Abstract
Introduction
The fundus examination is a non-invasive evaluation of the microcirculation of the retina. The aim of the present study is to develop and validate (reliability and validity) the ALTAIR software platform (Automatic image analyser to assess retinal vessel calibre) in order to analyse its utility in different clinical environments.
Methods and analysis
A cross-sectional study in the first phase and a prospective observational study in the second with 4 years of follow-up. The study will be performed in a primary care centre and will include 386 participants. The main measurements will include carotid intima-media thickness, pulse wave velocity by Sphygmocor, cardio-ankle vascular index through the VASERA VS-1500, cardiac evaluation by a digital ECG and renal injury by microalbuminuria and glomerular filtration. The retinal vascular evaluation will be performed using a TOPCON TRCNW200 non-mydriatic retinal camera to obtain digital images of the retina, and the developed software (ALTAIR) will be used to automatically calculate the calibre of the retinal vessels, the vascularised area and the branching pattern. For software validation, the intraobserver and interobserver reliability, the concurrent validity of the vascular structure and function, as well as the association between the estimated retinal parameters and the evolution or onset of new lesions in the target organs or cardiovascular diseases will be examined.
Ethics and dissemination
The study has been approved by the clinical research ethics committee of the healthcare area of Salamanca. All study participants will sign an informed consent to agree to participate in the study in compliance with the Declaration of Helsinki and the WHO standards for observational studies. Validation of this tool will provide greater reliability to the analysis of retinal vessels by decreasing the intervention of the observer and will result in increased validity through the use of additional information, especially in the areas of vascularisation and vessel branching patterns.
Trial registration number
Clinical Trials.gov Identifier: NCT02087605.
Keywords: VASCULAR MEDICINE, PRIMARY CARE
Strengths and limitations of this study.
A new software for retinal vessels evaluation will be developed, the automatic image analyser to assess retinal vessel calibre (ALTAIR) platform.
A complete evaluation of retinal vascularisation, including automatically calculating the calibre of the retinal vessels, the vascularised area and the branching pattern will be performed.
The interobserver and intraobserver reliability in determining the calibre of arterial and venous vessels, the vascularised surface and branching patterns using the ALTAIR software platform will be performed.
The concurrent validity of the ALTAIR software platform, by analysing the relationship between retinal parameters and other parameters of vascular structure and function, will be performed.
The design of the study during the first phase is transversal; as a result, causality relations cannot be derived, that is, only the associations among the analysed variables can be performed.
Introduction
Fundus examination is a non-invasive evaluation of the retinal microcirculation and of the vascular damage caused by multiple cardiovascular risk factors. Different tools have been developed to evaluate the thickness of the retinal arteries and veins,1–7 however, all of them require intervention of an observer to some extent. Moreover, the techniques and tools that are currently applied are manual or semiautomatic in nature, for which the observer has an important influence, thus providing limited information.
In population studies, an association has been found between the calibre of the retinal vessels and arterial hypertension,8 left ventricular hypertrophy,9 metabolic syndrome,10 stroke11 and coronary heart disease,12 especially in women.13 However, other studies disagree and show contradictory results regarding the evolution of the arteriosclerotic lesion and the calibre of the retinal vessels.14–16 In this way, Cuspidi et al17 and Masaidi et al18 failed to detect an association between the calibre of the retinal vessels and target organ injuries (cardiac, vascular and renal) in studies of two hypertensive populations. However, Torres et al19 reported a negative association between carotid intima-media thickness (IMT) and the thickness of the retinal arteries but a positive association with the veins.
Recently, our group developed and validated a semiautomatic tool, the arteriovenous index calculator, to evaluate the vascular calibre of the retinal vessels,20 with reduced influence of the observer. This tool showed high reliability when measuring the calibre of the retinal vessels with an intraclass correlation coefficient (ICC) for intraobserver and interobserver greater than 0.96 for veins, arteries and the arteriovenous ratio (AVR). These measures, especially the venous calibre and the AVR, were also shown to be independent variables associated with estimated cardiovascular risk, according to the Framingham scale and the microvascular kidney lesions evaluated according to the level of microalbuminuria. This positive association between the cardiovascular risk and the venous calibre is in line with several published studies showing an association between the AVR and the risk of coronary heart disease.12 13 21 However, longitudinal studies with a greater number of patients would help to clarify the discrepancies among previously published studies on cardiovascular risk, and vascular structure and function. Moreover, it should not be forgotten that these tools provide less information than retinal imaging on the thickness of the arteries and veins, their branching patterns and the vascularised areas, which may be relevant for evaluating the status of the vascular tree and may be the cause of some of the discrepancies previously reported.
A new and different approach to the study of the vascular systems is the characterisation of the blood vessel patterns in the normal circulation of the human retina.22 With this method, the distribution of the branching of the vascular system in a two-dimensional space can be analysed, and the geometrical complexity of the branching and the density of the retinal vessels can be quantified.23 Indeed, the current scientific literature contains a number of publications on this subject.24–27
In the present study, a novel software platform for image processing of the structural properties of the vessels is proposed using a human fundus red-free camera. The “Automatic image analyser to assess retinal vessel calibre” (ALTAIR) software platform employs analytical methods and artificial intelligence (AI) algorithms to detect the retinal parameters of interest. The sequence of the algorithms consists of a new methodology that can be used to determine the properties of the veins and arteries of the retina; together, this system unifies all of the methods for automation of the measuring processes of retinal vessels.
