Abstract
We present an extremely rare case of empyema necessitatis secondary to Aspergillus fumigatus infection. A 58-year-old woman presented to our hospital with a painful skin rash on the right thorax. Three fistulas communicating with the pleural space were found. Since she did not show a clinical improvement despite antituberculous and antibacterial treatment, we looked for other causes. Pleural fungus culture showed A. fumigatus and chest wall biopsy revealed numerous fungal hyphae. Treatment with necrotic tissue debridement and antifungal agents was successful.
Background
Empyema necessitatis is a rare complication of parapneumonic pleural effusion with invasion into the surrounding soft tissue of the chest wall through the parietal pleura, forming subcutaneous abscess, which can extend to the trachea, oesophagus, diaphragm, breast and pericardium. The incidence of empyema necessitatis is low, with the majority of cases reported in the pre-antibiotic era, and the increasing frequency of antibiotic use and routine empyema drainage has further lowered its incidence. Tuberculosis is the most common pathogen, with an incidence of 50–73%.1 The second most common aetiology was Streptococcus pneumoniae in the pre-antibiotic era; however, a review from 1966 revealed that the second most common pathogen is now actinomycosis, with an incidence of 24%.2 The third most common pathogen is reportedly Staphylococcus aureus, with an incidence of approximately 10%.3 Other known pathogens are Mycobacterium avium-intercellulare, Streptococcus milleri, Fusobacterium nucleatum and Pseudomonas cepacia.
However, empyema necessitatis due to fungal infection is extremely rare. Only one case of spontaneous empyema necessitatis caused by Aspergillus fumigatus was presented in 2011 in Taiwan,4 and no other case has been reported since the 1960s.
We report a case of a middle-aged woman with empyema necessitatis caused by A. fumigatus.
Case presentation
A 58-year-old woman was referred to our institution with a 2-month history of right-sided chest-wall pain and skin rash. She had been treated with empirical antibiotics for cellulitis for a week, but her pain was aggravated and the skin rash had become a fistula with a purulent discharge. When the patient was admitted to our institution, she complained of general weakness, night fever, cough, sputum and exertional dyspnoea.
The patient's medical history included a diagnosis of pulmonary tuberculosis followed by a full recovery 30 years earlier. She had been diagnosed with right breast cancer 22 years prior, with staging pT3N1M0, for which she had undergone modified radical mastectomy with adjuvant radiotherapy and chemotherapy. The pulmonary tuberculosis had been reactivated 13 years prior. The patient had taken antituberculosis medication for 6 months, after which the results from a serial sputum acid-fast bacilli (AFB) smear were negative.
Investigations
The patient's vital signs were as follows: blood pressure, 119/51 mm Hg; heart rate, 59/min; respiratory rate, 14/min; and temperature, 37.9°C. At the physical examination we found three skin fistulas on the 2nd–4th intercostal space with a surrounding skin rash on right chest wall (figure 1). Further, we detected decreased lung sound, crackling sounds in the entire right lung, resonant percussion and decreased tactile fremitus.
Figure 1.
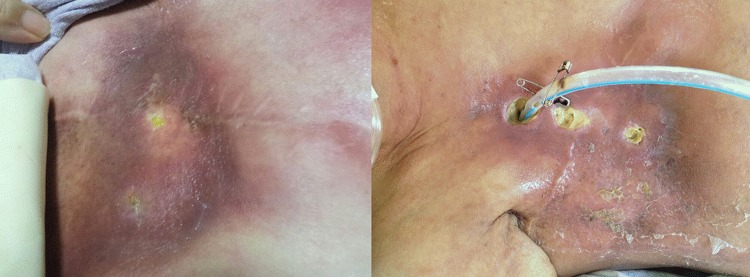
Skin rashes and ulcerative lesions on the right anterior chest wall. The ulcerative lesion had progressed, forming a fistula to the pleural cavity. A chest tube was inserted through the fistula.
The initial complete blood count results were as follows: white cell count, 15 220/µL; haemoglobin, 12.2 g/dL; platelets, 3 460 000/µL; and C reactive protein (CRP), 19.64 mg/dL. The arterial blood gas analysis with an oxygen supply of 1 L/min in a nasal prong revealed the following findings: pH, 7.453; PaCO2, 38.1 mm Hg; PaO2, 99.3 mm Hg; and HCO3−, 26.8 mEq/L. The chest radiography showed pulmonary consolidation at the right middle and lower lung field, and chest CT confirmed empyema necessitatis (figure 2). We found sinus tachycardia in the ECG. The ejection fraction was 53% and the estimated pulmonary artery systolic pressure was 39 mm Hg in echocardiography.
Figure 2.
