Abstract
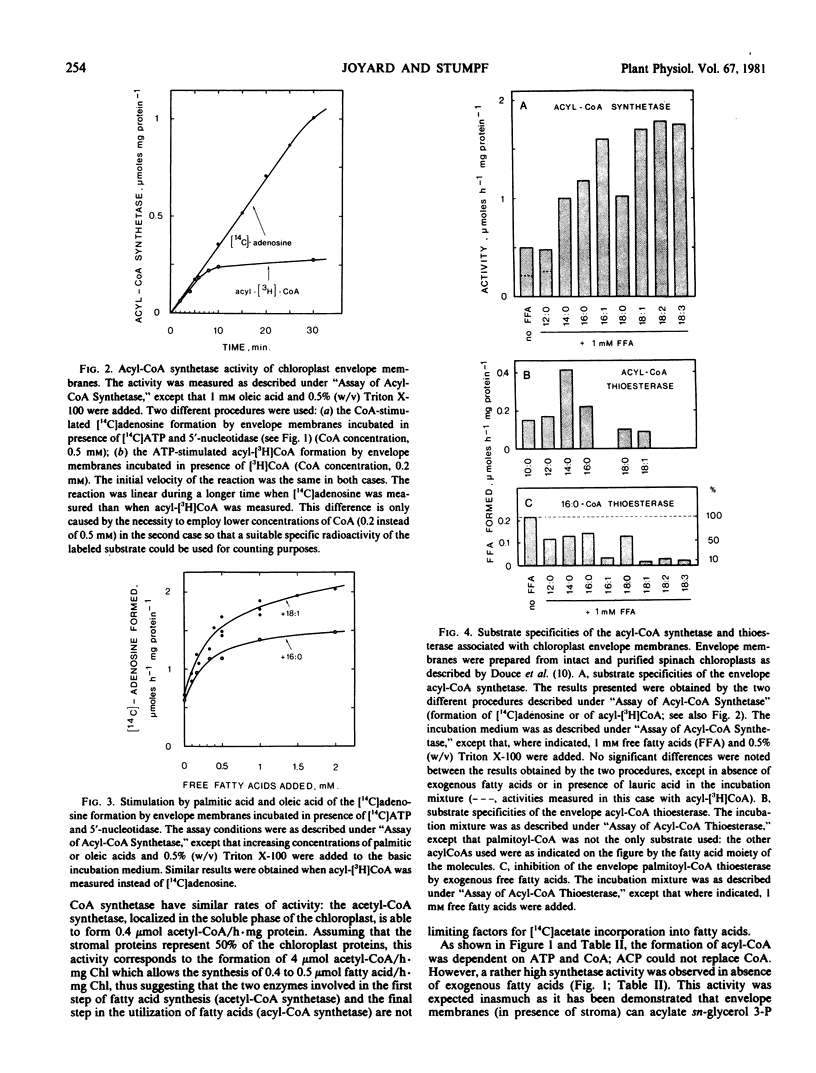
The chloroplast envelope is the site of a very active long-chain acylcoenzyme A (CoA) synthetase. Furthermore, we have recently shown that an acyl CoA thioesterase is also associated with envelope membrane (Joyard J, PK Stumpf 1980 Plant Physiol 65: 1039-1043). To clarify the interacting roles of both the acyl-CoA thioesterase and the acyl-CoA synthetase, the formation of acyl-CoA in envelope membranes was examined with different techniques which permitted the measurement of the actual rates of acyl-CoA formation. Using [14C]ATP or [14C]oleic acid as labeled substrates, it can be shown that the envelope acyl-CoA synthetase required both Mg2+ and dithiothreitol. Triton X-100 slightly stimulated the activity. The specificity of the acyl-CoA synthetase was determined either with [14C]ATP or with [3H]CoA as substrates. The results obtained in both cases were similar, that is, as substrates, the unsaturated fatty acids were more effective than saturated fatty acids, the velocity of the reaction increased from lauric acid to palmitic acid, and the maximum velocity was obtained with unsaturated C18 fatty acids.