Therefore, the general aim of the present study is the development and validation (reliability and validity) of the ALTAIR software platform in order to analyse its utility in different clinical settings. The following specific objectives will be studied:
Evaluation of interobserver and intraobserver reliability in determining the calibre of arterial and venous vessels, the vascularised surface and branching patterns using the ALTAIR software platform.
Evaluation of the concurrent validity of the ALTAIR software platform, in different populations and ethnicities, by analysing the relationship between retinal parameters and other parameters of vascular structure and function, including carotid IMT, pulse wave velocity (PWV) and the cardioankle vascular index (CAVI), as well as injuries in other target organs and the cardiovascular risk.
Evaluation of the evolution of target organ injuries and cardiovascular morbidity, and mortality according to the vascularisation parameters of the retina determined using the ALTAIR software platform.
Method and analysis
Study design
The first phase will be a cross-sectional study aimed at validating the developed tool. Subsequently, the second phase will consist of a prospective observational study with annual follow-up evaluations over 4 years. The study will be developed in a primary healthcare setting.
Subjects
Study population
The population under study will consist of participants from 35 to 74 years of age with a cardiovascular risk factor according to the 2013 European Society of Hypertension/European Society of Cardiology Guidelines.28 Participants are excluded due to the following criteria: psychic or cognitive disorders that interfere with the established requisites of the protocol; non-collaborative attitude; educational or comprehensive limitations; and severe comorbidities with a 12-month likelihood of life-threatening complications. A consecutive sampling of all patients sent to the research unit for cardiovascular risk evaluation will be performed, and those complying with the inclusion and exclusion criteria will be asked to participate until the estimated sample size is achieved. The participants will be mostly Caucasian, the majority ethnic group among patients attending in the health centre; however, at least 50 ethnic minority participants, to give more validity to the tool, will be included.
Sample size
The sample size has been set to detect a correlation coefficient among the PWV, the gold standard measure of arterial stiffness, and the retinal parameters of 0.15, with an α risk of 0.05 and a β risk of 0.20 and a 10% estimated loss regarding the difficulty of the technique or dropout on follow-up. As a result, a total of 386 patients will be included in the study. This number of patients will be adequate to detect a difference of 1 m/s on the PWV among the AVR tertiles, considering a SD of 2.22 m/s with an α risk of 0.05 and a β risk of 0.20.
Variables and measurement instruments
General and potentially effect-modifying variables, such as age, gender, occupation, smoking, alcohol consumption, personal history and drug use will be documented.
Laboratory determinations
Venous blood sampling will be performed between 8:00 and 9:00 after the individuals have fasted, abstained from smoking, and abstained from the consumption of alcohol and caffeinated beverages for the previous 12 h. Fasting plasma glucose, creatinine, uric acid, serum total cholesterol, high-density lipoprotein (HDL)-cholesterol and triglyceride concentrations will be measured using standard enzymatic automated methods. Low-density lipoprotein (LDL) cholesterol will be estimated by the Friedewald equation when the direct parameter is not available. Glycated haemoglobin will be measured with an immune-turbidimetric assay. High sensitive C reactive protein levels and fibrinogen concentrations will be determined by immunoturbidimetric assay. Blood samples will be collected in the health centre, and will be analysed at the University Hospital of Salamanca in external quality assurance programmes of the Spanish Society of Clinical Chemistry and Molecular Pathology.
Anthropometric measurements
Body weight will be determined on two occasions using a homologated electronic scale (Seca 770; Medical Scale and Measurement Systems, Birmingham, UK) following due calibration (precision±0.1 kg), with the patient wearing light clothing and shoeless. These readings will be rounded to 100 g. Height will be measured with a portable system (Seca 222; Medical Scale and Measurement Systems, Birmingham, UK), recording the average of two readings, and with the patient shoeless in the standing position. The values will be rounded to the closest centimetre. Body mass index (BMI) will be calculated as weight (kg) divided by height squared (m2). A value of >30 kg/m² will be taken to define obesity. Waist circumference will be measured using a flexible graduated measuring tape with the patient in the standing position without clothing. The upper border of the iliac crests will be located, and the tape will be wrapped around above this point, parallel to the floor, ensuring that it will be adjusted without compressing the skin. Adiposity indices, waist-height and waist-hip, will also be calculated.
Office or clinical blood pressure
Office blood pressure measurement will involve three measurements of systolic blood pressure (SBP) and diastolic blood pressure (DBP), using the average of the last two, with a validated OMRON model M10-IT sphygmomanometer (Omron Health Care, Kyoto, Japan), by following the recommendations of the European Society of Hypertension.29 Pulse pressure will be estimated with the mean values of the second and third measurements.
