Abstract
Background
The benefits of screening for non-small cell lung cancer (NSCLC) have been established for high-risk individuals, and recent guidelines advocate continued surveillance after curative therapy. Yet the optimal posttreatment surveillance strategy remains to be determined. We compared patterns of recurrence and modes of detection in surgically treated patients with pathologic early-stage and locally advanced NSCLC.
Methods
Consecutive patients who had undergone resection for stage I-IIIA NSCLC from 2004 to 2009 were identified from a prospectively maintained institutional database. All patients received interval chest computed tomography (CT) scans every 6 to 12 months after treatment.
Results
In total, 1640 patients were identified: 181 of 346 patients with stage IIIA NSCLC (52%) and 257 of 1294 patients with stage I-II NSCLC (20%) developed recurrences. Surveillance CT scan detected asymptomatic recurrences in 157 stage I-II patients (61%) and 89 stage IIIA patients (49%) (p=0.045). Symptoms led to detection of recurrences more often in stage IIIA patients (73 [40%]) than in stage I-II patients (81 [32%]). Distant were more common in stage IIIA patients than in stage I-II patients (153 [85%] vs. 190 [74%]; p=0.01). In stage IIIA patients, the risk of recurrence was highest during the first 2 years after surgery, but it remained substantial into year 4.
Conclusions
Stage IIIA patients had fewer recurrences detected by surveillance CT, a higher rate of symptomatic presentation, a markedly higher risk of recurrence, and a higher propensity for distant recurrence. Surveillance strategies may need to account for stage-specific differences.
Keywords: Recurrence, Surveillance, CT, NSCLC
Introduction
Despite recent advances in targeted therapies and multimodality approaches, non-small cell lung cancer (NSCLC) remains an aggressive disease with a high risk of recurrence [1]. For patients who have completed treatment with curative intent, the risk of both recurrence and a second primary lung tumor remains a long-term concern [2]. Surveillance for recurrences and new primary cancers is therefore a crucial component of cancer survivorship care and has been recommended by organizations such as the American Association for Thoracic Surgery (AATS) and the National Comprehensive Cancer Network (NCCN) [3, 4]. Whereas the surveillance regimens for other malignancies have the advantage of serum markers, such as prostate-specific antigen and carcinoembryonic antigen, no such biomarkers currently exist for lung cancer. The optimal surveillance regimen for NSCLC remains unclear, as demonstrated by the differing recommendations across various groups [5].
The first step toward designing a rational surveillance program is to gain a detailed understanding of the patterns of recurrences, as well as the timing of recurrences, to be able to answer the questions of where and when to look. In addition, how is another question that needs to be addressed. Examining the modalities in which recurrences are identified can inform the selection of modality for surveillance. Previous studies have suggested that the frequency of symptomatic presentation may limit the utility of routine surveillance imaging [6], whereas others have suggested that routine imaging with computed tomography (CT) is effective in detecting subclinical recurrences and metachronous cancers [7, 8]. However, the conclusions reached in these studies are limited by the heterogeneity of follow-up protocols and by small sample sizes.
Despite the range of prognoses across different stages of disease, most studies examining surveillance for recurrences have analyzed resected patients collectively, without distinguishing between stages. To help inform best practices for surveillance of lung cancer survivors, we compared the recurrence patterns of completely resected patients with pathologic locally advanced (stage IIIA) NSCLC with those of completely resected patients with early-stage disease (stages I-II) and examined the modality and timing in which the recurrences were detected.
Patients and Methods
A retrospective review of a prospectively maintained database was performed. Patients who underwent complete surgical resection for stage IIIA NSCLC from January 2004 to December 2009 and who had evaluable data on recurrence status were included in the study. Staging was based on the 7th edition of the American Joint Committee on Cancer staging system [9]. Baseline patient characteristics and treatments were obtained from the database. As some patients had undergone neoadjuvant therapy, all patients with IIIA disease (according to best stage [clinical or pathologic]) were included [10]. Patients who had not received induction therapy and had evidence of a stage IIIA malignancy in their final pathologic evaluation were included. In addition, those who had clinical stage IIIA disease (by either positron emission tomography (PET)/CT or positive tissue biopsy of N2 level lymph nodes) and who subsequently underwent induction therapy before complete surgical resection were included. Exclusion criteria were stage IIIB or IV disease, history of lung cancer, incomplete resection (R1 or R2), and carcinoid pathologic profile. A total of 346 patients fulfilled the study criteria. In total, 252 patients had final pathologic stage IIIA disease, and 94 had clinical stage IIIA disease that was downstaged in the final pathologic evaluation (pathologic stage 0-IIB), after neoadjuvant therapy.
Follow-up information—including recurrence, presence of metachronous tumors, method of detection, and subsequent treatments—was abstracted from the medical record. Recurrence-free status was censored at the last follow-up visit. Vital status was abstracted from the medical records and confirmed using the Social Security Death Index. Follow-up information was complete through May 2012.
