Abstract
Fluorescent in situ hybridization (FISH) is a method that uses fluorescent probes to detect specific nucleic acid sequences at the single cell level. Here we describe optimized protocols that exploit a highly sensitive FISH method based on branched DNA technology to detect mRNA and miRNA in human leukocytes. This technique can be multiplexed and combined with fluorescent antibody protein staining to addressa variety of questions in heterogeneous cell populations. We demonstrate antigen-specific upregulation of IFNγ and IL-2 mRNAs in HIV- and CMV-specific T cells. We show simultaneous detection of cytokine mRNA and corresponding protein in single cells. We apply this method to detect mRNAs for which flow antibodies against the corresponding proteins are poor or are not available. We use this technique to show modulation of a microRNA critical for T cell function, miR-155. We adapt this assay for simultaneous detection of mRNA and proteins by Image Stream technology.
Introduction
Single cell analysis is a rapidly growing field in biomedical research that yields an unprecedented level of information1,2. Flow cytometry has been the gold standard for high-throughput phenotypic and functional characterization of various cell types. While the major advantages of flow cytometry includea simple workflow using fluorescently labeled antibodies for the detection of intracellular or surface markers and high throughput, this technology is limited by availability of highly specific and sensitive reagents for antigens.
Advances in molecular biology have enabled techniques such as microarrays, quantitative PCR and RNA sequencing3–5. These technologies are sequence-based and not constrained by antibody availability, providing “unrestricted” tools for in-depth analysis of transcriptional signatures that define cell functions in physiologic or disease states. The major drawback of most of these approaches is the generation of transcriptional signatures in bulk populations of cells, which may provide insufficient and/or spurious information on the biologic entity of rare subsets within heterogeneous populations6. More recently, new methods such as microfluidic platforms and adaptations of RNAseq have been successfully used for single cell transcriptional analysis7,8. However, for these techniques, single cells must to be first live-sorted and the laborious methods required to isolate and amplify RNA can result in a significant loss of RNA species9.
Fluorescent in situ hybridization (FISH) is another method used for the detection of specific nucleic acid sequences at the single cell level10. It is based on fluorescently labeled probes and has been widely used in fluorescence microscopy to detect RNA or DNA sequences in cells adhered to coverslips or tissues11. Even though this powerful imaging technique enables detection of down to a single copy of a given nucleic acid sequence12 and simultaneous analysis of intracellular structures and localization, it has so far been poorly adaptable to flow cytometry. Flow cytometric identification of RNA species would have several major comparative advantages. Whereas microscopy is practically limited to analysis of a few hundred cells, flow cytometry allows high-throughput acquisition of up to millions of cells in a single sample and combination of larger varieties of fluorescent channels; acquisition of 10 to 15 fluorescent markers are now routinely performed on 4- or 5-laser cytometers.
Several adaptations of microscopy methods have been attempted in the past to detect FISH signals with flow cytometry13–15. However, they were limited to nucleic acid sequences with abundant expression, such as viral RNAs after acute infection14. More recently, another study16 achieved detection of lower levels of RNA and oncogene expression in cell lines, and identified viral genes (i.e. HIV) after in vitro infection of primary samples. Another limitation of previous experimental approaches is the inability to combine mRNA detection with antibody staining of proteins, a critical tool to define specific cell subsets in mixed populations.
Here we describe a novel flow-FISH method for high-throughput detection of mRNA and miRNA. This method is derived from the QuantiGene View RNA FISH cell assay (RNA-FISH) that was developed by Affymetrix for microscopy17. We show here that new protocols developed in our laboratory, when used in combination with this technique, enable simultaneous detection of several mRNA molecules in various leukocyte subsets from human blood identified by antibody staining of cell surface markers. We demonstrate sensitive and specific detection of cytokines such as IFNγ and IL-2 mRNA of HIV- and CMV-specific CD4 and CD8 T cells. We confirm specificity of cytokine mRNA detection by simultaneous IFNγ mRNA-FISH and IFNγ protein secretion capture assays. We highlight the ability of this assay to overcome the limitation of antibody availability by excellent detection of IL-21, a cytokine for which intracellular cytokine staining (ICS) with a fluorescent antibody is not sensitive enough to allow adequate quantitation. Furthermore, we use this assay to detect expression of Indoleamine 2,3-dioxygenase (IDO), an enzyme for which no flow cytometry antibody was available at the time these experiments were performed. Similarly, we show that this technique can be readily used for detection of small miRNA molecules that play major regulatory roles in eukaryotic cells without being translated into proteins. Finally, we prove that this approach can be used in combination with Image Stream technology, allowing for high-throughput subcellular compartmentalization studies of mRNA and protein co-expression. Taken together, these results show the power and high versatility of this novel technology to address biomolecular mechanisms in heterogeneous cell populations.
Results
Detection of mRNA and antibody staining by flow cytometry
One of the major limitations of typical FISH protocols is the variable requirement in hybridization times and conditions for different probe sets. In contrast, a specific algorithm is used for the QuantiGene View RNA method that allows for the design of probes that exhibit high sensitivity and specificity as well as the same hybridization temperature. This provides greater reproducibility between different probes and experimental designs and facilitates assay multiplexing. A prerequisite for the use of this technology in flow cytometry is the availability of hybridization and staining reagents compatible with cells in suspension. Initial testing revealed that cell buoyancy and cell lysis were major issues with the buffers developed for adherent cells and fluorescent microscopy, resulting in massive subsequent cell loss (Supplementary Fig. 1). Even though we were still able to reliably detect 18s ribosomal RNA with flow cytometry, such a large cell loss, along with the incompatibility between the initial FISH protocol and antibody staining, made this protocol unfavorable for analysis of primary cells in general and rare subpopulations, such as antigen-specific T cells.
Through a collaborative effort, we therefore developed alternative methods to process non-adherent cells. The cells were fixed with a methanol-based reagent before permeabilization. This new protocol was less harsh to the cells, leading to reduced cell loss and preservation of the main morphologic characteristics as indicated by forward and side scatter (Fig. 1a, b). We next performed head-to-head comparison of antibody staining using a standard surface-stain flow cytometry protocol or the new flow-RNA assay. To this purpose, we selected fluorochromes resistant to methanol fixation18 that stained for surface markers identifying main peripheral blood mononuclear cell (PBMC) subsets. In our hands, Brilliant Violet, Alexa and eFluorsdyes perform particularly well. We assessed the respective ability of specific antibody clones to recognize epitopes that withstand the procedures. As previously reported, the potential usability of an antibody depends on both the protein targeted and the specific clone. Although several manufacturers provide useful references as to which reagents are suitable with methanol-based flow protocols, in our experience this use still requires careful assessment by the investigator for each specific mRNA flow-FISH application. Once we had identified appropriate combinations, surface staining for PBMC subsets combined with the Flow RNA assay gave excellent results, with definition of cell populations very similar to a standard flow cytometry protocol (Fig. 1a,b). Furthermore, the new buffer used is compatible with dead cell viability dyes (Fig. 1b).
Figure 1. Antibody staining and detection of housekeeping genes.