PWV and central and peripheral augmentation index
These parameters will be estimated using the SphygmoCor System (AtCor Medical Pty Ltd, Head Office, West Ryde, Australia). With the patient sitting and resting his/her arm on a rigid surface, pulse wave analysis will be performed with a sensor in the radial artery, using mathematical transformation to estimate the aortic pulse wave. Central augmentation index (CAIx) will be estimated from aortic wave morphology using the following formula: increase in central pressure×100/pulse pressure, and it will be adjusted for heart rate at 75 bpm. Peripheral augmentation index (PAIx) is a measurement taken directly from the late systolic shoulder of the peripheral arterial waveform, and is defined as the ratio of the difference between the second peak and diastolic pressure to the difference between the first peak and diastolic pressure,30 it is age-dependent, and could be a useful index of vascular aging.31 PAIx will be calculated as follows: (second peak SBP2−DBP/first peak SBP−DBP)×100 (%), it will be corrected for heart rate at 75 bpm and it will be reported as PAIx75. Carotid and femoral artery pulse waves will be analysed, with the patient in a supine position, using the SphygmoCor System (Vx pulse wave velocity), estimating the delay as compared to the ECG wave and calculating PWV. Distance measurements will be taken with a measuring tape from the sternal notch to the carotid and femoral arteries at the sensor location and will be multiplied by 0.8. Subclinical organ damage of PWV will be defined as a carotid–femoral PWV >10 m/s.28 32
Assessment of vascular structure by carotid IMT
Carotid ultrasound to assess C-IMT will be performed by two investigators trained for this purpose before starting the study. A Sonosite Micromax ultrasound device paired with a 5–10 MHz multifrequency high-resolution linear transducer with Sonocal software will be used for performing automatic measurements of carotid IMT in order to optimise reproducibility. Measurements will be made of the common carotid after the examination of a 10 mm longitudinal section at a distance of 1 cm from the bifurcation, performing measurements in the proximal and in the distal wall in the lateral, anterior and posterior projections, following an axis perpendicular to the artery to discriminate two lines, one for the intima-blood interface and the other for the media-adventitious interface. A total of 6 measurements will be obtained of the right carotid and another 6 of the left carotid, using average values (average carotid IMT) and maximum values (maximum carotid IMT) automatically calculated by the software.33 The measurements will be obtained with the participant lying down, with the head extended and slightly turned opposite to the examined carotid artery. The reliability was evaluated before the study began, using the intraclass correlation coefficient, which showed values of 0.974 (95% CI 0.935 to 0.990) for intraobserver agreement on repeated measurements in 20 participants, and 0.897 (95% CI 0.740 to 0.959) for interobserver agreement. According to the Bland-Altman analysis, the mean difference for intraobserver agreement (95% limits of agreement) was 0.022 (95% CI −0.053 to 0.098) and intraobserver agreement was 0.012 (95% CI −0.034 to 0.059). The average IMT will be considered abnormal if it measures >0.90 mm, or if there are atherosclerotic plaques with a diameter of 1.5 mm or a focal increase of 0.5 mm or 50% of the adjacent IMT.28
CAVI and ankle-brachial index
CAVI and ankle-brachial index (ABI) will be measured using Vasera device VS-1500 (Fukuda Denshi). The PWV will be calculated, as well as CAVI, which gives a more accurate calculation of the atherosclerosis degree. CAVI integrates cardiovascular elasticity derived from the aorta to the ankle pulse velocity through an oscillometric method; it is used as a good measure of vascular stiffness and does not depend on blood pressure.34 CAVI values will be automatically calculated by substituting the stiffness parameter ß in the following equation to detect the vascular elasticity and the cardioankle PWV: stiffness parameter β=2ρ×1/(Ps–Pd)×ln (Ps/Pd)×PWV2, where ρ is the blood density, Ps and Pd are SBP and DBP in mm Hg, and the PWV is measured between the aortic valve and ankle. The average coefficient of the variation of the CAVI is less than 5%, which is small enough for clinical use and confirms that CAVI has favourable reproducibility.35 CAVI and ABI will be measured at rest. For the study, the lowest ABI and the highest CAVI and PWV obtained will be considered.
Renal assessment
Kidney damage will be assessed by measuring estimated-glomerular filtration rate using the CKD-EPI (chronic kidney disease epidemiology collaboration)36 equation and proteinuria, as assessed by the albumin/creatinine ratio following the criteria of the 2013 European Society of Hypertension/European Society of Cardiology Guidelines.28 Subclinical organ damage will be defined as a glomerular filtration rate below 30–60 mL/min/1.73 m2 or microalbuminuria (30–300 mg/24 h), or albumin–creatinine ratio (30–300 mg/g; 3.4–34 mg/mmol; preferably on morning spot urine). Renal disease will be defined as a glomerular filtration rate <30 mL/min/1.73 m2 (body surface area), proteinuria (>300 mg/24 h), or albumin/creatinine ratio >300 mg/24 h.28
Cardiac assessment
Electrocardiographic examination will be performed using a General Electric MAC 3.500 ECG System (Niskayuna, New York, USA), which automatically measures wave voltage and duration, and estimates the criteria of the Cornell voltage-duration product (Cornell VDP).37 Electrocardiographic left ventricular hypertrophy will be defined as a Sokolow-Lyon index >3.5 mV; RaVL >1.1 mV, Cornell voltage duration product >244 mV×ms or RaVL >1.1 mV.28
Cardiovascular risk assessment
Cardiovascular risk will be estimated using the score of the 2013 European Society of Hypertension/European Society of Cardiology Guidelines28 and the risk equation (D’Agostino scale) based on the Framingham study.38 Risk factors used include age, sex, total cholesterol, high-density lipoprotein cholesterol (HDL-C) and SBP as quantitative variables, and drug treatment for hypertension, smoking and history of diabetes mellitus as dichotomous variables.