All patients received follow-up by either the treating physician or a nurse practitioner with specialized training in lung cancer survivorship care. The surveillance regimen conformed to the NCCN guidelines [3] and consisted of history, physical examination, and routine CT scan of the chest and upper abdomen, with or without contrast, every 6 to 12 months for the first 2 years then annually thereafter. Other modalities of surveillance, such as PET/CT, bronchoscopy, and serum markers, were not performed for routine surveillance.
Recurrences were adjudicated by confirmatory imaging studies (PET/CT) or positive tissue biopsy results. Locoregional recurrences were defined as the appearance of new sites of disease limited to the ipsilateral regional lymph nodes, bronchial stump, staple line, or within the residual lobe, if a sublobar resection was performed. Recurrences at all other sites, including pleural and pericardial effusions, were classified as distant recurrences. Second primary lung tumors were distinguished from recurrences using the Martini-Melamed criteria: different histologic profile from the index tumor; same histologic profile as the index tumor but diagnosed two years later; or same histologic profile as the index tumor, diagnosed within two years, but in a separate lobe or segment, without involvement of intervening lymph nodes or metastasis [11, 12]. The institutional review board at Memorial Sloan-Kettering Cancer Center approved the study.
In a previous review of patients who underwent surgical resection for early-stage NSCLC at our institution, 1294 patients were included. The follow-up regimen, which comprised routine CT scanning, was the same as that described for advanced-stage patients [11].
Statistical Analysis
The primary endpoint was the development of either recurrence or metachronous lung cancer. The time to event was calculated from the date of surgical resection. If no new events occurred, then the patient was censored at the date of the last clinical follow-up. Overall survival was estimated using the Kaplan-Meier method, starting from the date of surgical resection for the primary tumor. Nonparametric log-rank tests were used to compare survival between groups. As the frequency of observations can affect time-to-event analysis, the annualized rate of recurrence was calculated over 12-month intervals from the time of surgery. The rate was calculated by dividing the total number of events in each year by the total follow-up time and presented as events per 100 person-years. Rates and patterns of recurrence were compared against a contemporary cohort of stage I and II patients treated during the same period at our institution, for which some data had been previously analyzed using the same methodology [11]. Comparisons between initial stage and recurrence patterns were performed using χ2 analysis, with the significance level set at 0.05. SAS 9.3 (SAS Institute, Cary, NC) was used to perform all analyses.
Results
For the analysis on Stage IIIA patients, a total of 346 patients received follow-up for a median of 35 months (range, 3–92 months). Baseline characteristics are summarized in Table 1. More than half of patients (195; 56%) underwent neoadjuvant therapy, most commonly chemotherapy alone. Approximately half of these patients (101; 51%) had final pathologic stage IIIA disease, whereas the remaining patients were downstaged. Less than half of all patients (151; 44%) had pathologic stage IIIA disease and did not undergo induction therapy before surgery. A majority of these patients (112; 74%) received adjuvant therapy after resection.
Table 1.
Patient Characteristics (N=346)
| Characteristic | Stage IIIA (N=346) | Stage I and II (N=1294) |
|---|---|---|
| Female | 193 (56) | 752 (58) |
| Age, years (mean ± SD) | 65 ± 10 | 69 ± 10 |
| FEV 1, % predicted (mean ± SD) | 87 ± 18 | 89 ± 21 |
| Current or past smoker | 308 (89) | 1098 (85) |
| Neoadjuvant therapy | ||
| Yes | 195 (56) | 0 |
| Chemotherapy | 168 (49) | |
| Chemoradiation | 26 (8) | |
| Radiation | 1 (0.2) | |
| Procedure | ||
| Lobectomy | 265 (77) | 955 (74) |
| Pneumonectomy | 46 (13) | 17 (1) |
| Bilobectomy | 19 (5) | 22 (2) |
| Wedge | 10 (3) | 188 (15) |
| Segmentectomy | 5 (1) | 111 (9) |
| Histologic profile | ||
| Adenocarcinoma | 227 (66) | 995 (77) |
| Squamous cell | 70 (20) | 222 (17) |
| Other | 25 (7) | 45 (3) |
| Large cell | 18 (5) | 32 (2) |
| Adenosquamous | 5 (1) | 0 |
| Bronchioloalveolar carcinoma | 1 (0.2) | 0 |
| Adjuvant therapy | ||
| Yes | 187 (54) | 202 (16) |
| Chemotherapy | 127 (37) | 192 (15) |
| Radiation | 40 (12) | 1 (<1) |
| Chemotherapy + radiation | 20 (6) | 1 (<1) |
| Unknown | 15 (4) | 8 (1) |
The average number of CT scans performed among all patients was 2.6 per person for each year of follow-up. This number most likely includes close follow-up imaging of patients with suspicious findings, as well as routine CT scans. When this number was broken down into patients with and patients without a diagnosis of recurrence or metachronous tumors, the average number of CT scans per year was 1.4 and 3.5, respectively.