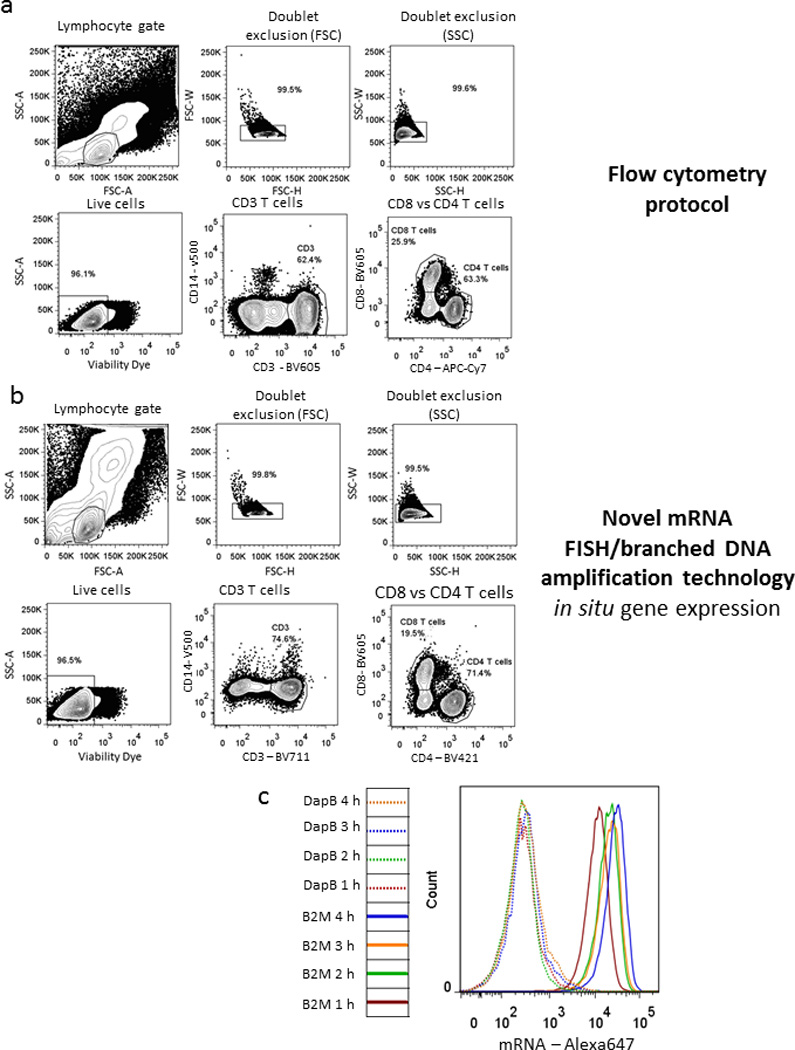
Comparison of physical and phenotypic characteristics of PBMCs stained with a dead cell dye and fluorescent antibodies for CD3, CD4, CD8 and CD14 using either (a) a standard surface stain flow cytometry protocol or (b) the new flow-RNA assay (representative out of 5). (c) Kinetic experiment of different hybridization incubation times following staining with either an irrelevant probe DapB or the housekeeping gene β2M (representative example out of 3).
The mRNA probes have optimal hybridization temperature of about 40°C. In order to identify the hybridization time that confers optimal signal-to-noise ratio for detection, we performed a kinetics experiment using either an irrelevant probe towards DapB (dihydopicolinate reductase of Bacillus Subtilis) or a probe specific for the housekeeping mRNAβ2-microglobulin (β2M). A plateau was reached between the 2- and 4-hour hybridization times (Fig. 1c), with excellent signal-to-noise ratio. We thus selected the 2-hour hybridization step for subsequent experiments. A schematic representation of the workflow can be found in Fig. 2.
Figure 2. Schematic representation of Flow-FISH methodology.
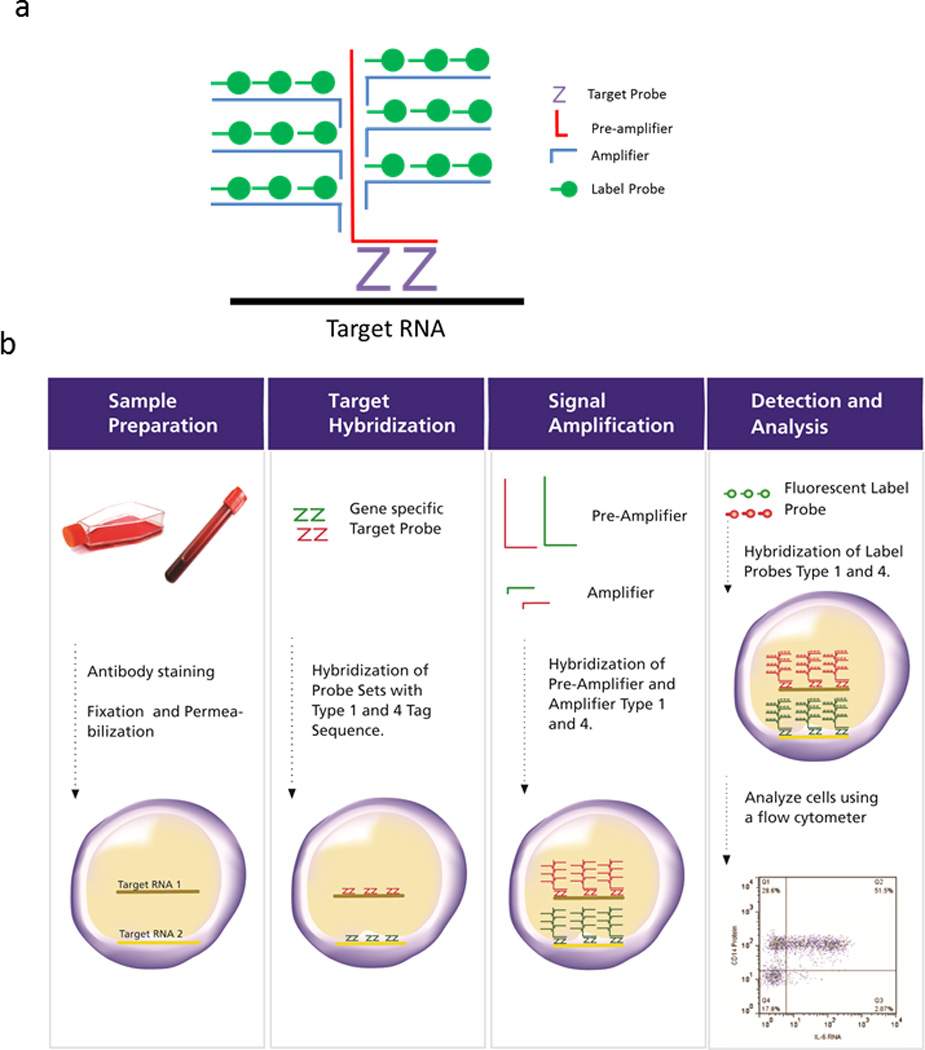
(a)Structure of target probe hybridization with the amplifying brunch DNA sequences and the label DNA probes. (b) Diagram representing the workflow of the flow-FISH assay
The sensitivity of the assay varies depending on the probes used (as different probes have different affinities) and the target sequence of interest (small RNA sequences are targeted by fewer probes resulting in potentially weaker signal). Based on our preliminary data shown in Supplementary Fig. 2 we can confidently detect about 10 mRNA copies per cell for the housekeeping gene HMBS (hydroxymethylbilane synthase). It is recommended that users should perform their own validation to identify the sensitivity of the assay based on the target of interest, the cell type, and the specific experimental design. Sequences of the probes used in our studies are detailed in Supplementary Table 1.
Detection of cytokine-producing antigen-specific T cells
In order to investigate the ability of this assay to detect antigen-specific T cell responses after stimulation with cognate antigen, we examined blood samples from HIV-infected subjects. PBMC were stimulated overnight with a HIV-Gag peptide pool, CMV lysate or PMA/Ionomycin, or left unstimulated. We then performed the flow-RNA assay to detect mRNA levels of IL-2 and IFNγin the CD4 and CD8 T cell subsets identified by the relevant surface markers. Results demonstrate that we can accurately identify cytokine mRNA-producing virus-specific CD4 T cells (Fig. 3a–d) and CD8 T cells (Supplementary Fig 2). In contrast to regular ICS where addition of Brefeldin and/or Monensin traps cytokines inside the cell for 6 to 12 hours, leading to accumulation of proteins produced over the duration of incubation, here we detect an accurate “snapshot” of mRNA expression at the specific time when the cells are fixed. Transcription and translation kinetics will also vary. Therefore, percentages of cytokine-producing cells after stimulation can differ between ICS and flow-mRNA assays. In spite of these differences, there was overall a good correlation between results when transcription (mRNA flow-FISH) and translation (ICS) of IFN-γ and IL-2 were examined in parallel (Supplementary Fig.4). The absence of cytokine secretion blockade in the flow-FISH approach is a significant advantage over standard ICS when cell-cell interactions are examined, as many cytokines work in autocrine and paracrine fashion to modulate activation signals.
Figure 3. IL-2 and IFNγ mRNA detection in antigen-specific CD4 T cells.