Retinal vascular evaluation
Using a non-mydriatic retinography, TOPCON TRC NW 200, (Topcon Europe BC, Capelle a/d Ijssel, The Netherlands) in the sitting position, a trained nurse will get nasal and temporal images centred in the papilla. Then, using the specific software developed (ALTAIR), the retinal vessels’ thickness, the AVR, the area vascularised and the pattern of branching will be automatically calculated.
Development of the ALTAIR platform: Automatic image analyser to assess retinal vessel calibre.
The platform, called ALTAIR “Automatic image analyser to assess retinal vessel calibre”, makes use of a methodology divided in different stages, which are described below, to determine the characteristics of interest of the veins and arteries of the retina. This methodology uses AI techniques and analytical algorithms to discover retinal parameters of interest.
The methodology is separated into two phases: (1) Digitisation of the retina, in which the different measures of the eye image are recognised. Here a data structure is created, which makes it possible to represent and process the retina. This phase is subdivided into the following steps as discussed below: load image and eye detection, processing, detection and segmentation. (2) Measurements with which we work with retinas, which have been previously identified. This phase includes extraction of knowledge and manual correction, or expert knowledge, if necessary.
Digitisation of the retina: To perform this phase, the following steps are necessary:
Load image and eye detection: The platform will automatically try to determine which eye (left or right) is the image, based on the detection of the macula. In this step, if the automatic detection has been wrong, the supervisor can modify this value by simply clicking on the correct eye.
Processing: In this step, the noise is reduced, contrast is improved, blurriness corrected and edges sharpened. Some of these actions can be carried out at the hardware level, which is to say, with the features included with the camera. During the testing, retinography will be performed using a Topcon TRC NW 200 non-mydriatic retinal camera, obtaining nasal and temporal images centred on the disk.
Detection limits: In this step, the platform is capable of locating the disk and identifying the centre and edges of the retina (figure 1). The identification of the papilla is vital since it helps as the starting point for the detection and identification of the different blood vessels. The platform builds a data structure that identifies each part of the retina based on the matrices of colours representing the images obtained. In this step, image processing techniques24 26 will be used to detect intensity based on the boundaries of the structures.
- Segmentation: In order to detect the limits, it becomes necessary to carry out a process of image segmentation. Segmentation is the process that divides an image into regions or objects of which the pixels have similar attributes. Each segmented region typically has a physical significance within the image. It is one of the most important processes in an automated vision system because it makes it possible to extract the objects from the image for subsequent description and recognition.39–41 This step can be considered the heart of the methodology proposed and used in the platform, and performs the following actions:
- Identification of vessels. Blood vessels are identified in the image by thresholding techniques. Their purpose is to remove pixels where the structuring element does not enter, in this case the blood vessels. The platform offers here a number of useful options for experts: threshold vessels, in order to modify the threshold level automatically taken, to new vessel detection. Recalculate vessels, recalculate the vessels taking as the threshold established with the previous parameter. Pencil/eraser thickness: sets thickness to draw or erase lines/vessels or to switch vessels. Connect: selecting this option allows the user to interact with the overall image of the retina, connecting those vessels in which the structure has been divided as they were an undetected section.
- Structure of vessel. At the end of this stage the entire arteriovenous tree is stored in a structured way, making it possible to know not only if a vessel passes through a point or not, but through which point each vessel passes, which one is its parent, etc.
Figure 1.
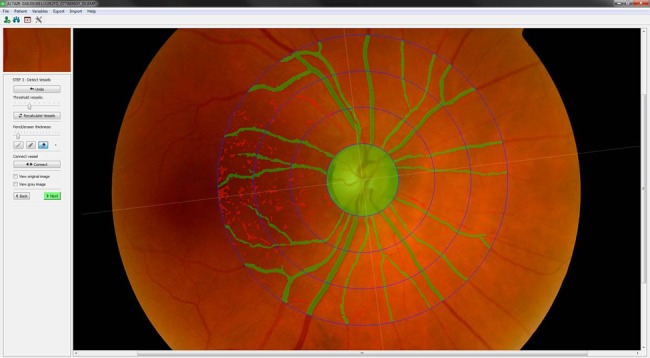
Detection and identification of vessels steps: locating the disk and identifying the centre and edges of the retina.
Figure 2.