Slightly more than half of the patients with stage IIIA disease (181; 52%) developed recurrences during the follow-up period (Table 2), the vast majority of which (153; 85%) were distant metastases (93 distant only, 60 both locoregional and distant). Only 28 patients (15%) developed locoregional recurrence only. In comparison, 257 stage I-II patients (20%) developed recurrences, 190 (74%) of which were distant metastases. The sites of recurrence are noted in Table 2. The most common sites of distant metastases were brain (n=47), pleural involvement (n=37), contralateral lung (n=33), and bone (n=29). Compared with patients with early-stage disease, patients with advanced-stage disease had more distant metastases than locoregional recurrences (p=0.01). The majority of patients who developed distant metastases had one site of disease (n=111; 72%).
Table 2.
Recurrence and Detection Characteristics
| Characteristic | Stage IIIA (N=181) | Stage I-II (N=257) |
|---|---|---|
| Recurrence location | ||
| Locoregional | 88 | 143 |
| Regional lymph nodes | 57 (65) | 69 (48) |
| Same side | 40 (45) | 62 (43) |
| Staple line | 14 (16) | 40 (28) |
| Distant | 153 | 190 |
| Brain | 47 (31) | 36 (19) |
| Pleural disease | 37 (24) | 67 (36) |
| Contralateral lung | 33 (22) | 44 (23) |
| Bone | 29 (19) | 17 (9) |
| Liver | 13 (8) | 22 (12) |
| Adrenal | 11 (7) | 15 (8) |
| Chest wall | 8 (5) | 15 (8) |
| Detection method | ||
| Surveillance CT | 89 (49) | 157 (61) |
| Symptoms | 73 (40) | 81 (32) |
| Other | 12 (7) | 17 (7) |
| Unknown | 9 (5) | 2 (1) |
| Treatment of recurrence | ||
| Chemotherapy | 74 (41) | 90 (35) |
| Radiation | 35 (19) | 37 (14) |
| Chemotherapy + radiation | 23 (13) | 30 (12) |
| None | 20 (11) | 17 (7) |
| Unknown | 12 (7) | 38 (15) |
| Surgery + radiation | 5 (3) | 18 (7) |
| Surgery | 6 (3) | 13 (5) |
| Other | 5 (3) | 14 (5) |
Data are no. (%)
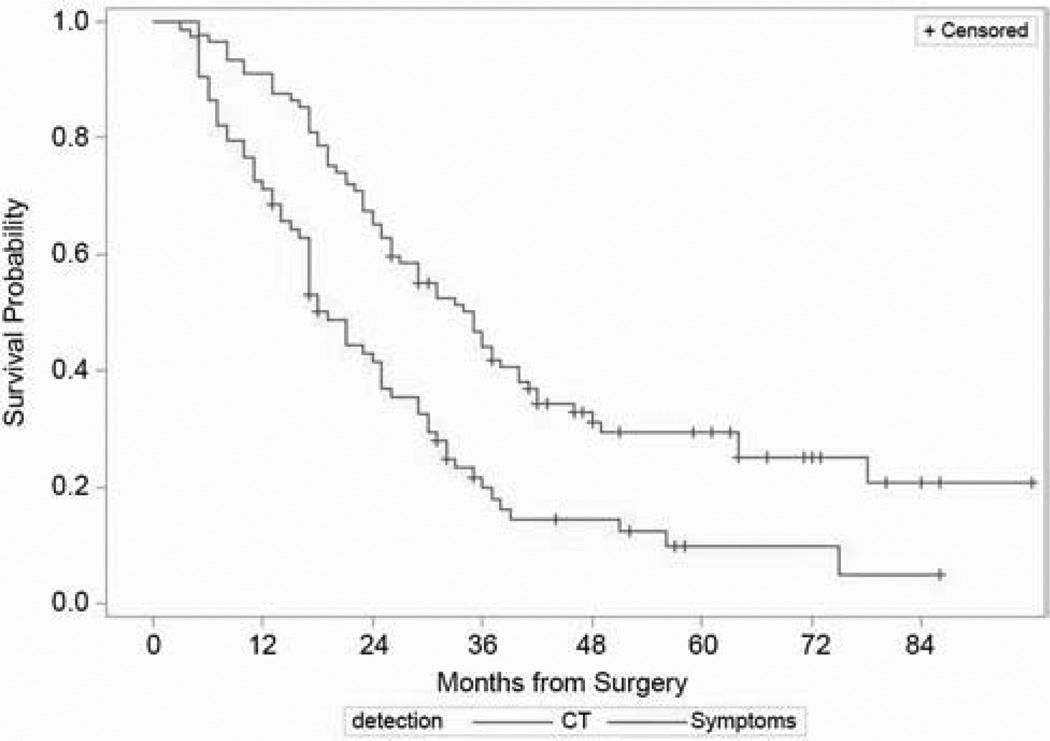
Nearly half of all recurrences in the stage IIIA cohort (89; 49%) were detected by scheduled surveillance CT scan during routine follow-up visits. Symptoms led to the diagnosis of recurrence (40%) outside of a regular follow-up visit in 73 patients (40%). The remaining recurrences were largely detected incidentally by imaging tests performed for indications other than the lung cancer. In contrast, a higher proportion of recurrences among early-stage patients (61%) were detected by surveillance CT scan (p=0.04), and only 32% (81/257) of detected recurrences in the early-stage cohort were identified as a result of symptoms outside of a regular follow-up visit. The overall survival from the time of surgery was longer for patients whose recurrences were detected by surveillance CT scans than for those who presented with symptoms (Figure 1; p<0.001)
Figure 1.
