Abstract
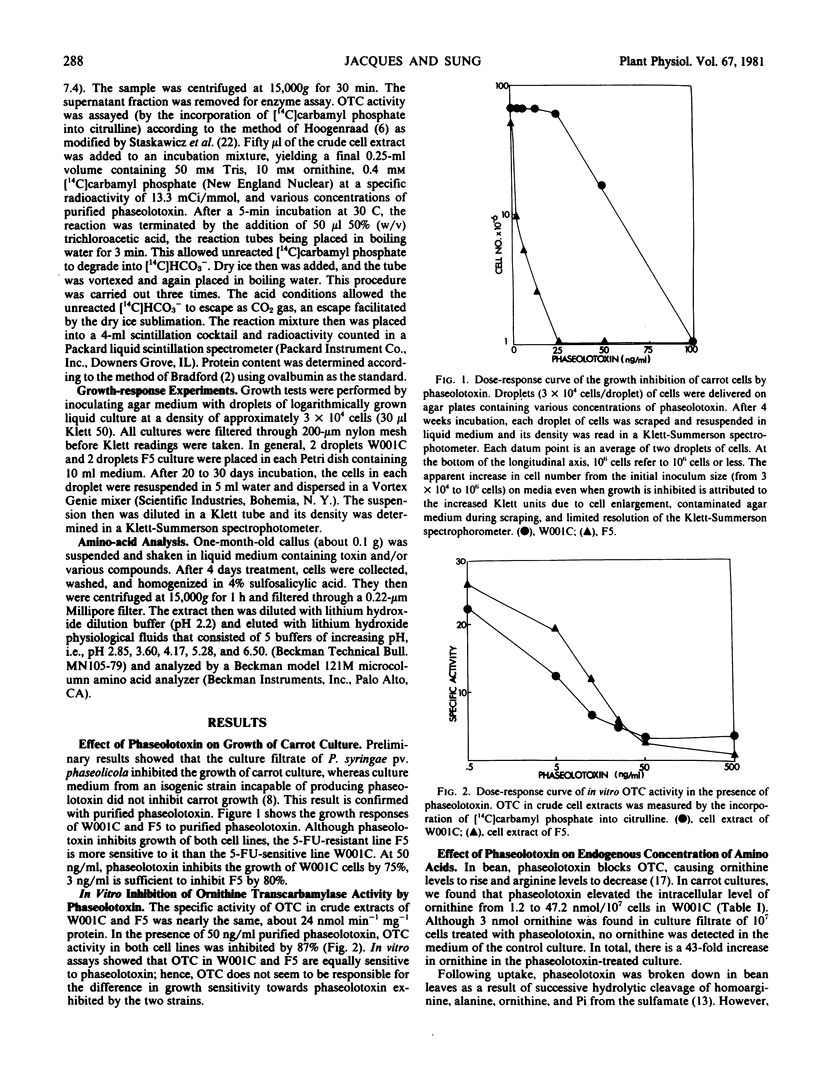
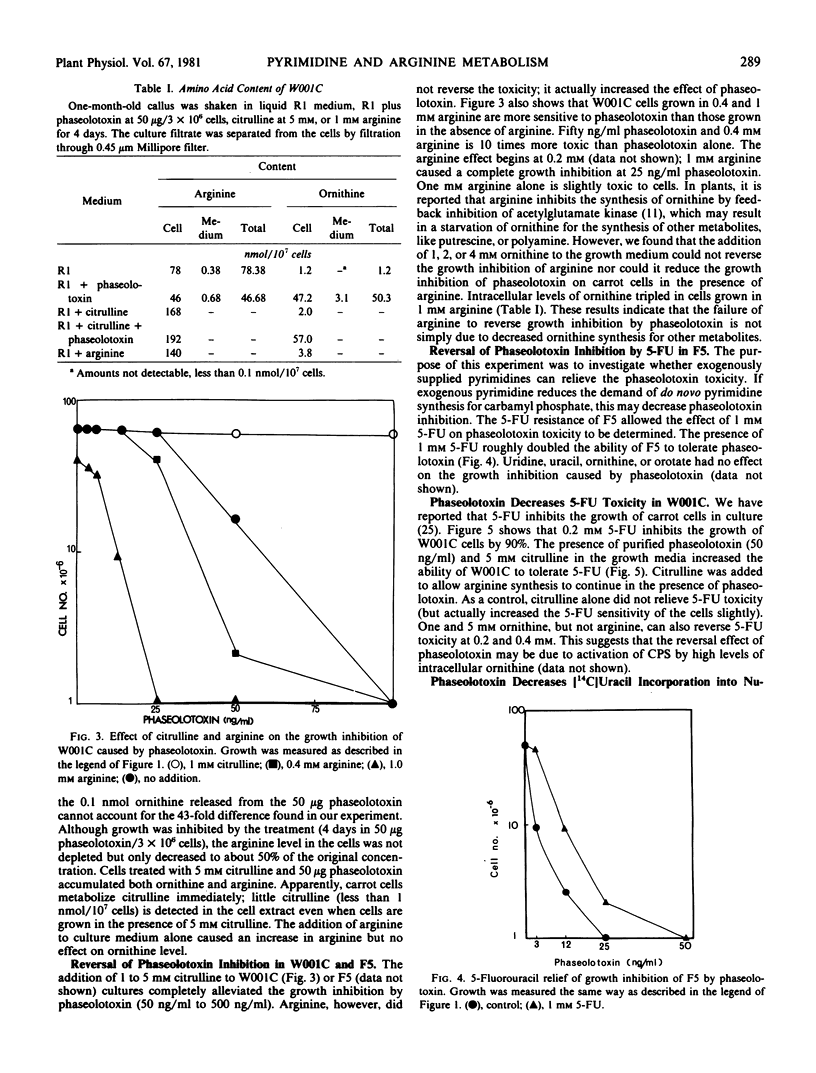
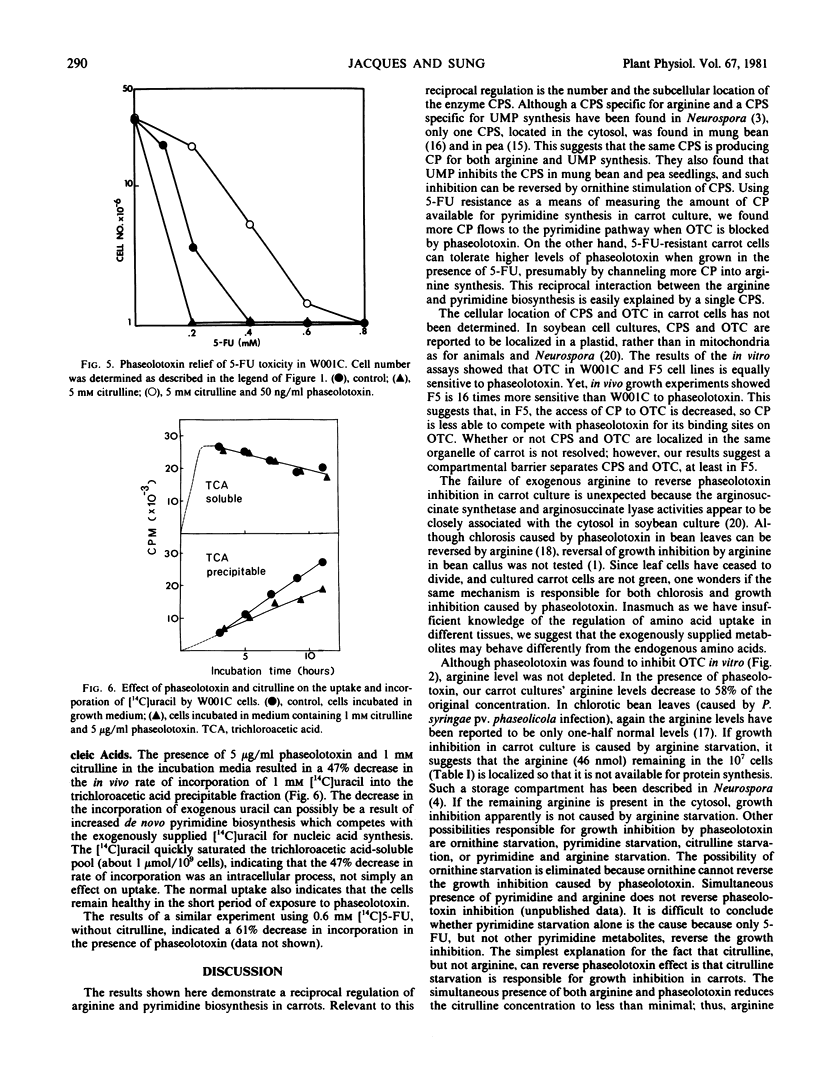
Purified phaseolotoxin inhibits the growth of carrot cells. Such inhibitions can be reversed completely by citrulline but not by arginine. This toxin inhibits ornithine transcarbamylase activity in vitro, which leads to an accumulation of ornithine and a decrease in arginine levels intracellularly. In carrot cells, 5-fluorouracil (5-FU) toxicity can be reduced by the addition of purified toxin and citrulline, or ornithine. The toxin also decreases the incorporation of [14C]uracil and [14C]5-FU into trichloroacetic acid precipitable material by 50%. Finally, a 5-FU-resistant line, F5 (Sung ZR, Jacques S 1980 Planta 148: 389-396), was found to be more sensitive to the toxin than were 5-FU-sensitive cells. One millimolar 5-FU roughly doubled the ability of F5 to tolerate phaseolotoxin. These results demonstrate a close regulation between the pyrimidine and arginine path-ways in carrots.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Davis R. H. Metabolite distribution in cells. Science. 1972 Nov 24;178(4063):835–840. doi: 10.1126/science.178.4063.835. [DOI] [PubMed] [Google Scholar]
- Goldstein A. S., Hoogenraad N. J., Johnson J. D., Fukanaga K., Swierczewski E., Cann H. M., Sunshine P. Metabolic and genetic studies of a family with ornithine transcarbamylase deficiency. Pediatr Res. 1974 Jan;8(1):5–12. doi: 10.1203/00006450-197401000-00002. [DOI] [PubMed] [Google Scholar]