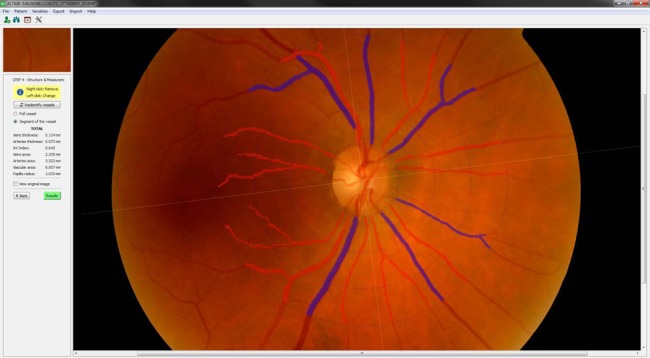
Detection and identification of vessels steps: cataloguing of veins and arteries.
Measurements
In this second phase, the results obtained are presented. The platform can display the following measures: thickness of the veins in µm, artery thickness in µm, AVR (ratio between the thickness of the arteries and veins), veins area in square µm, artery area in square µm, area of all vessels in square µm and radio papilla in millimeters.
The platform also generates internally combined parameters through quadrants and circles. These values are visible when it comes to exporting the CSV files either individually or together with other existing processed database image values taken earlier.
Results of its validity analysis must be consistent with the findings from researches focused on cardiovascular risk estimation as well as evaluation of target organ damage. The results obtained during the use of the platform will be connected and used to extract additional information by using reasoning models such as case-based reasoning (CBR).43 44
In conclusion, the platform is robust to changes in the appearance of retinal fundus images typically encountered in clinical environments, and is proposed as a unified platform to connect all the methods needed to automate all processes of measurement on the retinas.
Retinal software validation
To validate the retinal software platform, the following steps will be completed by the evaluators after previous training in imaging appreciation.
- Evaluation of the reliability or precision
- Intraobserver variability: To evaluate the measurement repeatability, the operator must measure the same image of an individual on at least two occasions. To this end, an operator will measure 100 images of a random subsample of 50 patients with a 1-week difference between the two measurements. In this case, the operator and the analysed images will be the same on both days, and the information from the previous measurement will be unknown.
- Interobserver variability: To evaluate the reproducibility of the measurement system, an operator other than the one who completed the assessment in phase 1 will evaluate the same 100 images previously analysed. The information from the results obtained in the previous phase will be unknown to this operator, and both operators will have the same experience in the subject and pertaining to the use of the software. Furthermore, both operators will receive the same preparatory training.
- Evaluating the validity (accuracy)
- To assess the degree of agreement between ALTAIR and the AV Index calculator software (1) previously validated by us, the evaluation of 100 images will be performed using both tools. In this way, we will be able to demonstrate that the new method, apart from providing the same results, is more objective and faster in elaborating the results.
- The measurement validity will be analysed in a total sample of 386 participants and 772 retinographies in regard to the relationship between the results of the carotid IMT as a measurement of vascular structure, the PWV, the gold standard measure of arterial stiffness, the CAVI, kidney function, electrocardiographic parameters and the estimated cardiovascular risk using different scales.
- The association between different estimated parameters of the retina with the evolution or onset of new lesions in the target organs will be analysed, as well as any cardiovascular events that occur during the 4-year follow-up of the second phase of this project.
Statistical analysis
The data corresponding to quantitative variables with a normal distribution will be presented with the mean and SD and with the median and an IQR if the distribution is asymmetric, while the qualitative variables will be presented according to the distribution of the frequencies. Normality will be evaluated with the Kolmogorov-Smirnov test. The Pearson's χ2 test will be used to analyse associations between the qualitative variables. The mean comparison, in the case of two groups, will be performed using the Student's t test for independent samples and, in the case of larger groups, will be performed using the ANOVA test. Post hoc contrasts will be performed with the least significant difference (LSD) method and an α value <0.05. Repeated data will be analysed with the Student's t test for paired data. The relationship between quantitative variables will be analysed according to the Pearson's or Spearman's correlation coefficient, depending on the type of distribution being considered. Finally, a multivariate analysis with multiple lineal regressions will be performed to analyse the association of the retinal parameters generated by ALTAIR with the vascular structure and function. To contrast the hypothesis, an α risk of 0.05 will be set as the limit of statistical significance. Statistical analysis will be performed using SPSS/PC+ software V.20.0.
For validation of the retinal software, we will evaluate the measurements of artery and vein thickness, vascularised surfaces and branching patterns from the three phases of validation, and theICC will be calculated as a comparative method. Using the Bland-Altman method, the limits of agreement between the measurements of the observers will be evaluated. The κ agreement coefficient will be analysed to categorise the variable. This coefficient will allow us to evaluate the degree of agreement between the two methods. The accordance validity will also be analysed via correlation and multiple regression analysis, by evaluating the degree of association with other parameters of vascular structure and function, and target organ injury.
Project schedule
This project will be on a 5-year plan, with the aim of developing and validating the ALTAIR software platform during the first year. Subsequently, a 4-year follow-up study will be performed to evaluate the evolution of target organ injuries, as well as the cardiovascular risk on the analysed retinal vascularisation parameters, that is, the artery and vein thickness, the vascularised surface and the branching patterns. An exhaustive evaluation of the participant will be performed at baseline and during the third and fifth years. A short evaluation will be performed during the second and fourth years.