Overall survival from the time of surgery among stage IIIA patients with recurrences detected by CT surveillance, compared with those presenting with symptoms (p<0.001).
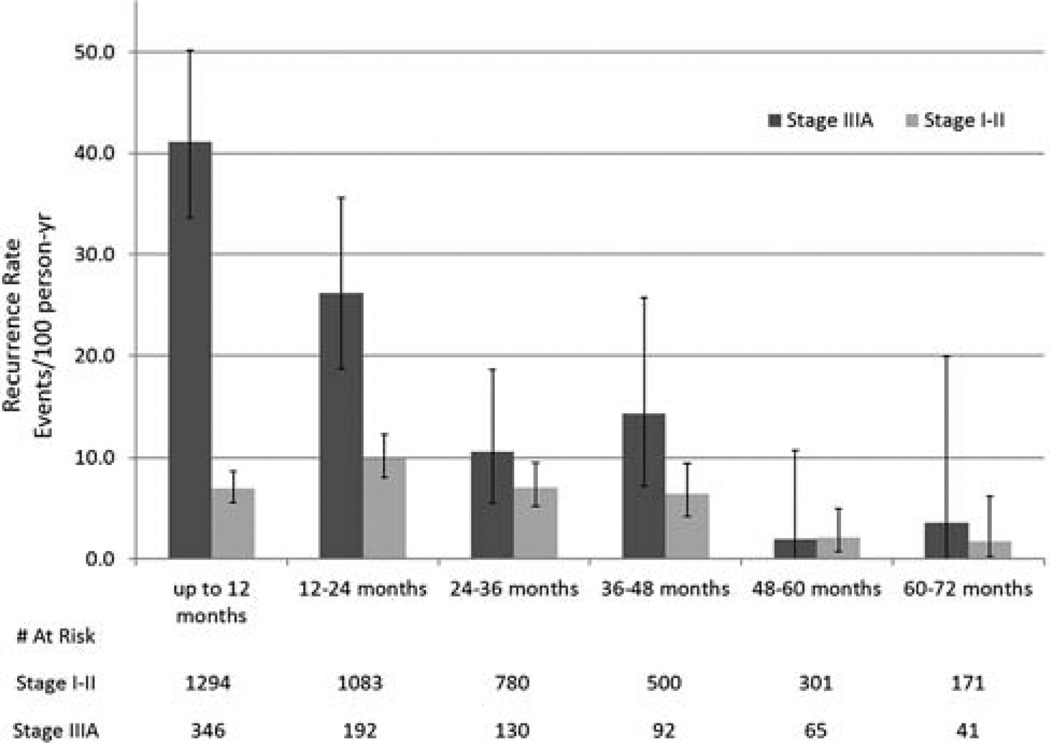
Rates of recurrence during each postoperative year were calculated and compared with those for patients treated for early-stage cancers (Figure 2). Although the rate of recurrence was highest during the first 2 years after surgery, the risk of recurrence remained elevated through postoperative year 4. The rate of recurrence during the first two years was 7 and 10 recurrences per 100 person-years, respectively; this dropped to 2 per 100 person-years after year 4. Compared with early-stage patients, stage IIIA patients had a significantly higher rate of recurrence during the first 2 years postresection. After year 4, rates of recurrence were similar between the two cohorts.
Figure 2.
Comparison of rates of recurrence after surgical resection for NSCLC.
Comment
Marked differences were found in the postresection patterns of recurrence between patients with locally advanced NSCLC and those with early-stage disease. The rate of recurrence was considerably higher for the stage IIIA cohort, peaking during the first 2 years; it was sustained, however, throughout the first 4 years for both cohorts. More than half of the stage IIIA patients developed recurrences, the vast majority of which were distant metastases, most commonly in the brain. In comparison, 1 of 5 patients with early-stage disease developed recurrence, and onequarter of those had locoregional disease only. Accordingly, surveillance CT scan was more successful at detecting recurrences in early-stage patients than in advanced-stage patients. To our knowledge, this is the largest series to examine the differences in postresection detection and recurrence patterns between patients with early-stage and those with locally advanced NSCLC.
Time Distribution of Recurrences
Several studies on cancer recurrence have demonstrated the importance of tailoring intensive surveillance to the patients and periods with the highest rates of recurrence [13, 14]. Demicheli et al. reported the recurrence dynamics of 1506 patients who underwent resection for early-stage NSCLC. Using Kernel-smoothed hazard functions, they found three discrete peaks of high rates of treatment failure: at 9 months, 2 years, and 4 years after surgery [14]. Applying the same methods to our data, we found notably similar results (data not shown). Although the authors suggest that the peaks in recurrence dynamics are attributable to differences in tumor biology and may correspond to changes in metastatic potential, this hypothesis remains untested. It is possible that the discrete peaks observed are artifacts secondary to the discrete follow-up intervals. Although recurrence is usually reported as if it were a continuous variable, events tend to cluster in the intervals during which imaging is performed, and interval censoring should be taken into account when interpreting the hazard of recurrence over time. For this reason, we annualized the observed rate of recurrence over 12-month intervals.