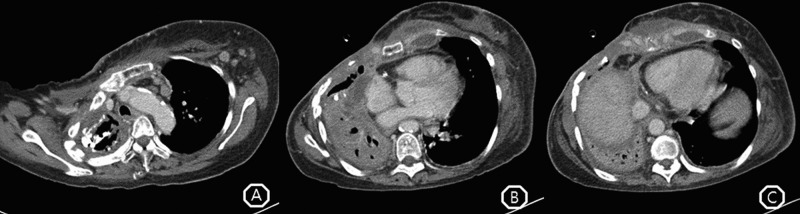
(A) Multiple lymph node enlargements were found at the left lower neck, mediastinum and axilla, which more likely suggested reactive change combined with infection or tuberculous lymphadenitis. (B) Anterior chest wall of right lung was opened by forming a fistulous tract to the skin, and lung volume was decreased. Patchy consolidation with intraparenchymal calcification in the right lung, and several complicated fluid collections with peripheral wall enhancement around the sternum, clavicle, ribs and lung parenchyma suggested probable chest wall tuberculous infection with parasternal cold abscess. (C) Pericardial enhancement with a small amount of pericardial fluid and pleural thickening in the right lung were found.
Differential diagnosis
We believed that Mycobacterium tuberculosis was the most likely pathogen, because tuberculosis is the most common aetiology of empyema necessitatis and the patient's medical history included recurrent pulmonary tuberculosis. A thoracic surgeon inserted a chest tube for natural drainage with a water-sealed bottle. We performed culture studies from the peripheral blood and pleural fluid, and we treated the patient with antituberculosis medications including isoniazid, rifampin, ethambutol and pyrazinamide combined with intravenous piperacillin/tazobactam for over a week. Despite undergoing treatment for bacterial infection and tuberculosis, the patient experienced aggravation of dyspnoea, cough, fever and tachycardia during the treatment period. The results of the culture studies were negative for bacteria and tuberculosis.
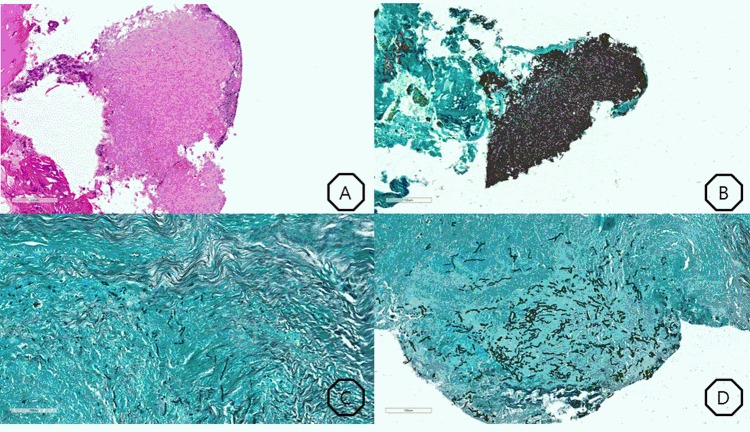
Therefore, we focused on unusual pathogens, and we considered fungal infection as one of the possibilities. We performed a serum aspergillus antigen (galactomannan) test; the galactomannan titre was 5.03, which indicated a positive result. Fungal culture studies of the pleural fluid revealed A. fumigatus. We started intravenous amphotericin-B and performed three biopsies of the soft tissue of the chest wall. Periodic acid-Schiff stain and Grocott’s methenamine silver staining of the biopsy specimens revealed numerous fungal hyphae, and the tissue finding was morphologically consistent with aspergillosis (figure 3).
Figure 3.
(A) Numerous hyphae, periodic acid-Schiff (PAS)×200. (B) Numerous hyphae, Grocott's methenamine silver (GMS)×200. (C) Intercostal muscle invasion, GMS×200. (D) Soft-tissue invasion with necrosis, GMS×200.
Treatment
We replaced the drug with liposomal amphotericin because of acute kidney injury in the third day since initiation of amphotericin-B, and we continued this treatment for 5 days. We started voriconazole for the patient following tissue confirmation. Treatment with serial antifungal agents resulted in improvements in subjective dyspnoea and fever, and the serum CRP decreased from 25.8 to 1.98 mg/dL. However, the follow-up CT still revealed persistent active inflammation in the right haemothorax and chest wall. Therefore, we planned an Eloesser procedure 5 weeks later to create a relatively permanent drainage opening in the chest wall. The second, third and fourth ribs were partially removed to the sternocostal junction. In the surgical field, the right lung was covered with a thick peel and the pleural cavity was filled with necrotic tissue. We carefully debrided all of the necrotic tissue without injuring the lung, and we generated a musculocutaneous flap at the superior and right lateral margins.