Quality control
In order to ensure data quality, the professionals in charge of assessment of retinal images and data collection will receive specific training. Regular external monitoring will then be performed to verify adequate application of methods, both in performing the different examinations and collecting the information.
Ethical and legal issues
The study has been approved by the clinical research ethics committee (CEIC) of the healthcare area of Salamanca (‘CEIC of Area de Salud de Salamanca’, 29 January 2014). Participants will be required to sign an informed consent form prior to inclusion in the study, in accordance with the Declaration of Helsinki.45 Participants will be informed of the objectives of the project and of the risks and benefits of the examinations made. None of the examinations pose life-threatening risks for the type of participants to be included in the study. The study includes the obtaining of biological samples; the study particpants therefore will be informed in detail. The confidentiality of the recruited participants will be ensured at all times in accordance with the provisions of current legislation on personal data protection (15/1999 of 13 December, LOPD), and the conditions contemplated by Act 14/2007 on biomedical research.
Discussion
In a previous study performed by our group,20 we identified a positive association between the cardiovascular risk, estimated with the Framingham scale, and the retinal vessel calibre, mainly the venous calibre, and this association was maintained in the multivariate analysis. The arterial calibre, which also demonstrated a positive association, and the AVR, which demonstrated a negative association, seem to play a less relevant role than the venous calibre in regard to the cardiovascular risk.
The positive association between the cardiovascular risk and the venous calibre is in accordance with several published studies showing an association between the AVR and coronary heart disease risk.12 13 21 For example, McGeechan et al21 showed that when including the arterial and venous thickness, the Framingham model improved coronary event prediction, but only in women. This finding was repeated in several studies and was recently reported in a meta-analysis,13 which seems to support the notion that microvascular disease could be more important than coronary heart disease in women than in men.46 However, these results have been variable; Wong et al,47 in the Beaver Dam Eye study, described the lack of an association between the AVR, cardiovascular morbidity and mortality in the examined subgroups. In addition, Wang et al48 identified an association between venous calibre and coronary death in men but not in women.
It is likely that some of these discrepancies are due to the different tools employed for evaluation of the retinal vessels, as all of these methods are manual or semiautomatic and, to a greater or lesser degree, are susceptible to the influence of the observer. The use of only particular information regarding the vascularisation of the retina may also influence the results. However, the ALTAIR software platform resolves these problems. On the one hand, this platform is practically an automated tool with low observer influence. In addition, we expect the interobserver and intraobserver variability to be low and, as a result, the reliability to be significantly elevated. On the other hand, by using additional information on retinal vascularisation, the vascularised surface, the vascularisation patterns and the artery and vein thickness, we expect to improve the validity of the tool and clarify the discrepancies reported in previous studies.
Study limitations
Data will be obtained from patients with a cardiovascular risk factor, who satisfy the inclusion criteria and who were referred by a family physician to the research unit for vascular risk assessment. Thus, this approach uses a consecutive sampling method with inclusion criteria, that is, a non-randomised sampling method. However, the size of the sample may buffer this limitation, and the real clinical conditions may lead us to a more real situation than that using more restrictive inclusion criteria for the study patients. The design of the study during the first phase is transversal; as a result, causality relations cannot be derived, that is, only the associations among the analysed variables can be performed. Therefore, non-statistically detected associations between variables may be possible due to the sample size.
Supplementary Material
Footnotes
Collaborators: Members of the ALTAIR group: LG-O, JIR-R, MAG-M, JAM-F, SR-G, JFdP-S, PC-S, MAM-C and JM-R María C Patino-Alonso, Emiliano Rodríguez-Sánchez, Diana Perez Arechaederra, Sara Mora Simón, Ángela de Cabo Laso, Carmela Rodriguez Martín, Luis F Valero Juan, Leticia Gómez-Sánchez, Cristina Agudo-Conde.
Contributors: LG-O and JMC-R came up with conception of the idea for the study. LG-O, JMC-R, JIR-R, MAG-M, JAMF, SR-G, JFdP-S and PC-S were involved in development of the protocol, organisation and funding. LG-O, JMC-R and MAG-M took part in writing of the manuscript. All the authors have read the draft critically, made contributions and approved the final text.
Funding: The project has been funded by the Institute of Health Carlos III of the Ministry of Economy and Competitiveness (Spain) through the Network for Prevention and Health Promotion in Primary Care (redIAPP, RD12/0005), cofinanced with European Union ERDF, the Autonomous Government of Castilla and León (GRS 907/B/14 and intensification of research program) and Vicente y Garcia Corselas Foundation (Call 2013).
Competing interests: None.
Patient consent: Obtained.
Ethics approval: The study has been approved by the clinical research ethics committee of the healthcare area of Salamanca.