Our data highlights the importance of two aspects of timing of surveillance regimens: intensity and duration. During the first 2 years after surgery—and, in particular, during the first year—the risk of recurrence was markedly higher for patients with stage IIIA disease than for patients with early-stage disease. The overall higher number of recurrences among stage IIIA patient likely reflects the higher risk in the first two years after surgery. Although the risk of recurrence among stage IIIA patients remained slightly higher in years 3 and 4, the difference between advanced-stage and early-stage patients was much less pronounced; and for both groups, the risk of recurrence didn’t appear to decrease until years 5 and 6. Although the rate of recurrence among stage IIIA patients was highest during the first 2 years after surgery, recurrences were not limited to the first 2 years—indeed, recurrences were detected up to seven years post-resection.
On the basis of the results of the National Lung Screening Trial (in which CT screening was performed on an annual basis), yearly imaging would appear to be sufficient if one were considering only for screening for second primaries [15]. However, an annual frequency would likely be too long an interval to monitor for recurrences, particularly in the higher-risk stage IIIA cohort. Given the peak of recurrences during the first 2 years in the stage IIIA cohort, it would seem that the greatest intensity of follow-up should be directed to that interval. For example, for IIIA patients, one could consider a stage-specific follow-up schedule based on risk: years 1 to 2, every 3 months; years 3 to 4, every 6 months; year 5 and onward, every year. For early-stage patients, the schedule for years 1 to 2 could be every 6 months, with the frequency remaining the same for the remaining years. Over time, the risk of recurrence decreases, and the importance of screening for second malignancies increases, but the need for continued follow-up remains.
Sites of Recurrence and Implications for Surveillance
The vast majority of recurrences among stage IIIA patients were distant. Our results were consistent with the previous series by Taylor et al., who also reported on the high frequency of lung, brain, and bone metastases after surgical resection for NSCLC [16]. Of the distant recurrences in our series, nearly half involved either pleural recurrences (n=37) or contralateral lung metastases (n=33). If one considers that an additional 28 patients had locoregional recurrences only, then more than half of all recurrences would have been detectable by chest CT scan. However, that the remaining recurrences occurred outside the domain of a chest CT scan and would not have been detected by that modality raises some troubling questions. Including the adrenals and the entire liver as part of a chest CT protocol could be helpful. Skeletal metastases could be detected by bone scan or PET/CT imaging, as could liver or adrenal metastases
Currently, PET/CT is not recommended as a routine surveillance method for asymptomatic patients. The role of PET/CT for follow-up after resection for NSCLC has been investigated by various groups [17, 18]. Compared with CT alone, PET/CT was more sensitive in the detection of recurrences, and this improved sensitivity was largely attributable to the detection of extrathoracic metastatic disease [18]. However, with this higher sensitivity comes higher costs, greater radiation exposure, and the risk of false-positives. Similarly, the brain was the most common site of recurrence, yet neither CT nor PET scan is capable of capturing such recurrences. No studies investigators have examined the value of routine brain MRI for surveillance purposes.
The underlying question in surveillance is whether active surveillance for recurrences—and, in particular, distant recurrences—can effect an improvement in outcomes. Should high-risk patients (i.e., stage IIIA patients) undergo intensive follow-up that includes a PET scan and brain MRI every 3 months for the first 2 years? At present, there is no evidence that such an aggressive surveillance regimen would result in improved survival. As a result, many have argued against intensive surveillance, citing increased costs as well as unnecessary and ineffective interventions [19]. In theory, earlier detection of locoregional disease would allow for earlier curative intervention. Whether earlier detection of metastatic recurrent disease translates to increased survival remains unclear. The ability of early detection to have an effect on survival is solely dependent on the availability of meaningful treatment options. For now, the design of a rational follow-up regimen—assuming that we are interested in monitoring for both recurrences and second primaries—should be based on the observed risks of both, in terms of site and timing. CT-detected recurrences were associated with longer overall survival, compared with symptomatic recurrences. However, this correlation may reflect a more aggressive nature of symptomatic recurrences, rather than an actual benefit of imaging surveillance. Without a comparison group that did not undergo the same surveillance regimen, it is impossible to isolate the effect of CT surveillance from these data. The efficacy of surveillance regimens should be evaluated prospectively. The primary goal of our study was to describe the manner of detection and recurrence patterns of lung cancer, in order to inform the design of future prospective trials, and it is important to caution against any overinterpretation of a survival benefit from these retrospective data.