- Hoogenraad N. J., Sutherland T. M., Howlett G. J. Effect of the transition-state analogue, delta-N-(phosphonacetyl)-L-ornithine on citrulline synthesis in isolated rat-liver mitochondria and on urea synthesis in isolated rat hepatocytes. Eur J Biochem. 1979 Oct;100(1):309–315. doi: 10.1111/j.1432-1033.1979.tb02062.x. [DOI] [PubMed] [Google Scholar]
- Hoogenraad N. J. Synthesis and properties of delta-N-(phosphonacetyl)-L-ornithine. A transition-state analog inhibitor of ornithine transcarbamylase. Arch Biochem Biophys. 1978 May;188(1):137–144. doi: 10.1016/0003-9861(78)90366-1. [DOI] [PubMed] [Google Scholar]
- Lovatt C. J., Albert L. S. Regulation of Pyrimidine Biosynthesis in Intact Cells of Cucurbita pepo. Plant Physiol. 1979 Oct;64(4):562–569. doi: 10.1104/pp.64.4.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan G. A., Neuhard J. Pyrimidine metabolism in microorganisms. Bacteriol Rev. 1970 Sep;34(3):278–343. doi: 10.1128/br.34.3.278-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal T. D., Naylor A. W. Partial purification and properties of carbamoyl phosphate synthetase of Alaska pea (Pisum sativum L. cultivar Alaska). Biochem J. 1969 Jun;113(2):271–279. doi: 10.1042/bj1130271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong B. L., Jackson J. F. Pyrimidine nucleotide biosynthesis in Phaseolus aureus. Enzymic aspects of the control of carbamoyl phosphate synthesis and utilization. Biochem