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Contributor Information
Collaborators: JM-R María C Patino-Alonso, Emiliano Rodríguez-Sánchez, Diana Perez Arechaederra, Sara Mora Simón, Ángela de Cabo Laso, Carmela Rodriguez Martín, Luis F Valero Juan, Leticia Gómez-Sánchez, and Cristina Agudo-Conde
References
- 1.Hubbard LD, Brothers RJ, King WN et al. . Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the atherosclerosis risk in communities study. Ophthalmology 1999;106:2269–80. [DOI] [PubMed] [Google Scholar]
- 2.Ikram MK, de Jong FJ, Vingerling JR et al. . Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam Study. Invest Ophthalmol Vis Sci 2004;45:2129–34. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen TT, Wang JJ, Sharrett AR et al. . Relationship of retinal vascular caliber with diabetes and retinopathy: the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2008;31:544–9. [DOI] [PubMed] [Google Scholar]
- 4.Pose-Reino A, Gomez-Ulla F, Hayik B et al. . Computerized measurement of retinal blood vessel calibre: description, validation and use to determine the influence of ageing and hypertension. J Hypertens 2005;23:843–50. [DOI] [PubMed] [Google Scholar]
- 5.Tikellis G, Wang JJ, Tapp R et al. . The relationship of retinal vascular calibre to diabetes and retinopathy: the Australian Diabetes, Obesity and Lifestyle (AusDiab) study. Diabetologia 2007;50:2263–71. [DOI] [PubMed] [Google Scholar]
- 6.Cheung CY, Ikram MK, Sabanayagam C et al. . Retinal microvasculature as a model to study the manifestations of hypertension. Hypertension 2012;60:1094–103. [DOI] [PubMed] [Google Scholar]
- 7.Ortega M, Barreira N, Novo J et al. . Sirius: a web-based system for retinal image analysis. Int J Med Inform 2010;79:722–32. [DOI] [PubMed] [Google Scholar]
- 8.Tanabe Y, Kawasaki R, Wang JJ et al. . Retinal arteriolar narrowing predicts 5-year risk of hypertension in Japanese people: the Funagata study. Microcirculation 2010;17:94–102. [DOI] [PubMed] [Google Scholar]
- 9.Tikellis G, Arnett DK, Skelton TN et al. . Retinal arteriolar narrowing and left ventricular hypertrophy in African Americans. the Atherosclerosis Risk in Communities (ARIC) study. Am J Hypertens 2008;21:352–9. [DOI] [PubMed] [Google Scholar]
- 10.Wong TY, Duncan BB, Golden SH et al. . Associations between the metabolic syndrome and retinal microvascular signs: the atherosclerosis risk in communities study. Invest Ophthalmol Vis Sci 2004;45:2949–54. [DOI] [PubMed] [Google Scholar]
- 11.Yatsuya H, Folsom AR, Wong TY et al. . Retinal microvascular abnormalities and risk of lacunar stroke: atherosclerosis risk in communities study. Stroke 2010;41:1349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong TY, Klein R, Sharrett AR et al. . Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA 2002;287:1153–9. [DOI] [PubMed] [Google Scholar]
- 13.McGeechan K, Liew G, Macaskill P et al. . Meta-analysis: retinal vessel caliber and risk for coronary heart disease. Ann Intern Med 2009;151:404–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabanayagam C, Shankar A, Klein BE et al. . Bidirectional association of retinal vessel diameters and estimated GFR decline: the Beaver Dam CKD Study. Am J Kidney Dis 2011;57:682–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabanayagam C, Tai ES, Shankar A et al. . Retinal arteriolar narrowing increases the likelihood of chronic kidney disease in hypertension. J Hypertens 2009;27:2209–17. [DOI] [PubMed] [Google Scholar]
- 16.van Hecke MV, Dekker JM, Nijpels G et al. . Are retinal microvascular abnormalities associated with large artery endothelial dysfunction and intima-media thickness? The Hoorn Study. Clin Sci (Lond) 2006;110:597–604. [DOI] [PubMed] [Google Scholar]
- 17.Cuspidi C, Meani S, Salerno M et al. . Retinal microvascular changes and target organ damage in untreated essential hypertensives. J Hypertens 2004;22:2095–102. [DOI] [PubMed] [Google Scholar]
- 18.Masaidi M, Cuspidi C, Giudici V et al. . Is retinal arteriolar-venular ratio associated with cardiac and extracardiac organ damage in essential hypertension? J Hypertens 2009;27:1277–83. [DOI] [PubMed] [Google Scholar]
- 19.Torres FS, Fuchs SC, Maestri MK et al. . Association between carotid intima-media thickness and retinal arteriolar and venular diameter in patients with hypertension: a cross-sectional study. Atherosclerosis 2013;229:134–8. [DOI] [PubMed] [Google Scholar]
- 20.García-Ortiz L, Recio-Rodríguez JI, Parra-Sanchez J et al. . A new tool to assess retinal vessel caliber. Reliability and validity of measures and their relationship with cardiovascular risk. J Hypertens 2012;30:770–7. [DOI] [PubMed] [Google Scholar]
- 21.McGeechan K, Liew G, Macaskill P et al. . Risk prediction of coronary heart disease based on retinal vascular caliber (from the Atherosclerosis Risk In Communities [ARIC] Study). Am J Cardiol 2008;102:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masters BR. Fractal analysis of the vascular tree in the human retina. Annu Rev Biomed Eng 2004;6:427–52. [DOI] [PubMed] [Google Scholar]