The current recommendations from the AATS and NCCN call for routine chest CT imaging after surgical resection for NSCLC [3, 4]. As with any surveillance program, the ideal follow-up protocol should maximize the early detection of events, at the lowest possible cost, at both the individual and societal levels. To date, no large, prospective, randomized, controlled trials on follow-up methods for lung cancer have been completed. Benamore et al. compared 40 stage III patients who underwent the INT 0139 and INT 1060 intergroup trials and received intense surveillance against 35 patients with the same stage followed by chest radiograph. Although more recurrences were detected in the trial group, there was no significant difference in overall survival (p=0.67) [20]. A recent meta-analysis examined the effect of intensive surveillance on survival from several heterogeneous series [21]. Although there was a trend toward improved survival in groups that received intensive follow-up, the results were not conclusive. The series studied included patients with different treatments and stages of disease, and they used dissimilar definitions of “intensive” surveillance. The IFCT-0302 trial is an ongoing, multi-institution, randomized, controlled trial examining the benefits of surveillance CT and fiberoptic bronchoscopy, compared with chest x-ray alone [22]. The results of this study have not yet been published.
As this study was observational in nature, our analysis is limited by its retrospective nature and its inherent biases. Although the experience represents that at a single institution, it is one of the largest attempts to explore these questions. Furthermore, as we performed a descriptive analysis, no inferences about effects on survival outcomes can be made—nor were any intended to be made. Any effect on survival outcomes would necessarily require prospective evaluation.
Patients with stage IIIA NSCLC had markedly higher rates of postresection recurrence than did patients with early-stage disease, particularly during the first 2 years. However, the risk of recurrence did not decrease after the first 2 years—it persisted at least into the fourth year. Compared with patients with early-stage disease, patients with advanced-stage disease were more likely to have distant metastases and less likely to have recurrences detected by surveillance imaging. Although surveillance CT scans detected more than half of all recurrences, the remaining recurrences occurred outside the area routinely assessed by chest CT imaging. These findings raise questions about whether it may be warranted to individualize surveillance routines on the basis of risk.
Acknowledgment
The authors wish to thank Drs. Manjit S. Bains, Nabil Rizk, Robert Downey, Prasad S. Adusumilli, David Finley, and Raja Flores, whose patients were included in the analysis. The authors also express their appreciation to David Sewell for his support in manuscript preparation.
Financial Support: NIH/NCI Cancer Center Support Grant P30 CA008748
Discussion
101. STAGE-SPECIFIC DIFFERENCES IN PATTERNS OF RECURRENCE AND THEIR MODE OF DETECTION IN EARLY STAGE VERSUS LOCALLY ADVANCED NON-SMALL CELL LUNG CANCER. Paper presented by Feiran Lou, M.D., New York, New York. feiranl@gmail.com
Discussion by Julius Toth, M.D., Toronto, Ontario, Canada. jtoth@southlakeregional.org
Dr. J. Toth (Toronto, Ontario, Canada): That was a nicely-done paper.
I was wondering if any of your strategies looked into the initial surgical management variations between the patients as to whether or not that had any impact on the locoregional versus the distant metastases, especially looking at the high locoregional recurrence rate of the early-stage lung cancers. Were these treated with a wedge, a segment, were they done VATS, were they done open, and if you throw that into the mix, will that alter your follow-up strategy at all?
101. STAGE-SPECIFIC DIFFERENCES IN PATTERNS OF RECURRENCE AND THEIR MODE OF DETECTION IN EARLY STAGE VERSUS LOCALLY ADVANCED NON-SMALL CELL LUNG CANCER. Response by Feiran Lou, M.D., New York, New York. feiranl@gmail.com
Dr. Lou: Thank you. That is a very interesting question that is definitely worth looking into. However, the difference in recurrence as a function of the extent of resectionwas not one of our main questions to answer for this study. We did have about 75% of patients undergoing lobectomy in this population and pretty much none for wedge resections for stage IIIA patients, but we did not specifically compareat the locoregional recurrence rates.
Dr. Toth: Thank you.
101. STAGE-SPECIFIC DIFFERENCES IN PATTERNS OF RECURRENCE AND THEIR MODE OF DETECTION IN EARLY STAGE VERSUS LOCALLY ADVANCED NON-SMALL CELL LUNG CANCER. Paper presented by Feiran Lou, M.D., New York, New York. feiranl@gmail.com
Discussion by Paula A. Ugalde, M.D., Quebec, Ontario, Canada. paugalde20@gmail.com
Dr. P. Ugalde (Quebec, Ontario, Canada): Did you notice any difference in the pattern of recurrence between the stage IIIA patients who had neoadjuvant treatment versus the patients who had adjuvant treatment?
101. STAGE-SPECIFIC DIFFERENCES IN PATTERNS OF RECURRENCE AND THEIR MODE OF DETECTION IN EARLY STAGE VERSUS LOCALLY ADVANCED NON-SMALL CELL LUNG CANCER. Response by Feiran Lou, M.D., New York, New York. feiranl@gmail.com
Dr. Lou: We did not specifically look at that. Again, we had about 50% of patients who underwent neoadjuvant therapy and another half who did not. It would be interesting to look at, but we did not look at those.