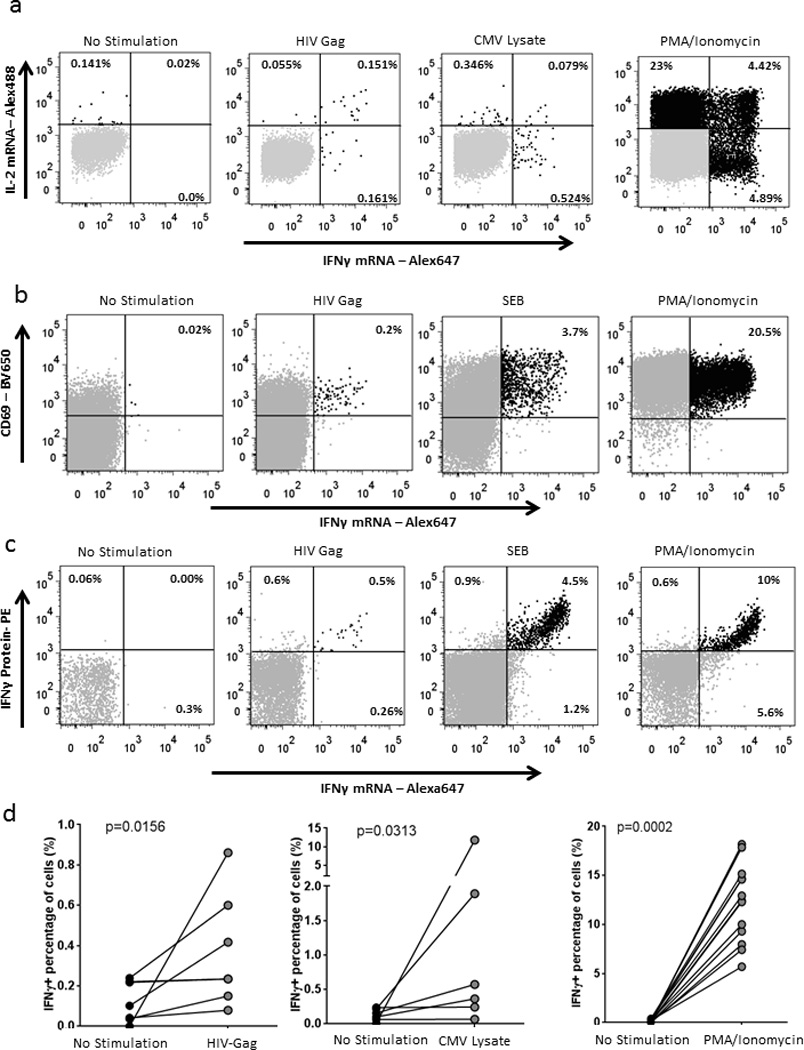
(a) IL-2 and IFN-γ mRNA detection in CD4 T cells from PBMCs of an HIV-infected individual stimulated with a HIV-Gag peptide pool, CMV lysate or PMA/Ionomycin for 12 hours (one representative experiment out of five). (b) IFN-γ mRNA detection in combination with CD69 antibody staining from PBMCs of an HIV-infected subject left incubated with medium alone or stimulated with an HIV-Gag peptide pool, SEB or PMA/Ionomycin for 12 hours. (c) Combined detection of IFN-γ protein, using a 45 minute capture assay, and IFN-γmRNA in CD4 T cells from PBMCs of an HIV-infected person incubated with medium alone or stimulated with an HIV-Gag peptide pool, SEB or PMA/Ionomycin for 12 hours (one representative experiment out of five). (d) Collective data of IFN-γ mRNA detection after HIV-Gag peptide pool, CMV lysate or PMA/Ionomycin stimulation. Statistical analysis was performed using a Wilcoxon matched-pairs test.
In order to prove that the detection of mRNA is specific to activated cells, we utilized two different approaches. We first used an antibody against CD69, a surface molecule upregulated on most T cells upon stimulation, in combination with mRNA detection. The large majority of IFN-γ mRNA+ cells were also CD69+, indicating specific detection of mRNA in the activated population (Fig. 3b). We next performed the flow-RNA assay in combination with a cytokine capture assay (Miltenyi Biotec, used per manufacturer’s instructions) that allows trapping of the molecule of interest by a primary antibody coated on the cell surface, followed by detection with a secondary fluorescent-labeled antibody. The IFN-γ capture phase lasts for 45 minutes and therefore stains cells that have secreted IFN-γ within this time frame. Fig. 3c shows combined detection of IFN-γ protein by IFN-γ capture and mRNA production in HIV-specific T cells stimulated with HIV Gag or T cells stimulated with Staphylococcal enterotoxin B (SEB) or Phorbol 12-myristate 13-acetate (PMA). These data show overall very good consistency, however after SEB and PMA/Ionomycin stimulation we observed a small percentage of cells harboring IFNγ mRNA but on which no IFN-γ protein was detected by the capture assay: about 20% of the CD4 T cells expressing IFNγ mRNA upon SEB stimulation and 35% of the CD4 T cells expressing IFNγ mRNA upon PMA stimulation were negative by IFNγ capture. This difference may be due to both a difference in sensitivity as well as different in kinetics with a delay between transcription and translation. These data show that the flow-RNA assay is sensitive enough to detect antigen-specific T cells and can be used to detect cytokine transcription and translation in the same cells, which can be very useful for studies of functional regulation of immune cells.
Sensitive detection of molecules with poor antibody staining
One of the major limitations of antibody-based flow cytometry is that although thousands of suitable antibodies are commercially available, these still do not cover the full panel of molecules of interest present within a cell. Moreover, antibodies have variable sensitivities and often do not perform well at detecting weakly expressed antigens. Flow-RNA offers the potential to stain for a much more diverse selection of molecules. In order to test this, we first decided to stain for the cytokine IL-21, as the low sensitivity of its detection by ICS has been a hurdle for many investigators. IL-21 is a cytokine critical for CD4 T cell help to B cells and CD8 T cells, but is also implicated in several autoimmune diseases19, including idiopathic thrombocytopenic purpura (ITP). ITP is characterized by abnormal production of antibodies that attack platelets. Theseauto-antibodies have been linked to abnormal T follicular helper cells that produce increased amounts of IL-2120. We thus decided to test the ability of the mRNA FISH assay to detect IL-21 compared to ICS in spleen mononuclear cell samples from two subjects that had undergone splenectomy for ITP refractory to medical treatment and from one individual who had surgery for traumatic splenic rupture. Despite maximal stimulation with PMA/Ionomycin, ICS assays on splenocytes detected only small levels of IL-21 protein in all three subjects investigated (Fig. 4), whereas robust production of IL-2 was identified. In contrast to ICS, them RNA flow-FISH assay was able to detect a much greater percentage of IL-21-secreting cells after PMA/Ionomycin stimulation, whereas the fraction of IL-2-secreting CD4 T cells was overall similar with the two techniques. Interestingly, this massive production of IL-21 was present in the two ITP subjects (Fig. 4a,b) but very low in the control sample (Fig 4c). Detection of IL-21 mRNA was restricted to CD4 T cells and not CD8 T cells (Supplementary Fig. 5), consistent with the known role of CD4 T cells as the main producers of IL-21. We were able to simultaneously use three different probes to detect mRNA levels of IL-2 (Alexa 488), IL-21(Alexa 647) and IFN-γ (Alexa 750) in CD4 T cells from these patients (Supplementary Fig. 6), allowing for detailed investigation of co-expression patterns at the single cell level.
Figure 4. Detection of IL-21 mRNA in CD4 T cells.
