kanga, Broulaye Kone, Suzanne Kouadio, Guy César Kouaho, Jeanne Eliam Kouakou, Sidonie Ngatchou, Touré Pety, Zenica Seoue, and Mamourou Kone (Abidjan, Côte d'Ivoire); Laurence Borand, Pinn Chou, Kearena Chhim, Meng Ly Ek, Viseth Horm Srey, Seng Hout, Sethikar Im, Saroeum Keo, Vannith Lim, Sopheak Ngin, Vara Ouk, Vibol Ung, and the Magna and Maryknoll associations (Phnom Penh, Cambodia); Gail Ashford, Promise Duma, Portia Duma, Sarita Lalsab, Shini Legote, Tshepiso Mabena, Joseph Makhura, Modise Maphutha, Selvan Naidoo, and Mandisa Nyati (Soweto, South Africa). The scientific board includes Bernard Koffi Ngoran (Abidjan, Côte d'Ivoire), Koum Kanal (Phnom Penh, Cambodia), Lynn Morris (Johannesburg, South Africa), Séverine Blesson (ANRS, Paris, France), Camille Aubron-Olivier (Gilead Sciences, Paris, France), Gilles Peytavin (Paris, France), Koen Van Rompay (Davis, CA, USA), and Valériane Leroy (Bordeaux, France). The independent committee includes John Sullivan (Worcester, MA, USA), Philippe Lepage (Brussels, Belgium), Laurent Mandelbrot (Paris, France), Marie-Louise Newell (London, United Kingdom) and Anne-Marie Taburet (Paris, France).
References
- World Health Organization. 2010. Antiretroviral therapy for HIV infection in adults and adolescents. Recommendations for a public health approach, 2010 revision. Available at http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf (last accessed 4 April 2014)
- Blum MR, Chittick GE, Begley JA, Zong J. Steady-state pharmacokinetics of emtricitabine and tenofovir disoproxil fumarate administered alone and in combination in healthy volunteers. J Clin Pharmacol. 2007;47:751–759. doi: 10.1177/0091270007300951. [DOI] [PubMed] [Google Scholar]
- Chittick GE, Zong J, Begley JA, Alianti JR, Sorbel JJ, Blum MR. Pharmacokinetics of emtricitabine/tenofovir disoproxil fumarate and tacrolimus at steady state when administered alone or in combination. Int J Clin Pharmacol Ther. 2008;46:627–636. doi: 10.5414/cpp46627. [DOI] [PubMed] [Google Scholar]
- Zong J, Chittick GE, Wang LH, Hui J, Begley JA, Blum MR. Pharmacokinetic evaluation of emtricitabine in combination with other nucleoside antivirals in healthy volunteers. J Clin Pharmacol. 2007;47:877–889. doi: 10.1177/0091270007300808. [DOI] [PubMed] [Google Scholar]
- Gilead Sciences. 2012. Emtriva® prescribing information. Available at http://www.gilead.com/pdf/emtriva_pi.pdf (last accessed 4 April 2014)
- Wang LH, Begley J, St Claire RL, III, Harris J, Wakeford C, Rousseau FS. Pharmacokinetic and pharmacodynamic characteristics of emtricitabine support its once daily dosing for the treatment of HIV infection. AIDS Res Hum Retroviruses. 2004;20:1173–1182. doi: 10.1089/aid.2004.20.1173. [DOI] [PubMed] [Google Scholar]
- Valade E, Tréluyer J-M, Bouazza N, Ghosn J, Foissac F, Benaboud S, Fauchet F, Viard J-P, Urien S, Hirt D. Population pharmacokinetics of emtricitabine in HIV-1-infected adult patients. Antimicrob Agents Chemother. 2014;58:2256–2261. doi: 10.1128/AAC.02058-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. 2012. Use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Available at http://whqlibdoc.who.int/hq/2012/WHO_HIV_2012.6_eng.pdf?ua=1 (last accessed 4 April 2014)
- World Health Organization. 2010. Rapid advice: use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Available at http://whqlibdoc.who.int/publications/2009/9789241598934_eng.pdf?ua=1 (last accessed 4 April 2014)
- Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44:989–1008. doi: 10.2165/00003088-200544100-00001. [DOI] [PubMed] [Google Scholar]