Dr. Ugalde: Can you please tell me if the neoadjuvant treatment that you do in your service is chemoradiation or just chemo and if the adjuvant treatment in that context would be chemo plus or minus radiation?
Dr. Lou: Yes. Patients who received neoadjuvant therapy, a vast majority of them received chemotherapy only, and the same isis true for adjuvant.
Dr. Ugalde: Thank you.
101. STAGE-SPECIFIC DIFFERENCES IN PATTERNS OF RECURRENCE AND THEIR MODE OF DETECTION IN EARLY STAGE VERSUS LOCALLY ADVANCED NON-SMALL CELL LUNG CANCER. Paper presented by Feiran Lou, M.D., New York, New York. feiranl@gmail.com
Discussion by Joshua H. Burack, M.D., Brooklyn, New York. jburack@downstate.edu
Dr. J. Burack (Brooklyn, New York): Feiran, I enjoyed your presentation immensely.
Could you give us some information as to when you recommend the first thoracic CAT scan following lung resection?
Dr. Lou: The first CAT scan?
Dr. Burack: The initial postoperative one. At what time interval does the Memorial group usually do their first postop CT scan of the chest?
101. STAGE-SPECIFIC DIFFERENCES IN PATTERNS OF RECURRENCE AND THEIR MODE OF DETECTION IN EARLY STAGE VERSUS LOCALLY ADVANCED NON-SMALL CELL LUNG CANCER. Response by Feiran Lou, M.D., New York, New York. feiranl@gmail.com
Dr. Lou: Most of the patients we analyzed had the first scan in the first 6 months. In particular, for the first 2 years, they had 2 scans per year, so it would be at month 6 and month 12.
Dr. Burack: Thank you.
101. STAGE-SPECIFIC DIFFERENCES IN PATTERNS OF RECURRENCE AND THEIR MODE OF DETECTION IN EARLY STAGE VERSUS LOCALLY ADVANCED NON-SMALL CELL LUNG CANCER. Paper presented by Feiran Lou, M.D., New York, New York. feiranl@gmail.com
Discussion by Steven Herman, M.D., Brooklyn, New York. steven.herman@downstate.edu
Dr. S. Herman (Brooklyn, New York): Feiran, that was an excellent presentation, nicely presented, and very clear.
I have a couple of questions. From the data that you have given, when looking at the patients with resected early stage lung cancer who were followed and presented with new pulmonary recurrences, are those perhaps new cancers or are these local recurrences? Certainly if you have done lobectomy, there is no local parenchymal recurrence. Given the fact that we know the propensity of patients who have had one lung carcinomas for having new ones, what we are doing when we are looking at CT scans in following treated early stage lung cancer patients could actually be lung surveillance rather than cancer recurrence detection.
Secondly, in the more advanced cases, where we have the IIIA's, should we be doing studies like PET scans or head CT’s in addition to or instead of chest CT scans to pick up these patients’ recurrence sites prior to symptom presentation, which is often too late to do anything about, even in advanced cases.
101. STAGE-SPECIFIC DIFFERENCES IN PATTERNS OF RECURRENCE AND THEIR MODE OF DETECTION IN EARLY STAGE VERSUS LOCALLY ADVANCED NON-SMALL CELL LUNG CANCER. Response by Feiran Lou, M.D., New York, New York. feiranl@gmail.com
Dr. Lou: Thank you, Dr. Herman.
For the first question regarding second primary tumors, we did also look into that aspect in our data. It's not presented here, but we actually found only 6 second primary lung cancers in stage IIIA patients, and the criteria we used were the Martini-Melamed criteria. All of the cases were detected by CT surveilane. Interestingly in early-stage patients we found a 6% rate of second primary lung cancers. So there seemed to be a slighter higher proportion of second primary lung cancers detected in earlier-stage patients. The rate of second primary lung cancers that develop over time in early-stage patients, not surprisingly, was actually constant in all the years following surgery. Forthis reason, we recommend continued surveillance, at least yearly, for the purpose of second primary lung cancers. But, we do recommend increased frequency of surveillance in patients treated for stage IIIA cancers in the first 2 years for the purpose of detecting recurrences.
Your second question was regarding?
Dr. Herman: Alternate testing, like head scans or other things, rather than just chest CT scans.