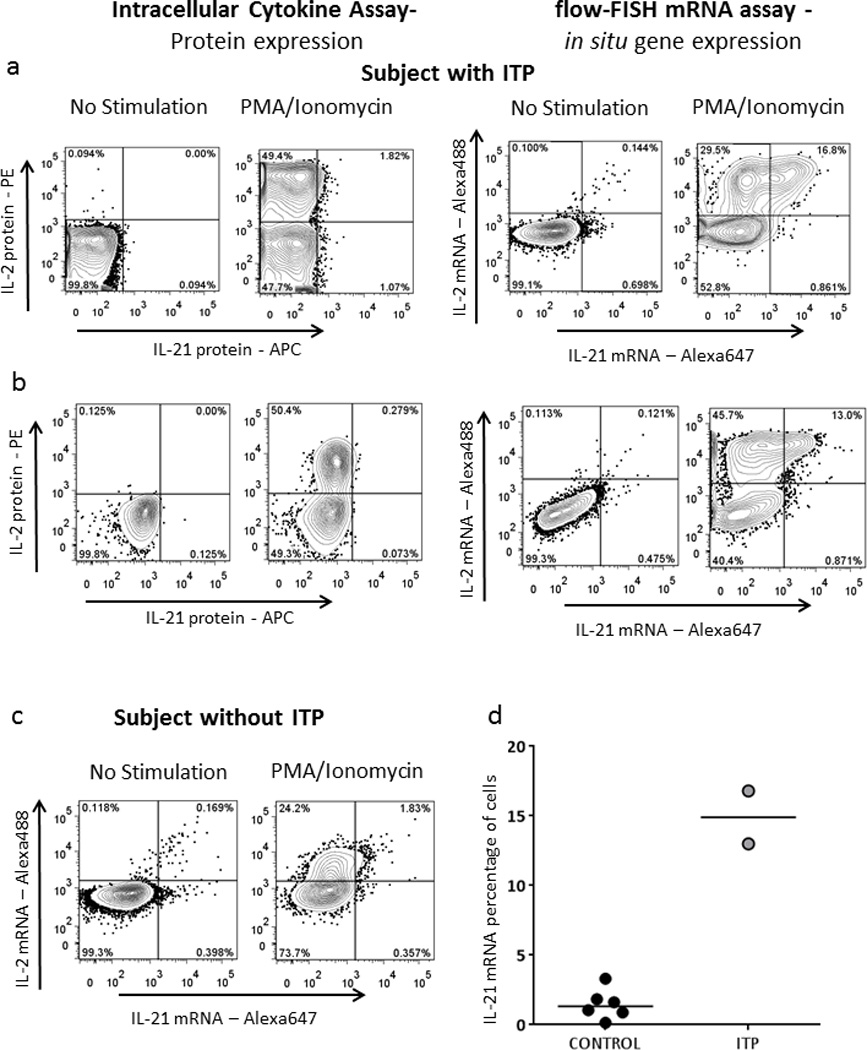
Lymphocytes isolated from a spleen sample of two subjects suffering from idiopathic thrombocytopenic purpura (ITP) (a, b) and one subject who underwent surgery for traumatic splenic rupture (c). Cells were incubated with culture medium alone or stimulated overnight with PMA/ionomycin, with addition of brefeld in and monensin only for the ICS assay. Following incubation, cells were stained either following a standard ICS protocol or with the new flow-RNA FISH protocol for dead cell dye, surface lineage markers and either cytokine-specific antibodies or cytokine-specific probe sets. (d) Collective data on IL-21 mRNA expression after PMA/Ionomycin stimulation in CD4 T cells from 6 negative control and 2 ITP subjects.
We next decided to investigate expression of an enzyme for which there was no commercially available antibody for flow cytometry at the time that this experiment was performed, Indoleamine 2,3-dioxygenase (IDO). An antibody detecting intracellular IDO became recently available (eBioscience). IDO is critical for metabolism of tryptophan, an essential amino acid. Catabolism of tryptophan by IDO results in an immunosuppressive environment that has been implicated in the pathogenesis of cancer, autoimmunity and chronic infections21, 22. Stimulation of PBMCs with the TLR7/8 agonist ligand R848 or with combination of IFN-γ and LPS for 8 hours led to robust upregulation of IDO mRNA in monocytes (Fig. 5a,b). We also found a strong correlation between IDO mRNA expression in CD14+ cells by flow-FISH and the relative mRNA levels as measured by qRT-PCR (Fig. 5c), These data show the unique capacity of the flow-FISH assay to detect mRNA expression of molecules that cannot be detected with antibody-based flow cytometry.
Figure 5. Detection of Indoleamine 2,3-dioxygenase (IDO) in monocytes.
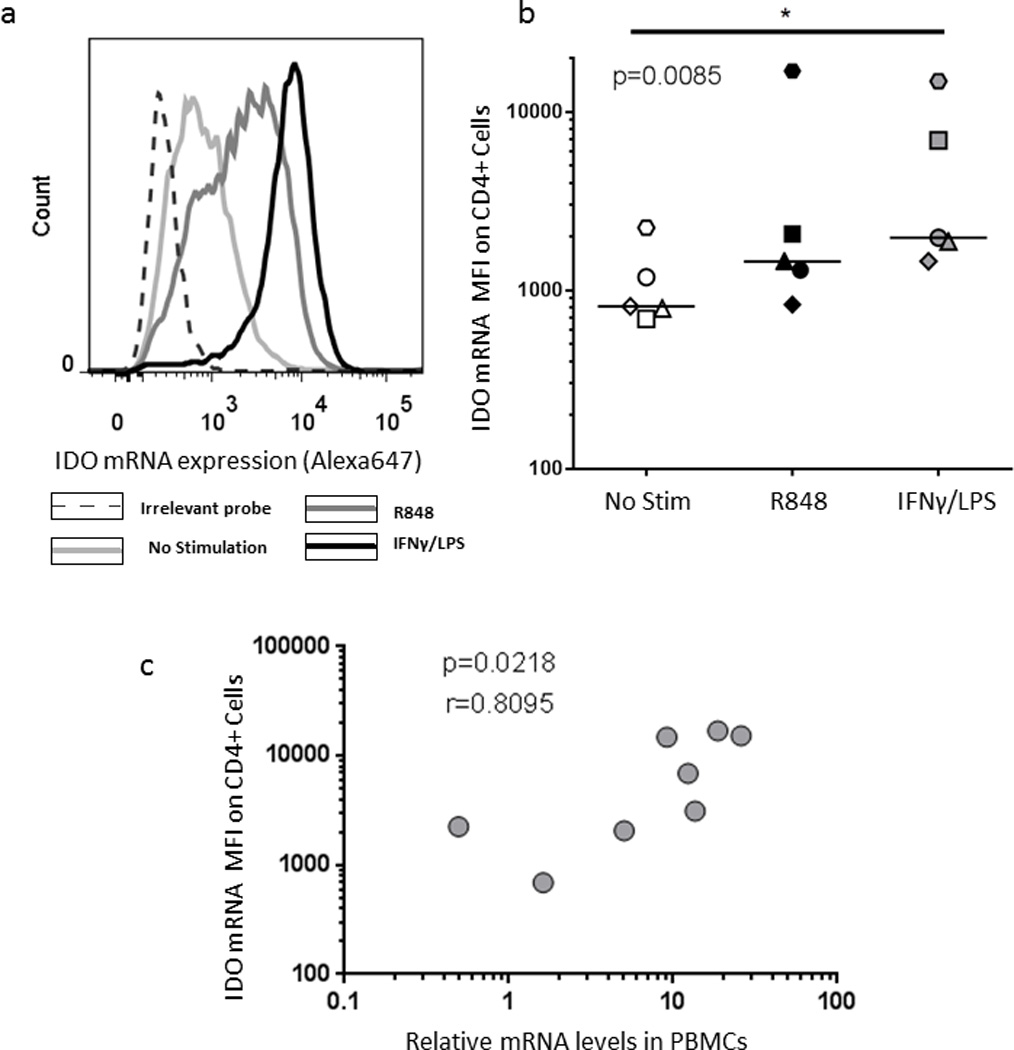
PBMCs from an HIV negative subject were stimulated for 8 hours with either R848 or IFNγ + LPS. IDO mRNA detection in monocytes was achieved after gating on the CD14 positive cell subset within the monocyte gate. (a) Representative example and (b) collective data on five different subjects. Statistical analysis used Friedman test with a Dunn’s post test comparison. (c) Correlation between IDO mRNA mean fluorescent intensity (MFI) in CD14+ cells and IDO relative mRNA expression as measured by qPCR in PBMCs from the same experiment. Statistical analysis used the Spearman rank sum test.
miRNA detection in primary T cells by flow cytometry
We next utilized this technique to detect miRNA expression in primary T cells after PMA/ionomycin stimulation. miRNAs are small non-coding RNAs that control gene expression at the post-transcriptional level and play a critical role in regulating cell functions. Here we used flow-FISH to detect two miRNA targets, miR-21 and miR-155 that have been shown to control immune responses23, 24. Stimulation of CD4 and CD8 T cells for 12 hours did not significantly enhance miR-21 expression but led to increased expression of miR-155 (Fig. 6). This is consistent with the literature that showed miR-155 to be one of the few miRNA up-regulated in T cells after stimulation while miR-21 is not significantly enhanced25. miRNA expression was measured in activated CD69+ CD4 (Fig. 6d) and CD8 T cells (Fig. 6c) proving the ability of flow-RNA to provide insight into single-cell co-expression of miRNAs in combination with mRNAs molecules and proteins of interest. These data show the unprecedented capabilities of this novel method to also quantify small non-coding miRNA molecules that act as critical regulators of mRNA translation.
