Abstract
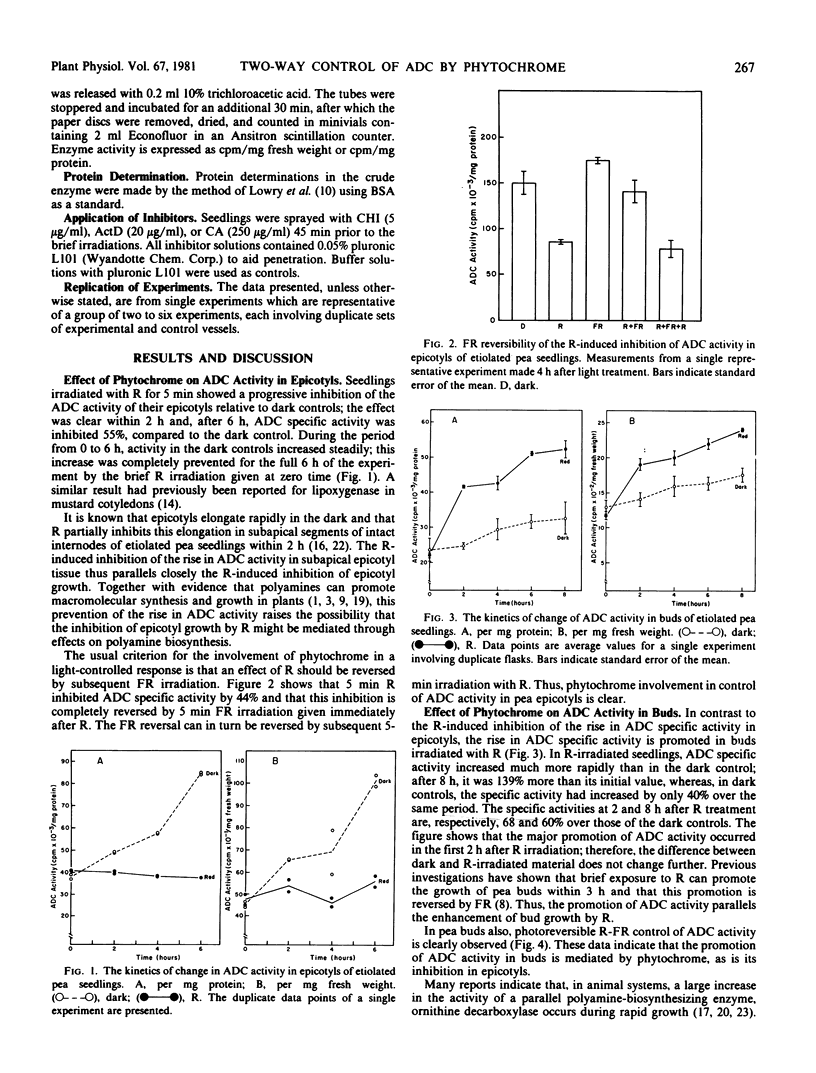
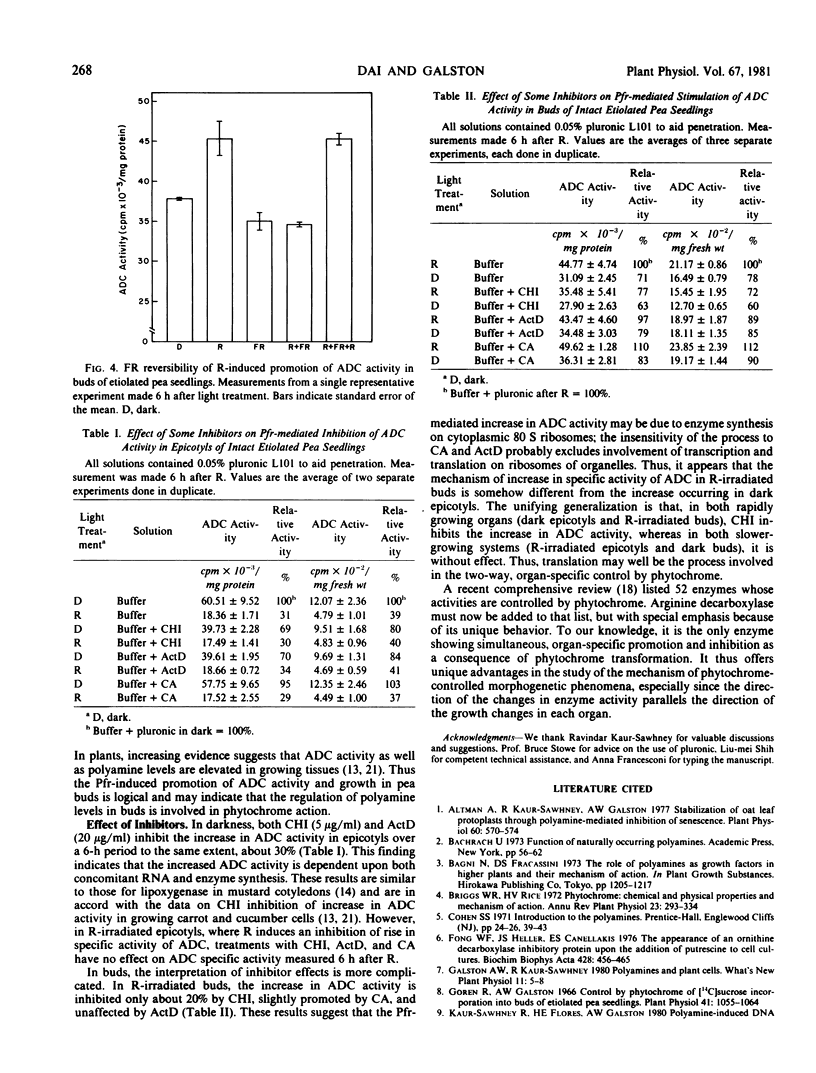
The specific activity of arginine decarboxylase (ADC; l-arginine carboxylase; EC 4.1.1.19) rises steadily over an 8 hour experimental period in the growing buds and subapical epicotyl internodes of 6-day-old totally etiolated pea seedlings. Treatment with red light (R) completely annuls this rise in epicotyls but increases it in buds, thus paralleling the opposite effects of R on the growth of these two organs. Far red light (FR) reverses both effects of R on ADC and is, in turn, reversed by R, indicating phytochrome control. Effects in both organs are clearly seen within 2 hours. By 6 hours after R, the post-irradiation rise in ADC specific activity in buds is 3 times greater than that of the dark controls. Over the same period, ADC specific activity in epicotyls is inhibited by 56% relative to dark controls, reflecting zero net change after R and a continued rise in the dark. Cycloheximide inhibits the rise in ADC activity in both rapidly growing organs (epicotyls in dark and buds after R) but is without effect in both slower growing organs. Actinomycin D inhibits only in dark grown epicotyls, whereas chloramphenicol produces no inhibition in any system tested.
ADC is the first enzyme to show a two-way, organ-specific response to phytochrome conversion from Pr to Pfr. This finding is discussed in relation to the growing evidence that polyamines formed from arginine may be important growth regulators in plants, as well as in microbial and animal cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman A., Kaur-Sawhney R., Galston A. W. Stabilization of Oat Leaf Protoplasts through Polyamine-mediated Inhibition of Senescence. Plant Physiol. 