- Hirt D, Urien S, Rey E, Arrivé E, Ekouévi DK, Coffié P, Leang SK, Lalsab S, Avit D, Nerrienet E, McIntyre J, Blanche S, Dabis F, Tréluyer J-M. Population pharmacokinetics of emtricitabine in human immunodeficiency virus type 1-infected pregnant women and their neonates. Antimicrob Agents Chemother. 2009;53:1067–1073. doi: 10.1128/AAC.00860-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn PM, Mirochnick M, Shapiro DE, Bardeguez A, Rodman J, Robbins B, Huang S, Fiscus SA, Van Rompay KKA, Rooney JF, Kearney B, Mofenson LM, Watts DH, Jean-Philippe P, Heckman B, Thorpe E, Jr, Cotter A, Purswani M PACTG 394 Study Team. Pharmacokinetics and safety of single-dose tenofovir disoproxil fumarate and emtricitabine in HIV-1-infected pregnant women and their infants. Antimicrob Agents Chemother. 2011;55:5914–5922. doi: 10.1128/AAC.00544-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbers APH, Hawkins DA, Gingelmaier A, Kabeya K, Rockstroh JK, Wyen C, Weizsäcker K, Sadiq ST, Ivanovic J, Giaquinto C, Taylor GP, Moltó J, Burger DM PANNA network. The pharmacokinetics, safety and efficacy of tenofovir and emtricitabine in HIV-1-infected pregnant women. AIDS Lond Engl. 2013;27:739–748. doi: 10.1097/QAD.0b013e32835c208b. [DOI] [PubMed] [Google Scholar]
- Stek AM, Best BM, Luo W, Capparelli E, Burchett S, Hu C, Li H, Read JS, Jennings A, Barr E, Smith E, Rossi SS, Mirochnick M. Effect of pregnancy on emtricitabine pharmacokinetics. HIV Med. 2012;13:226–235. doi: 10.1111/j.1468-1293.2011.00965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirnschall G, Harries AD, Easterbrook PJ, Doherty MC, Ball A. The next generation of the World Health Organization's global antiretroviral guidance. J Int AIDS Soc. 2013;16:18757. doi: 10.7448/IAS.16.1.18757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. 2013. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Available at http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf?ua=1 (last accessed 4 April 2014)
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- Le Saux T, Chhun S, Rey E, Launay O, Weiss L, Viard J-P, Pons G, Jullien V. Quantification of seven nucleoside/nucleotide reverse transcriptase inhibitors in human plasma by high-performance liquid chromatography with tandem mass-spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;865:81–90. doi: 10.1016/j.jchromb.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Kuhn E, Lavielle M. Maximum likelihood estimation in nonlinear mixed effects models. Comput Stat Data Anal. 2005;49:1020–1038. [Google Scholar]
- Comets E, Brendel K, Mentré F. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Programs Biomed. 2008;90:154–166. doi: 10.1016/j.cmpb.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn. 2001;28:481–504. doi: 10.1023/a:1012299115260. [DOI] [PubMed] [Google Scholar]
- Schinazi RF. Assessment of the relative potency of emtricitabine and lamivudine. Letter to the editor. J Acquir Immune Defic Syndr. 2003;34:243–245. doi: 10.1097/00126334-200310010-00017. [DOI] [PubMed] [Google Scholar]
- Dunlop W. Serial changes in renal haemodynamics during normal human pregnancy. Br J Obstet Gynaecol. 1981;88:1–9. doi: 10.1111/j.1471-0528.1981.tb00929.x. [DOI] [PubMed] [Google Scholar]
- Davison JM, Dunlop W. Renal hemodynamics and tubular function normal human pregnancy. Kidney Int. 1980;18:152–161. doi: 10.1038/ki.1980.124. [DOI] [PubMed] [Google Scholar]
- Mirochnick M, Capparelli E. Pharmacokinetics of antiretrovirals in pregnant women. Clin Pharmacokinet. 2004;43:1071–1087. doi: 10.2165/00003088-200443150-00002. [DOI] [PubMed] [Google Scholar]
- Davison JM. Kidney function in pregnant women. Am J Kidney Dis. 1987;9:248–252. doi: 10.1016/s0272-6386(87)80117-8. [DOI] [PubMed] [Google Scholar]
- Maynard SE, Thadhani R. Pregnancy and the kidney. J Am Soc Nephrol. 2009;20:14–22. doi: 10.1681/ASN.2008050493. [DOI] [PubMed] [Google Scholar]