Figure 6. miRNA detection in CD4 and CD8 T cells with flow cytometry.
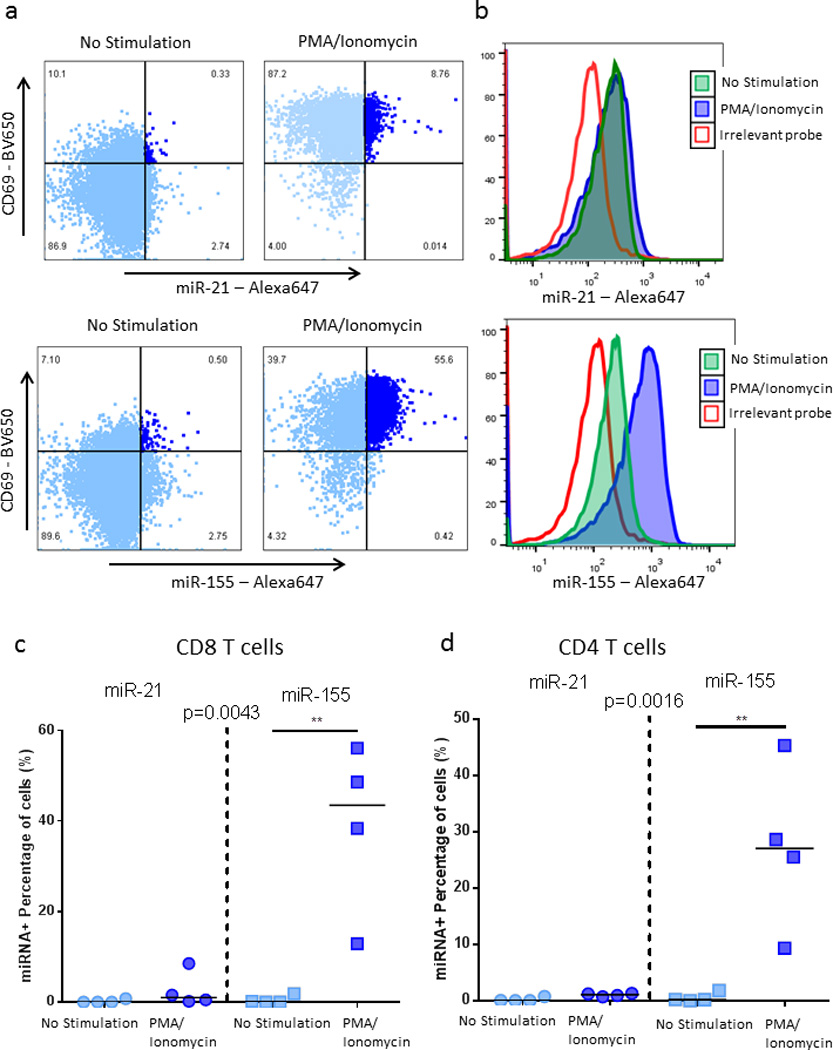
PBMCs from HIV-negative subjects were left unstimulated or were stimulated overnight with PMA/Ionomycin. (a, b) Representative example of miR-21 and miR-155 expression in CD8 T cells. (c, d)Collective data on four subjects for the detection of miR-21 and miR-155 in CD69+ activated CD8 and CD4 T cells. Statistical analysis used Friedman test with a Dunn’s post test comparison.
Combined mRNA and protein visualization using Image Stream
One of the disadvantages of any traditional flow cytometry technique compared to microscopy is that one cannot visualize subcellular localization of molecules. The newly developed ImageStream technology (Amnis) combines the advantages of high-throughput flow stream analyses with microscopy capabilities. Although not reaching the spatial definition of standard fluorescent microscopy, this platform allows much easier acquisition of statistically significant cell numbers in heterogeneous populations. Developing combined detection of multiple mRNAs and proteins with this technology thus has the potential of considerably expanding the versatility of its applications. In order to assess the applicability of the flow-RNA protocol to the ImageStream platform, we again used a cytokine protein capture assay combined with cytokine mRNA quantitation. We were able to simultaneously detect IFN-γ protein and IFN-γ mRNA in blood CD4 T cells from a healthy subject (Fig.7). These data show the potential application of flow-RNA to perform ImageStream experiments for high-throughput co-vizualization of mRNA, miRNA and protein at the single cell level and briefly demonstrate the potential for flow-FISH in colocalization studies. This approach may be applied to investigations of cellular localization in protein-mRNA or miRNA-target mRNA interaction studies.
Figure 7. Combined detection of IFNγ protein and mRNA with ImageStream.
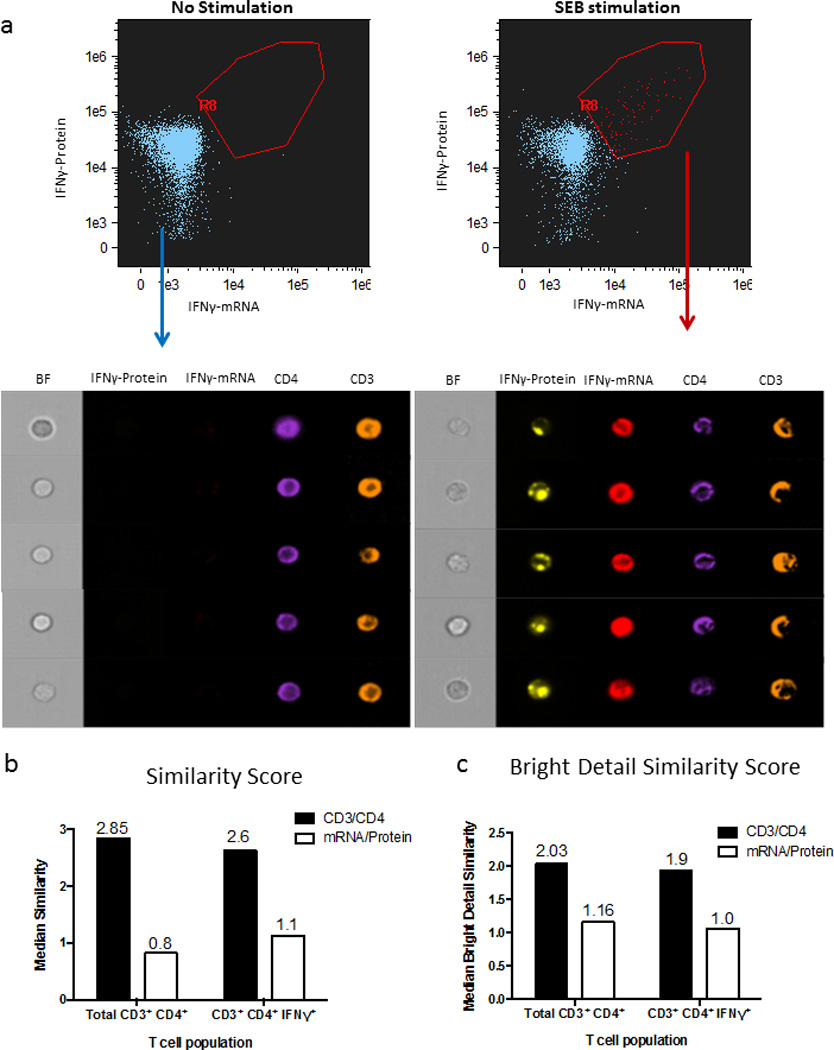
(a) PBMCs from an HIV-negative subject were left unstimulated or stimulated with SEB for 12 hours. At the end of this incubation time, a 45-minute capture assay was performed for detection of IFNγ-protein, followed by IFN-γmRNA staining with the flow-FISH assay. CD4 T cells were then acquired on an ImageStream instrument. Images illustrate expression of IFN-γ protein, IFN-γ mRNA, CD4 and CD3 in the lymphocyte gate in unstimulated cells (left panels) and in stimulated cells (right panels).(b–c) Co-localization analysis for CD3/CD4 in black and IFNg protein/mRNA in white using two different analytical tools (b) Similarity score (the log transformed Pearson’s correlation coefficient, measuring the spatial linear correlation between two total stains within a cell mask) and (c) Bright Detail Similarity score (the log transformed Pearson’s correlation coefficient, measuring the spatial linear correlation between the only the bright regions of two stains within a cell mask). We found a low correlation between secreted IFNg protein and internal mRNA using both measures (median score ~1). In comparison, surface stained CD3 and CD4 were well correlated (median score ~2).