Outcome and follow-up
A follow-up chest CT showed removal of complicated fluid collection, total atelectasis of the right lung parenchyma after the Eloesser procedure, and the absence of new pulmonary lesions by aspergillosis (figure 4). The patient took voriconazole in the outpatient department for 12 months without recurrence of empyema necessitatis and without any significant adverse events.
Figure 4.
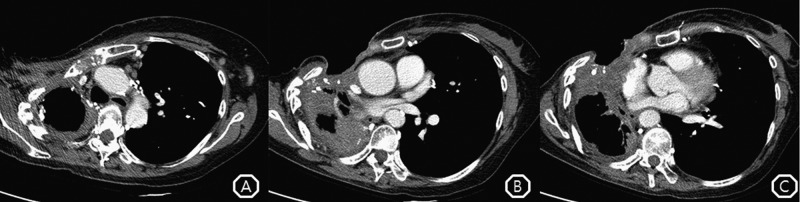
(A) Follow-up chest CT showed no change in the multiple lymph node enlargements. (B) The patient still had open state of the anterior chest wall with right lung atelectasis, but the extent of the low-attenuated lesion in the anterior chest wall was decreased. (C) Aeration and parenchymal consolidation in the right lower lobe were improved.
Discussion
Pleural aspergillosis is a relatively rare entity and is not associated with pulmonary aspergillus infections such as allergic bronchopulmonary aspergillosis, bronchopneumonic aspergillosis, or aspergilloma. This condition is defined as pleural or pleural space involvement by direct invasion of the Aspergillus spp, excluding reactive pleural effusion, by parenchymal inflammation. A review of pleural aspergillosis cases revealed several predisposing factors for pleural aspergillosis: previous pulmonary tuberculosis, bronchopleural fistula, open or closed pleural instrumentation and lung resection.5 Our patient had a history of pulmonary tuberculosis without bronchopleural fistula or pulmonary intervention.
Conventionally, invasive aspergillus infection was thought to only occur under conditions in which the host was immunocompromised.6 However, a report of six cases in 1981 suggested that immunocompetent patients with fibrotic pleura could be candidates for pleural aspergillosis.7 In general, patients with pleural aspergillosis have a prolonged history of using antimycobacterial agents and antibiotics without clinical improvement and negative results for AFB and bacterial culture studies. In the majority of previous cases, the diagnosis of fungal infection in the pleura was established by smear examination and culture study of the complicated pleural effusion to reveal acute, septate, branching hyphae or numerous growths of Aspergillus. Compared to invasive pulmonary aspergillosis or aspergilloma, histological confirmation was uncommon in these cases. Radiographic appearances are found infrequently at the early stage of this disease. Serum aspergillus antigens could also be used for an earlier diagnosis but the data are inconclusive; furthermore, there were studies reporting false-positive galactomannan tests in cases being treated with piperacillin/tazobactam, as in our case.8 Additionally, in our case, a positive culture result was not sufficient evidence for invasive infection because the pleural fluid was not sterile due to the fistula. Therefore, we performed biopsy to confirm tissue invasion of Aspergillus after getting positive results from serum galactomannan and pleural culture.
Despite the different aetiologies of empyema necessitatis, the optimal treatment is surgical debridement with antibiotic therapy. The reported durations of antibiotics prescribed for the patient vary according to the pathogen: S. pneumonia, 10 days;2 Fusobacterium, 4 weeks;9 methicillin-resistant Staphylococcus aureus, 5 weeks;10 M. tuberculosis, 6–9 months;11 12 actinomycosis, 6–18 months;13 14 and M. abscessus, 18 months.15 In the previously reported case of empyema necessitatis due to A. fumigatus, the patient received itraconazole for more than 1 month.4 However, we maintained voriconazole for a longer duration because the patient showed severe presentation initially and her chest wall lesion remained little improved over several weeks. We emphasise that the aetiology as well as the extent of the lesion should be considered when planning the treatment duration for empyema necessitatis.
Surgical management should be considered for patients in whom fungal empyema necessitatis is poorly controlled despite appropriate medical treatment. Moreover, early surgical intervention may be beneficial for all pleural aspergillosis patients because reducing the fungal burden may shorten the duration of antifungal medication. Many surgical techniques are available, but a consensus has not been reached regarding which surgical method should be selected for fungal empyema necessitatis patients. The Eloesser procedure appears to be the most physiological method when drainage is necessary because it allows negative pressure in the chest wall during empyema drainage.