1977 Oct;60(4):570–574. doi: 10.1104/pp.60.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong W. F., Heller J. S., Canellakis E. S. The appearance of an ornithine decarboxylase inhibitory protein upon the addition of putrescine to cell cultures. Biochim Biophys Acta. 1976 Apr 23;428(2):456–465. doi: 10.1016/0304-4165(76)90054-4. [DOI] [PubMed] [Google Scholar]
- Goren R., Galston A. W. Control by phytochrome of C-sucrose incorporation into buds of etiolated pea seedlings. Plant Physiol. 1966 Jun;41(6):1055–1064. doi: 10.1104/pp.41.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marcus A. Photocontrol of Formation of Red Kidney Bean Leaf Triphosphopyridine Nucleotide Linked Triosephosphate Dehydrogenase. Plant Physiol. 1960 Jan;35(1):126–128. doi: 10.1104/pp.35.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague M. J., Armstrong T. A., Jaworski E. G. Polyamine Metabolism in Embryogenic Cells of Daucus carota: II. Changes in Arginine Decarboxylase Activity. Plant Physiol. 1979 Feb;63(2):341–345. doi: 10.1104/pp.63.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelze-Karow H., Schopfer P., Mohr H. Phytochrome-mediated repression of enzyme synthesis (lipoxygenase): a threshold phenomenon. Proc Natl Acad Sci U S A. 1970 Jan;65(1):51–57. doi: 10.1073/pnas.65.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki Y., Ichihara A. Induction of ornithine decarboxylase in cultured mouse L cells. I. Effects of cellular growth, hormones, and actinomycin D. J Biochem. 1976 Sep;80(3):557–562. doi: 10.1093/oxfordjournals.jbchem.a131311. [DOI] [PubMed] [Google Scholar]