Discussion
Fluorescent in situ hybridization (FISH) enables detection of nucleic acid sequences at the single cell level. Until now, this powerful technology has been largely limited to fluorescent microscopy imaging, thus limiting its use for analysis of heterogeneous cell populations, which require acquisition of large numbers of events. Even though a number of attempts have been made to adapt this method to flow cytometry, they overall showed reduced sensitivity, often relatively poor specificity, time-consuming experimental design, and lack of versatility with different nucleic acid targets requiring individualized protocols. Critically, they did not allow simultaneous detection of multiple targets in the same sample, and were hampered by the lack of compatibility between RNA-FISH and antibody staining for cell surface markers. Here we show the application of a novel, powerful RNA-FISH technology in various optimized flow cytometry protocols that overcome several significant hurdles toward the study of cell function, gene expression, and transcriptional regulation in heterogeneous cell samples.
The approach presented here is derived from reagents initially developed for fluorescent microscopy (QuantiGene View RNA) that have been successfully used to detect DNA, mRNA and miRNAs sequences17. Direct application of the QuantiGene View RNA protocol to PBMC samples was accompanied by severe cell loss. Furthermore, addition of protease, which enhances accessibility of probes to their target, resulted in poor antibody staining for cell surface markers, probably because of alteration in antigen conformation.
We therefore made use of a new protocol using a methanol-based permeabilization step. We show here that this procedure is compatible with dead cell dyes and, critically, antibody staining for a variety of cell surface proteins. The depth of data provided is thus similar to standard polychromatic flow cytometry. Reagents currently available allow multiplexing for three different sets of probes for the fluorochromes Alexa 488, 647 and 750. The method is compatible with a wide array of small molecule dyes such as Brilliant Violet, Alexa dyes and eFluors, but not with protein-based fluorescent dyes, such as PE, PerCP and APC, and their tandem dye conjugates. Methanol is also known to denature some epitopes, such that some monoclonal antibodies are necessarily incompatible with this method.
After having optimized hybridization times and combination of mRNA probes, surface antibody staining and dead cell dye, we applied the protocol to PMBCs isolated from HIV-infected individuals. We were able to accurately identify CD4 and CD8 T cells and to detect mRNA expression for the cytokines IFN-γ and IL-2 by HIV and CMV-specific T cells after cognate antigen stimulation, with sensitivity comparable to standard ICS for the corresponding cytokine proteins. This mRNA upregulation was exclusively observed within CD69+ cells, confirming that it occurred upon activation. In order to compare transcription and translation levels of the same cytokine, we were next able to combine an IFN-γ capture assay that traps secreted proteins on the cell surface with mRNA detection. Besides further confirming the validity of the assay, we believe that this combined assay opens up interesting possibilities to study regulation of cytokine or chemokine production in mixed populations of immune cells. Depending on the question to be addressed, combination with the capture assay can also overcome one current limitation of the mRNA-FISH assay, which is its incompatibility with intracellular staining for proteins. Technically, this limitation can probably be overcome by modification of the fixation and permeabilization procedures, but this will require further investigation.
A major strength of this assay is the potential to detect many mRNAs of interest without being limited by antibody availability. This has great applicability for studies on animal species for which antibody availability for flow cytometry is limited. All FISH probes are derived by an algorithm that enables them to have similar hybridization temperatures and therefore allows combination of different probes and reproducibility between different assays. We were able to detect a combination of three different probes for the cytokines IL-2, IL-21 and IFN-γ in the same sample, highlighting the unique multiplexing ability of this method. Besides the large panel of ready-made probes available, custom-designed probes can also be ordered for the gene of interest.
We also assessed the ability of the flow-RNA assay to detect expression of mRNAs for which detection with antibody is either poor or not available. Detection of IL-21 mRNA was clearly superior to identification of IL-21 protein by standard ICS assay. The potential significance of this finding is illustrated by our results on spleen samples from two subjects with ITP, an autoimmune disease in which IL-21 is known to play a role. Whereas ICS revealed little, we found a marked upregulation of IL-21 transcription in CD4+ splenocytes upon stimulation in these patients, contrasting with low induction in a subject with no underlying autoimmune disorder. This technique can thus be used to define altered gene expression patterns in primary cells in the context of human diseases. We could also clearly detect up-regulation of transcription of the immunomodulating enzyme IDO in monocytes after stimulation with TLR agonists, whereas no antibody was commercially available to detect IDO by flow cytometry at the time of these experiments.
Recently, it has become increasingly clear that non-coding RNAs, including microRNA (miRNAs) and long non-coding RNAs, play broad and critical regulatory roles in cell regulation, including immune responses. We thus also investigated the ability of mRNA flow-FISH to detect such nucleic acids sequences for which there is no protein translation. miRNAs control gene expression at the post-transcriptional level by translational repression or enhanced target degradation. In spite of the short sequence of miRNAs (around 22 nucleotides), we successfully detected miR-155 modulation in CD4 and CD8 T cells after stimulation. The implication of miRNAs in many diseases makes them attractive targets for immunotherapeutic interventions and biomarker development for cancer and autoimmune diseases26,27. Our proof-of-principle experiments indicate the unprecedented capabilities of this assay to simultaneously detect expression of miRNA, mRNA and protein at the single cell level, providing a tool for in-depth analysis of miRNA function in combination with their translational and expressional effects in both normal and pathologic conditions.
As flow cytometry does not show localization of protein expression within cells, we finally explored the potential of the flow-RNA assay to be used with ImageStream technology. We successfully detected IFN-γ mRNA and protein levels on CD4 T cells after SEB stimulation, with excellent signal/noise ratios for the different parameters measured. Thus this assay can be used with this novel instrument combining some of the advantages of both polychromatic flow cytometry and fluorescent microscopy, including compartmentalization studies.
In conclusion, this novel mRNA/miRNA flow-FISH assay presents major advantages over previous technologies including: an algorithm-based controlled design of probes that allows reproducibility and combination of various probes with similar hybridization conditions; the straightforward combination with fluorescent antibody staining that allows simultaneous phenotyping of heterogeneous cell populations; the design of probes for molecules for which no antibody is available; the user-friendly protocol that has many similarities with standard antibody-staining procedures for regular flow cytometry; and the processing and data acquisition with standard laboratory equipment and cytometers. Our data illustrate the versatility of this technique and its broad applicability for analysis of normal cell functions and disease states.
Methods
Human Subjects
Peripheral blood was obtained from HIV-infected and uninfected individuals at the Massachusetts General Hospital (MGH), Boston. The study was approved by the MGH Institutional Review Board and written informed consent was obtained from all study participants prior to enrollment in the study. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density centrifugation. Frozen PBMCs were thawed and were cultured in RPMI 1640 medium (Invitrogen) supplemented with HEPES, penicillin, streptomycin and 10% human AB serum (Gemini Bioproducts) (R10 medium).
Non-identifiable excess spleen tissue was obtained at Massachusetts General Hospital under approval by the Partners Human Resource Committee. These surgeries were performed for medical or surgical indications not related to hematologic or autoimmune diseases, with the exception of two patients who underwent splenectomy for refractory idiopathic thrombocytopenic purpura (ITP), which is currently an uncommon indication for such an intervention. Spleens were dissected into small fragments and subsequently mechanically disrupted to single cell suspension using a 70µm cell strainer and a syringe plunger. Following washing in R10 medium containing 2mM EDTA, cells were strained once more and erythrocytes were lysed using RBC lysis buffer (Qiagen). Cells were immediately cryopreserved for subsequent use.
Cell stimulation
Cells were resuspended in 500uL R10 medium before being stimulated with an HIV-Gag peptide pool (2µg/mL/peptide), CMV lysate (1µg/mL), SEB (1µg/mL), PMA/Ionomycin (1×, Cell Stimulation Cocktail, eBioscience), TLR 7/8 agonist R848(10ng/ml) or recombinant IFNγ(100ng/ml) + LPS(10ng/ml).