- 23.Cheung CY, Ong S, Ikram MK et al. . Retinal vascular fractal dimension is associated with cognitive dysfunction. J Stroke Cerebrovasc Dis 2014;23:43–50. [DOI] [PubMed] [Google Scholar]
- 24.Chen B, Tosha C, Gorin MB et al. . Analysis of autofluorescent retinal images and measurement of atrophic lesion growth in Stargardt disease. Exp Eye Res 2010;91:143–52. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez CI, Hornero R, Lopez MI et al. . A novel automatic image processing algorithm for detection of hard exudates based on retinal image analysis. Med Eng Phys 2008;30:350–7. [DOI] [PubMed] [Google Scholar]
- 26.Heneghan C, Flynn J, O'Keefe M et al. . Characterization of changes in blood vessel width and tortuosity in retinopathy of prematurity using image analysis. Med Image Anal 2002;6:407–29. [DOI] [PubMed] [Google Scholar]
- 27.Jagoe R, Blauth CI, Smith PL et al. . Automatic geometrical registration of fluorescein retinal angiograms. Comput Biomed Res 1990;23:403–9. [DOI] [PubMed] [Google Scholar]
- 28.Mancia G, Fagard R, Narkiewicz K et al. . 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013;31:1281–357. [DOI] [PubMed] [Google Scholar]
- 29.O'Brien E, Asmar R, Beilin L et al. . Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self blood pressure measurement. J Hypertens 2005;23:697–701. [DOI] [PubMed] [Google Scholar]
- 30.Munir S, Guilcher A, Kamalesh T et al. . Peripheral augmentation index defines the relationship between central and peripheral pulse pressure. Hypertension 2008;51:112–18. [DOI] [PubMed] [Google Scholar]
- 31.Kohara K, Tabara Y, Oshiumi A et al. . Radial augmentation index: a useful and easily obtainable parameter for vascular aging. Am J Hypertens 2005;18:11S–4S. [DOI] [PubMed] [Google Scholar]
- 32.Van Bortel LM, Laurent S, Boutouyrie P et al. . Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 2012;30:445–8. [DOI] [PubMed] [Google Scholar]
- 33.Gomez-Marcos MA, Recio-Rodriguez JI, Patino-Alonso MC et al. . Protocol for measuring carotid intima-media thickness that best correlates with cardiovascular risk and target organ damage. Am J Hypertens 2012;25:955–61. [DOI] [PubMed] [Google Scholar]
- 34.Shirai K, Hiruta N, Song M et al. . Cardio-ankle vascular index (CAVI) as a novel indicator of arterial stiffness: theory, evidence and perspectives. J Atheroscler Thromb 2011;18:924–38. [DOI] [PubMed] [Google Scholar]
- 35.Shirai K, Utino J, Otsuka K et al. . A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J Atheroscler Thromb 2006;13:101–7. [DOI] [PubMed] [Google Scholar]
- 36.Levey AS, Stevens LA, Schmid CH et al. . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okin PM, Roman MJ, Devereux RB et al. . Electrocardiographic identification of increased left ventricular mass by simple voltage-duration products. J Am Coll Cardiol 1995;25:417–23. [DOI] [PubMed] [Google Scholar]
- 38.D'Agostino RB Sr, Vasan RS, Pencina MJ et al. . General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743–53. [DOI] [PubMed] [Google Scholar]
- 39.Goldbaum MH, Katz NP, Nelson MR et al. . The discrimination of similarly colored objects in computer images of the ocular fundus. Invest Ophthalmol Vis Sci 1990;31:617–23. [PubMed] [Google Scholar]
- 40.Kalviainen H, Hirvonen P, Xu L et al. . Probabilistic and non-probabilistic Hough transforms. Image Vision Comput 1995;13:239–52. [Google Scholar]
- 41.Lee S, Wang Y, Lee E. A computer algorithm for automated detection and quantification of microaneurysms and haemorrhages in color retinal images. SPIE Conference on Image Perception and Performance 1999;3663:61–71. [Google Scholar]
- 42.Jensen FV, Nielsen TD. Bayesian networks and decision graphs. Springer, 2009. [Google Scholar]
- 43.De Paz JF, Rodríguez S, Bajo J et al. . CBR system for diagnosis of patient. IEEE Computer Society Press, 2009:807–12. [Google Scholar]
- 44.Rodríguez S, De Paz JF, Bajo J et al. . Applying CBR sytems to micro-array data classification. Proceedings of IWPACBB 2008; Springer Velag, Advances in Soft Computing Series, 2008. [Google Scholar]
- 45.WMA. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–4. [DOI] [PubMed] [Google Scholar]
- 46.Wang JJ, Liew G, Wong TY et al. . Retinal vascular calibre and the risk of coronary heart disease-related death. Heart 2006;92:1583–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong TY, Knudtson MD, Klein R et al. . A prospective cohort study of retinal arteriolar narrowing and mortality. Am J Epidemiol 2004;159:819–25. [DOI] [PubMed] [Google Scholar]
- 48.Wang JJ, Liew G, Klein R et al. . Retinal vessel diameter and cardiovascular mortality: pooled data analysis from two older populations. Eur Heart J 2007;28:1984–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