Dr. Lou: There have been some studies looking at the efficacy of PET-CT versus CT surveillance in the setting of recurrence. They have been smaller studies, but in general they found that there is an increase in the detection rate of recurrence in PET-CT compared to CT alone. Whether or not that means or translates to an increase in survival is something that we cannot answer right now. Most guidelines currently do not recommend PET-CT scanning for the purpose of surveillance, but it is something that is definitely worth looking into in the future.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation: Presented at the 50th Annual Meeting of the Society of Thoracic Surgeons, Orlando, FL, January 25–29, 2014.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Sugimura H, Nichols FC, Yang P, et al. Survival after recurrent nonsmall-cell lung cancer after complete pulmonary resection. Ann Thorac Surg. 2007;83:409–418. doi: 10.1016/j.athoracsur.2006.08.046. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network guidelines for surveillance following therapy for NSCLC. [Accessed October 4, 2010]; Available at http://www.nccn.org. [Google Scholar]
- 4.Jacobson FL, Austin JH, Field JK, et al. Development of the American Association for Thoracic Surgery guidelines for low-dose computed tomography scans to screen for lung cancer in North America: recommendations of the American Association for Thoracic Surgery Task Force for Lung Cancer Screening and Surveillance. J Thorac Cardiovasc Surg. 2012;144:25–32. doi: 10.1016/j.jtcvs.2012.05.059. [DOI] [PubMed] [Google Scholar]
- 5.Colt HG, Murgu SD, Korst RJ, et al. Follow-up and surveillance of the patient with lung cancer after curative-intent therapy. Chest. 2013;143:e437s–e454s. doi: 10.1378/chest.12-2365. [DOI] [PubMed] [Google Scholar]
- 6.Walsh GL, O'Connor M, Willis KM, et al. Is follow-up of lung cancer patients after resection medically indicated and cost-effective? Ann Thorac Surg. 1995;60:1563–1572. doi: 10.1016/0003-4975(95)00893-4. [DOI] [PubMed] [Google Scholar]
- 7.Lamont JP, Kakuda JT, Smith D, et al. Systematic postoperative radiologic follow-up in patients with non-small cell lung cancer for detecting second primary lung cancer in stage IA. Arch Surg. 2002;137:935–939. doi: 10.1001/archsurg.137.8.935. [DOI] [PubMed] [Google Scholar]
- 8.Westeel V, Choma D, Clement F, et al. Relevance of an intensive postoperative follow-up after surgery for non-small cell lung cancer. Ann Thorac Surg. 2000;70:1185–1190. doi: 10.1016/s0003-4975(00)01731-8. [DOI] [PubMed] [Google Scholar]
- 9.Lung . In: American Joint Committee on Cancer Staging Manual. 7th Edition. Edge SB, Byrd DR, Compton CC, et al., editors. New York, New York: Springer; 2010. p. 253. [Google Scholar]
- 10.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 11.Lou F, Huang J, Sima CS, et al. Patterns of recurrence and second primary lung cancer in early-stage lung cancer survivors followed with routine computed tomography surveillance. J Thorac Cardiovasc Surg. 2013;145:75–82. doi: 10.1016/j.jtcvs.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 12.Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg. 1975;70 [PubMed] [Google Scholar]
- 13.Mollberg NM, Ferguson MK. Postoerative surveillance for non-small cell lung cancer resected with curative intent: developing a patient-centered approach. Ann Thorac Surg. 2013;95:1112–1121. doi: 10.1016/j.athoracsur.2012.09.075. [DOI] [PubMed] [Google Scholar]
- 14.Demicheli R, Fornili M, Ambrogi F, et al. Recurrence dynamics for non-small-cell lung cancer. J Thorac Oncol. 2012;7:723–730. doi: 10.1097/JTO.0b013e31824a9022. [DOI] [PubMed] [Google Scholar]
- 15.National Lung Screening Trial Research Team. Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor MD, Nagji AS, Bhamidipati CM, et al. Tumor recurrence after complete resection for non-small cell lung cancer. Ann Thorac Surg. 2012;93:1813–1820. doi: 10.1016/j.athoracsur.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 17.Choi S, Kim YT, Kim SK, et al. Positron emission tomography–computed tomography for postoperative surveillance in non-small cell lung cancer. Ann Thorac Surg. 2011;92:1826–1832. doi: 10.1016/j.athoracsur.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Dane B, Grechushkin V, Plank A, et al. PET/CT vs. non-contrast CT alone for surveillance 1-year post lobectomy for stage I non-small-cell lung cancer. Am J Nucl Med Mol Imaging. 2013;3:408–416. [PMC free article] [PubMed] [Google Scholar]
- 19.Furman M, Lambert LA, Sullivan ME, et al. Rational follow-up after curative cancer resection. J Clin Oncol. 2013;31:1130–1133. doi: 10.1200/JCO.2012.46.4438. [DOI] [PubMed] [Google Scholar]
- 20.Benamore R, Shepherd FA, Leighl N, Pintilie M, Patel M, Feld R, Herman S. Does intensive follow-up alter outcome in patients with advanced lung cancer? J Thorac Oncol. 2007;2:273–281. doi: 10.1097/01.JTO.0000263708.08332.76. [DOI] [PubMed] [Google Scholar]
- 21.Calman L, Beaver K, Hind D, et al. Survival benefits from follow-ups of patients with lung cancer: a systematic review and meta-analysis. J Thorac Oncol. 2011;6:1993–2004. doi: 10.1097/JTO.0b013e31822b01a1. [DOI] [PubMed] [Google Scholar]
- 22.Westeel V, Lebitasy MP, Mercier M, et al. IFCT-0302 trial: randomised study comparing two follow-up schedules in completely resected non-small cell lung cancer. Rev Mal Respir. 2007;24:645–652. doi: 10.1016/s0761-8425(07)91135-3. [DOI] [PubMed] [Google Scholar]