Flow-FISH method
PBMCs were stained with viability dye (Invitrogen LIVE/DEAD) for 30 minutes at room temperature. Surface antibodies were then added to stain for cell populations of interest [BV711 CD3(5 µl per 100 µl of sample, clone: OKT3), BV421 CD4(5 µl per 100 µl of sample, clone: OKT4), V500 CD8 (5ul per 100 µl of sample, clone: SK1) and BV605 CD14(5 µl per 100 µl of sample, clone: M5E2)]. Assay was performed as per manufacturer’s instructions (Affymetrix, Inc., http://media.ebioscience.com/data/pdf/literature/quantigene-flowrna-user-manual.pdf). Briefly, PBMCs were fixed with a 4% paraformaldehyde solution for 1 hour at room temperature. Cells were then permeabilized with ice-cold methanol, followed by further fixation with a 4% paraformaldehyde solution for 30 minutes at room temperature. All buffers used in the following steps were included in the manufacturer’s kit. To detect mRNA, probes comprised of ~20 oligonucleotide base pairs against the mRNA of interest were used. Currently, there are three different types of probes with unique tag sequences, named type 1, type 4 and type 6, respectively. Each tag sequence allows the hybridization of specific complementary branched DNA nanostructure with different excitation/emission spectra: type 1 – 650/668, type 4 – 495/519 and type 6 – 749/775. Probes were diluted 1:20 in target probe diluent, added to the cell suspension and allowed to hybridize to the target RNA for 2 hours at 40°C. Excess probe was removed by adding wash buffer followed by centrifugation at 800×G for 5 minutes. Signal amplification was achieved by performing sequential hybridization with DNA branches (i.e. Pre-Amplifier and Amplifier). The first DNA branch in the PreAmp Mix was added at a 1:1 ratio and was allowed to hybridize for 1.5 hours at 40°C. Samples were washed with wash buffer, and then the second DNA branch in the Amp Mix was added at a 1:1 ratio and hybridized for 1.5 hours at 40°C. Samples were washed with wash buffer before addition of the Label Probes. For signal detection, fluorochrome-conjugated Label Probes were diluted 1:100 in Label Probe diluent, added to the samples and allowed to hybridize for 1 hour at 40°C. Finally, PMBCs were washed with wash buffer, transferred to the storage buffer and subsequently analyzed on a LSR Fortessa, using FACS DIVA software version 7(BD Biosciences, San Jose CA). Flow cytometric data were analyzed with Flow Jo Version 7.5.5 (TreeStar).As negative controls we used probes that target irrelevant sequences for dihydrodipicolinate reductase/dapB gene of Bacillus Subtilis that should not be encountered in human samples. For the experiments where alterations of mRNA levels were investigated after stimulation the gates were set according to the unstimulated control.
Optimization of target probe hybridization time
In order to determine the optimal hybridization time in terms of the signal to noise ratio, we varied the target probe hybridization times from 1 to 4 hours. Both a negative control probe, dapB (Bascillus subtillus), and a probe for housekeeping gene B2M (type 1 probe) were used.
mRNA detection in activated CD4 T cells
PBMCs were either left unstimulated or were stimulated with HIV Gag, SEB or PMA/Ionomycin overnight (16 hours). The next day, cells were stained with viability dye and a panel of surface antibodies [(CD3-BV711 (5 µl per 100 µl of sample, clone: OKT3), CD4-BV421(5 µl per 100 µl of sample, clone: OKT4), CD8-BV605 (5 µl per 100 µl of sample, clone: RPA-T8), CD14-V500 (5 µl per 100 µl of sample, clone: M5E2 and CD69-BV650(5 µl per 100 µl of sample, clone: FN50)]. The flow-FISH protocol was followed as outlined above, and IFNγ mRNA type 4 probes were detected in activated CD4 T cells (CD4+/CD69+) on the LSR Fortessa (Figure 2b).
For detection of IL-21 CD4 T cells from spleen samples of subjects with idiopathic thrombocytopenic purpura (ITP) we stimulated cells for 6 hours with phorbol 12-myristate 13-acetate (PMA) /Ionomycin. The flow-FISH protocol was followed as outlined above, and IL-2 (type 4 probes), IL-21 (type 1 probes) and IFNγ (type 6 probes) mRNA on CD4 and CD8 T cells were measured on a BD Fortessa (Figure 3). We also performed intracellular cytokine staining from the same samples in order to compare IL-21 detection between ICS and the new flow-FISH protocol. Briefly, cells were stimulated with PMA/Ionomycin for 6 hours in the presence of Monensin and Brefeldin A (5µg/mL). Subsequently cells were stained with blue viability dye (Invitrogen Life Technologies) and fluorescent antibodies against CD3 (5 µl per 100 µl of sample, clone: OKT3), CD4(5 µl per 100 µl of sample, clone: OKT4), CD8 (5ul per 100 µl of sample, clone: SK1), CD14 (5 µl per 100 µl of sample, clone: M5E2) and CD19 (5 µl per 100 µl of sample, clone: HIB19). Intracellular staining for IFNγ, IL-2 and IL-21 was performed using BD Cytofix/Cytoperm Fixation/Permeabilization Solution according to manufacturer’s instructions. Cells were then analyzed on the LSR Fortessa.
Simultaneous detection of cytokine mRNA and protein
In order to determine whether the mRNA we were detecting correlated with the cells producing the protein, PBMCs were stimulated overnight with either HIV-Gag peptide pools, SEB or PMA/Ionomycin. Cells were then labeled with IFNγ catch reagent using the Miltenyi IFNγ capture assay kit and protocol. Cells were allowed to secrete cytokines for 45 minutes before commencing staining. Cells were stained with viability dye and surface antibodies [CD3-BV711 (5 µl per 100 µl of sample, clone: OKT3), CD4-BV421 (5 µl per 100 µl of sample, clone: OKT4), CD8-V500 (5ul per 100 µl of sample, clone: SK1) and CD69-BV650 (5 µl per 100 µl of sample, clone: FN50)], and the flow-FISH was followed. After completing washes outlined at the end of the protocol, cells were labeled with anti-IFNγ PE detection reagent following the Miltenyi protocol. The PE detection reagent was added after the flow-FISH protocol was completed because, if added before the fixation and permeabilization steps, the methanol would have degraded the protein dye and no IFNγ protein signal would have been observed. Cells were then analyzed on the LSR Fortessa.
IDO mRNA detection with Flow-FISH and qRT-PCR
PBMCs from HIV-negative subjects were stimulated with R848 or IFNγ + LPS for 8 hours. After 8 hours cells were split in two and half of them were processed with the flow-FISH assay for detection of IDO mRNA (type 4 probe) on CD14 cells [CD3-BV711 (5 µl per 100 µl of sample, clone: OKT3), CD19-V500 (5 µl per 100 µl of sample, clone: HIB19) and CD14-BV605 (5 µl per 100 µl of sample, clone: M5E2)] and the other half was lysed in RLT buffer containing 1% beta-mercaptoethanol followed by mRNA extraction with the Qiagen mRNA isolation kit. qRT-PCR for IDO and GAPDH housekeeping gene was performed using Taqman probes from ABI according to the manufacturer’s instructions.
Detection of miRNA with flow-FISH
PBMCs from HIV-negative subjects were either left unstimulated or were stimulated for 12 hours with PMA/Ionomycin. Flow-FISH protocol was followed as described above. Probes specific for miR-21 and miR-155 (type 1) were used. Cells were stained with surface antibodies [CD3-BV711 (5 µl per 100 µl of sample, clone: OKT3), CD4-BV421(5 µl per 100 µl of sample, clone: OKT4), CD8-V500 (5ul per 100 µl of sample, clone: SK1) and CD69-BV650 (5 µl per 100 µl of sample, clone: FN50)] and miRNA probes were detected in activated CD4 T cells (CD4+/CD69+) on a LSR Fortessa.
Imaging flow cytometry to visualize mRNA and protein
In order to visualize simultaneous detection of IFNγmRNA and protein PBMCs were left unstimulated or were stimulated with SEB for 12 hours. Flow-FISH in combination with the IFNγ capture assay were performed as described above. For each sample 30,000 cells were collected on a five-laser two-camera ImageStreamMark II imaging flow cytometer (Amnis, Seattle, WA). This instrument combines the immunofluorescent sensitivity and capacity of a traditional flow cytometer with bright field high resolution microscopy and image analysis. Since the ImageStream has an alternate excitation capacity than the LSR Fortessa, alternate antibody fluorochrome conjugates were necessary to achieve optimal detection. For this reason we used CD8-BV785 (5 µl per 100 µl of sample, clone: RPA-T8), CD3-BV605 (5 µl per 100 µl of sample, clone: OKT3) and CD4-BV421 (5 µl per 100 µl of sample, clone: OKT4). Images were captured with INSPIRE acquisition software (Amnis v.6.0.154.0) utilizing 40× magnification.