J. 1972 Sep;129(3):583–593. doi: 10.1042/bj1290583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil S. S., Tam L. Q. Mode of Action of the Toxin from Pseudomonas phaseolicola: I. Toxin Specificity, Chlorosis, and Ornithine Accumulation. Plant Physiol. 1972 May;49(5):803–807. doi: 10.1104/pp.49.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil S. S., Youngblood P., Christiansen P., Moore R. E. Phaseotoxin A: an antimetabolite from Pseudomonas phaseolicola. Biochem Biophys Res Commun. 1976 Apr 19;69(4):1019–1027. doi: 10.1016/0006-291x(76)90474-5. [DOI] [PubMed] [Google Scholar]
- Shargool P. D., Steeves T., Weaver M., Russell M. The localization within plant cells of enzymes involved in arginine biosynthesis. Can J Biochem. 1978 Apr;56(4):273–279. doi: 10.1139/o78-042. [DOI] [PubMed] [Google Scholar]
- Staskawicz B. J., Panopoulos N. J., Hoogenraad N. J. Phaseolotoxin-insensitive ornithine carbamoyltransferase of Pseudomonas syringae pv. phaseolicola: basis for immunity to phaseolotoxin. J Bacteriol. 1980 May;142(2):720–723. doi: 10.1128/jb.142.2.720-723.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung Z. R. Mutagenesis of cultured plant cells. Genetics. 1976 Sep;84(1):51–57. doi: 10.1093/genetics/84.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung Z. R. Turbidimetric measurement of plant cell culture growth. Plant Physiol. 1976 Mar;57(3):460–462. doi: 10.1104/pp.57.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]