The results obtained suggest that the acyl-CoA thioesterase associated with envelope membranes could be an ultimate control to prevent the transport (outside of the chloroplast) or the insertion (into chloroplast lipids) of fatty acids with chains shorter than C16.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROOKS J. L., STUMPF P. K. A SOLUBLE FATTY ACID SYNTHESIZING SYSTEM FROM LETTUCE CHLOROPLASTS. Biochim Biophys Acta. 1965 Feb 1;98:213–216. doi: 10.1016/0005-2760(65)90027-5. [DOI] [PubMed] [Google Scholar]
- Banis R. J., Tove S. B. Solubilization of a long chain fatty acyl-CoA synthetase from chicken adipose tissue microsomes. Biochim Biophys Acta. 1974 May 29;348(2):210–220. doi: 10.1016/0005-2760(74)90232-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CANVIN D. T., BEEVERS H. Sucrose synthesis from acetate in the germinating castor bean: kinetics and pathway. J Biol Chem. 1961 Apr;236:988–995. [PubMed] [Google Scholar]
- Cooper T. G. Activation of fatty acids in castor bean endosperm. J Biol Chem. 1971 Jun 10;246(11):3451–3455. [PubMed] [Google Scholar]
- Douce R., Holtz R. B., Benson A. A. Isolation and properties of the envelope of spinach chloroplasts. J Biol Chem. 1973 Oct 25;248(20):7215–7222. [PubMed] [Google Scholar]
- Joyard J., Douce R. Mn2+-dependent ATPase of the envelope of spinach chloroplasts. FEBS Lett. 1975 Mar 1;51(1):335–340. doi: 10.1016/0014-5793(75)80920-3. [DOI] [PubMed] [Google Scholar]
- Joyard J., Douce R. Site of synthesis of phosphatidic acid and diacyglycerol in spinach chloroplasts. Biochim Biophys Acta. 1977 Feb 23;486(2):273–285. doi: 10.1016/0005-2760(77)90023-6. [DOI] [PubMed] [Google Scholar]
- Joyard J., Stumpf P. K. Characterization of an acyl-coenzyme a thioesterase associated with the envelope of spinach chloroplasts. Plant Physiol. 1980 Jun;65(6):1039–1043. doi: 10.1104/pp.65.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinig H., Liedvogel B. Fatty acid synthesis by isolated chromoplasts from the daffodil. [14C]Acetate incorporation and distribution of labelled acids. Eur J Biochem. 1978 Feb;83(2):499–505. doi: 10.1111/j.1432-1033.1978.tb12116.x. [DOI] [PubMed] [Google Scholar]
- Kleinig H., Liedvogel B. On the energy requirements of fatty acid synthesis in spinach chloroplasts in the light and in the dark. FEBS Lett. 1979 May 15;101(2):339–342. doi: 10.1016/0014-5793(79)81039-x. [DOI] [PubMed] [Google Scholar]
- MUDD J. B., McMANUS T. T. Metabolism of acetate by cellfree preparations from spinach leaves. J Biol Chem. 1962 Jul;237:2057–2063. [PubMed] [Google Scholar]
- Mancha M., Stokes G. B., Stumpf P. K. Fat metabolism in higher plants. The determination of acyl-acyl carrier protein and acyl coenzyme A in a complex lipid mixture 1,2. Anal Biochem. 1975 Oct;68(2):600–608. doi: 10.1016/0003-2697(75)90655-7. [DOI] [PubMed] [Google Scholar]
- Murakami S., Strotmann H. Adenylate kinase bound to the envelope membranes of spinach chloroplasts. Arch Biochem Biophys. 1978 Jan 15;185(1):30–38. doi: 10.1016/0003-9861(78)90140-6. [DOI] [PubMed] [Google Scholar]
- Ohlrogge J. B., Kuhn D. N., Stumpf P. K. Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1194–1198. doi: 10.1073/pnas.76.3.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge J. B., Shine W. E., Stumpf P. K. Fat metabolism in higher plants. Characterization of plant acyl-ACP and acyl-CoA hydrolases. Arch Biochem Biophys. 1978 Aug;189(2):382–391. doi: 10.1016/0003-9861(78)90225-4. [DOI] [PubMed] [Google Scholar]
- Polokoff M. A., Bell R. M. Millipore filter assay for long-chain fatty acid:CoASH ligase activity using 3H-labeled coenzyme A. J Lipid Res. 1975 Sep;16(5):397–402. [PubMed] [Google Scholar]
- Roughan P. G., Holland R., Slack C. R. The role of chloroplasts and microsomal fractions in polar-lipid synthesis from [1-14C]acetate by cell-free preparations from spinach (Spinacia oleracea) leaves. Biochem J. 1980 Apr 15;188(1):17–24. doi: 10.1042/bj1880017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Slack C. R., Holland R. High rates of [1-14C]acetate incorporation into the lipid of isolated spinach chloroplasts. Biochem J. 1976 Sep 15;158(3):593–601. doi: 10.1042/bj1580593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Slack C. R. Long-chain acyl-coenzyme A synthetase activity of spinach chloroplasts is concentrated in the envelope. Biochem J. 1977 Feb 15;162(2):457–459. doi: 10.1042/bj1620457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMIRNOV B. P. [Biosynthesis of higher fatty acids from acetates in isolated chloroplasts of Spinacia oleracea leaves]. Biokhimiia. 1960 May-Jun;25:545–555. [PubMed] [Google Scholar]
- STUMPF P. K., JAMES A. T. The biosynthesis of long-chain fatty acids by lettuce chloroplast preparations. Biochim Biophys Acta. 1963 Feb 19;70:20–32. doi: 10.1016/0006-3002(63)90715-7. [DOI] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G., Balasingham N. Labelling studies in vivo on the metabolism of the acyl and glycerol moieties of the glycerolipids in the developing maize leaf. Biochem J. 1977 Feb 15;162(2):289–296. doi: 10.1042/bj1620289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf P. K., Boardman N. K. Fat metabolism in higher plants. XXXIX. Effect of adenosine triphosphate and triton X-100 on lipid synthesis by isolated spinach chloroplasts. J Biol Chem. 1970 May 25;245(10):2579–2587. [PubMed] [Google Scholar]
- Vijay I. K., Stumpf P. K. Fat metabolism in higher plants. XLVI. Nature of the substrate and the product of oleyl coenzyme A desaturase from Carthamus tinctorius. J Biol Chem. 1971 May 10;246(9):2910–2917. [PubMed] [Google Scholar]