However, surgical interventions may be associated with treatment-related morbidity and mortality. In elderly patients with damaged lungs or chronic lung disease, tube drainage is the best surgical management. In bacterial empyema necessitatis, 7 days of antibiotics and tube drainage should be adequate for clinical improvement of the patient.16 Further, in cases of tuberculous pleurisy and tuberculous empyema, patients with adequate tube drainage, symptomatic improvement and afebrile status were detected within 14 days of therapy.17 18
In summary, our case suggests that A. fumigatus has the potential to cause empyema and empyema necessitatis as well as parenchymal invasive aspergillosis. Physicians should not rule out fungal infection prior to making an effort to evaluate Aspergillus infection in patients with empyema necessitatis.
Learning points.
Immunocompetent as well as immunocompromised patients may have empyema necessitatis due to aspergillus.
A positive fungal culture study result is not confirmative evidence because of the risk of contamination. Surgical biopsy is required to confirm soft tissue invasion of Aspergillus.
The clinical suspicion is paramount in such cases, especially when the predisposing conditions are pointing in the direction of an uncommon aetiology.
Aspergillus should be considered as a pathogen for patients with refractory empyema necessitatis despite treatment with antituberculous or antibacterial medication.
Chest tube insertion is beneficial for fungal empyema necessitatis patients who cannot tolerate surgical intervention.
Footnotes
Contributors: HWL wrote the case report and performed the literature search. C-HL reviewed the draft of the manuscript and made the decision to submit the manuscript for publication. YWK and JC were involved in the conception, design and literature search.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Sindel EA. Empyema necessitatis. Bull Seaview Hosp 1940;6:1–49. [Google Scholar]
- 2.Freeman AF, Ben-Ami T, Shulman ST. Streptococcus pneumoniae empyema necessitatis. Pediatr Infect Dis J 2004;23:177–9. [DOI] [PubMed] [Google Scholar]
- 3.Llamas-Velasco M, Dominguez I, Ovejero E et al. Empyema necessitatis revisited. Eur J Dermatol 2010;20:115–19. [DOI] [PubMed] [Google Scholar]
- 4.Chen CH, Ho C, Liu HC et al. Spontaneous empyema necessitatis caused by Aspergillus fumigatus in an immunocompetent patient. JRSM Short Rep 2011;2:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meredith HC, Cogan BM, McLAULIN B. Pleural aspergillosis. AJR Am J Roentgenol 1978;130:164–6. [DOI] [PubMed] [Google Scholar]
- 6.Samarakoon P, Soubani A. Invasive pulmonary aspergillosis in patients with COPD: a report of five cases and systematic review of the literature. Chron Respir Dis 2008;5:19–27. [DOI] [PubMed] [Google Scholar]
- 7.Hillerdal G. Pulmonary aspergillus infection invading the pleura. Thorax 1981;36:745–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanriover MD, Metan G, Altun B et al. False positivity for Aspergillus antigenemia related to the administration of piperacillin/tazobactam. Eur J Intern Med 2005;16:489–91. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi Y, Kiyota M, Ebi N. Empyema necessitatis caused by Fusobacterium necrophorum. Chest 2013;144(4_MeetingAbstracts):223A. [Google Scholar]
- 10.Mizell KN, Patterson KV, Carter JE. Empyema necessitatis due to methicillin-resistant Staphylococcus aureus: case report and review of the literature. J Clin Microbiol 2008;46:3534–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee WS, Jean SS, Bai KJ et al. Empyema necessitatis due to Mycobacterium tuberculosis. J Microbiol Immunol Infect 2014:pii: S1684-1182(13)00237-5. [Google Scholar]
- 12.Jover F, Andreu L, Cuadrado JM et al. Tuberculous empyema necessitatis in a man infected with the human immunodeficiency virus. South Med J 2002;95:751–2. [PubMed] [Google Scholar]
- 13.Reyes CV, Thompson KS, Jensen J. Fine needle aspiration biopsy of mastitis secondary to empyema necessitatis. Acta cytologica 2011;43:873–6. [DOI] [PubMed] [Google Scholar]
- 14.Hooker TP, Hammond M, Corral K. Empyema necessitatis: review of the manifestations of thoracic actinomycosis. Cleve Clin J Med 1992;59: 542–8. [DOI] [PubMed] [Google Scholar]
- 15.Jo KW, Kim JW, Hong Y et al. A case of empyema necessitatis caused by Mycobacterium abscessus. Resp Med Case Rep 2012;6:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akgul AG, Orki A, Orki T et al. Approach to empyema necessitatis. World J Surg 2011;35:981–4. [DOI] [PubMed] [Google Scholar]
- 17.Ferrer J. Pleural tuberculosis. Eur Respir J 1997;10:942–7. [PubMed] [Google Scholar]
- 18.Madeo J, Patel R, Gebre W et al. Tuberculous empyema presenting as a persistent chest wall mass: case report. Germs 2013;3:21–5. [DOI] [PMC free article] [PubMed] [Google Scholar]