Post-acquisition data analysis was performed using IDEAS software (Amnis v.6.0.333.0). Single, focused cells were gated using Gradient RMS (focus), then Area vs. Aspect Ratio in the Bright field (BF) channel. Next, CD3+ events were gated based on Intensity (vs. BF area). From the CD3+ population, a bi-variate intensity plot of CD4 vs CD8 was generated to gate the CD8+ population.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the clinical and laboratory staff at the Massachusetts General Hospital and all study participants for their invaluable role in this project. We would like to thank Dr. Frederic Preffer for his input on this manuscript.
This study was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (PO1 AI-080192 and UM1AI100663 (CHAVI-ID); D.E.K; 1R56AI095088-01 to DGK), the National Heart Lung and Blood Institute of the National Institutes of Health (RO1 HL-092565; D.E.K) and The Office of the Director, National Institutes of Health (NIH 1S10OD012027-01A1; Dr Frederic Preffer). D.E.K is supported by a Research Scholar Career Award of the Quebec Health Research Fund (FRQS). FP was supported by a fellowship grant of the Massachusetts General Hospital Executive Committee on Research and the Harvard Global Health Institute (HGHI). DGK was supported by a Grand Challenges Exploration award from Bill and Melinda Gates Foundation.
Footnotes
AUTHORSHIP
Contributions: F.P. and D.E.K were responsible for the overall design and conduct of experiments; F.P, C.L, Q.N., B.N., S.R., D.G.K and D.E.K provided intellectual input and contributed to the experimental design; F.P, M.G.H, M.G., H.L.E., M.H., M.L., S.M.M, D.Z.S., S.M., and D.E.K did experiments; A.E.B. analyzed data; F.P and D.E.K wrote the paper; and D.E.K provided supervision.
Competing financial interests statement
C.L., Q.N., B.N., S.R.: these authors are all currently employed by Affymetrix Inc. However, this does not alter the authors' adherence to all the Nature Communications policies on sharing data and materials. The remaining authors declare that they have no competing financial interests.
References
- 1.Kalisky T, Quake SR. Single-cell genomics. Nature methods. 2011;8:311–314. doi: 10.1038/nmeth0411-311. [DOI] [PubMed] [Google Scholar]
- 2.Levsky JM, Shenoy SM, Pezo RC, Singer RH. Single-cell gene expression profiling. Science. 2002;297:836–840. doi: 10.1126/science.1072241. [DOI] [PubMed] [Google Scholar]
- 3.Slonim DK, Yanai I. Getting started in gene expression microarray analysis. PLoS computational biology. 2009;5:e1000543. doi: 10.1371/journal.pcbi.1000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.VanGuilder HD, Vrana KE, Freeman WM. Twenty-five years of quantitative PCR for gene expression analysis. BioTechniques. 2008;44:619–626. doi: 10.2144/000112776. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nature reviews. Genetics. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Bodovitz S. Single cell analysis: the new frontier in 'omics'. Trends in biotechnology. 2010;28:281–290. doi: 10.1016/j.tibtech.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Citri A, Pang ZP, Sudhof TC, Wernig M, Malenka RC. Comprehensive qPCR profiling of gene expression in single neuronal cells. Nature protocols. 2012;7:118–127. doi: 10.1038/nprot.2011.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Picelli S, et al. Full-length RNA-seq from single cells using Smart-seq2. Nature protocols. 2014;9:171–181. doi: 10.1038/nprot.2014.006. [DOI] [PubMed] [Google Scholar]
- 9.Deng Q, Ramskold D, Reinius B, Sandberg R. Single-cell RNA-seq reveals dynamic, random monoallelic gene expression in mammalian cells. Science. 2014;343:193–196. doi: 10.1126/science.1245316. [DOI] [PubMed] [Google Scholar]
- 10.Langer-Safer PR, Levine M, Ward DC. Immunological method for mapping genes on Drosophila polytene chromosomes. Proceedings of the National Academy of Sciences of the United States of America. 1982;79:4381–4385. doi: 10.1073/pnas.79.14.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Speicher MR, Carter NP. The new cytogenetics: blurring the boundaries with molecular biology. Nature reviews. Genetics. 2005;6:782–792. doi: 10.1038/nrg1692. [DOI] [PubMed] [Google Scholar]
- 12.Namekawa SH, Lee JT. Detection of nascent RNA, single-copy DNA and protein localization by immuno FISH in mouse germ cells and preimplantation embryos. Nature protocols. 2011;6:270–284. doi: 10.1038/nprot.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauman JG, Bayer JA, van Dekken H. Fluorescent in-situ hybridization to detect cellular RNA by flow cytometry and confocal microscopy. Journal of microscopy. 1990;157:73–81. doi: 10.1111/j.1365-2818.1990.tb02948.x. [DOI] [PubMed] [Google Scholar]
- 14.Crouch J, Leitenberg D, Smith BR, Howe JG. Epstein-Barr virus suspension cell assay using in situ hybridization and flow cytometry. Cytometry. 1997;29:50–57. [PubMed] [Google Scholar]
- 15.Patterson BK, et al. Detection of HIV-1 DNA and messenger RNA in individual cells by PCR-driven in situ hybridization and flow cytometry. Science. 1993;260:976–979. doi: 10.1126/science.8493534. [DOI] [PubMed] [Google Scholar]
- 16.Hanley MB, Lomas W, Mittar D, Maino V, Park E. Detection of low abundance RNA molecules in individual cells by flow cytometry. PloS one. 2013;8:e57002. doi: 10.1371/journal.pone.0057002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itzkovitz S, van Oudenaarden A. Validating transcripts with probes and imaging technology. Nature methods. 2011;8:S12–S19. doi: 10.1038/nmeth.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulz KR, Danna EA, Krutzik PO, Nolan GP. Single-cell phospho-protein analysis by flow cytometry. Current protocols in immunology / edited by John E. Coligan … [et al.] 2012;Chapter 8(Unit 8 17):11–20. doi: 10.1002/0471142735.im0817s96. [DOI] [PubMed] [Google Scholar]
- 19.Shekhar S, Yang X. The darker side of follicular helper T cells: from autoimmunity to immunodeficiency. Cellular & molecular immunology. 2012;9:380–385. doi: 10.1038/cmi.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jernas M, Nookaew I, Wadenvik H, Olsson B. MicroRNA regulate immunological pathways in T-cells in immune thrombocytopenia (ITP) Blood. 2013;121:2095–2098. doi: 10.1182/blood-2012-12-471250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barth H, Raghuraman S. Persistent infectious diseases say - IDO. Role of indoleamine-2,3-dioxygenase in disease pathogenesis and implications for therapy. Critical reviews in microbiology. 2014;40:360–368. doi: 10.3109/1040841X.2012.742037. [DOI] [PubMed] [Google Scholar]
- 22.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends in immunology. 2013;34:137–143. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seddiki N, Brezar V, Ruffin N, Levy Y, Swaminathan S. Role of miR-155 in the regulation of lymphocyte immune function and disease. Immunology. 2013 doi: 10.1111/imm.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tili E, Michaille JJ, Croce CM. MicroRNAs play a central role in molecular dysfunctions linking inflammation with cancer. Immunological reviews. 2013;253:167–184. doi: 10.1111/imr.12050. [DOI] [PubMed] [Google Scholar]
- 25.Bronevetsky Y, et al. T cell activation induces proteasomal degradation of Argonaute and rapid remodeling of the microRNA repertoire. The Journal of experimental medicine. 2013;210:417–432. doi: 10.1084/jem.20111717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baumjohann D, Ansel KM. MicroRNA-mediated regulation of T helper cell differentiation and plasticity. Nature reviews. Immunology. 2013;13:666–678. doi: 10.1038/nri3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindsay MA. microRNAs and the immune response. Trends in immunology. 2008;29:343–351. doi: 10.1016/j.it.2008.04.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


