Abstract
Introduction
Myocardial dysfunction is common after out-of-hospital cardiac arrest (OHCA) and high-sensitivity troponin T (hs-TnT) levels may provide incremental prognostic information to established risk indices.
Methods
A total of 155 patients with OHCA and a shockable rhythm (98% ventricular fibrillation; OHCA-VF/VT) had blood samples drawn within six hours of admission. Blood samples were also available after 24 hours, 48 hours, and 96 hours in subsets of patients. The endpoints of the study were hospital mortality and neurological status and mortality after one year.
Results
Admission hs-TnT levels were higher than the 99-percentile of the general population (14 ng/L) in all patients (range 18 to 17837 ng/L). Admission hs-TnT levels were associated with acute coronary artery occlusion, time to return of spontaneous circulation, heart failure, and renal function. Admission hs-TnT levels were higher in one-year non-survivors compared to survivors (median 747 (quartile 1 to 3, 206 to 1061) ng/L versus 345 (184 to 740) ng/L, P =0.023) and in patients with a poor versus a favorable neurological outcome (739 (191 to 1061) ng/L versus 334 (195 to 716) ng/L, P =0.028). However, hs-TnT measurements did not add prognostic information to established risk variables in multivariate analyses. hs-TnT levels measured during the hospitalization for OHCA-VF/VT correlated closely with admission levels (r ≥0.63) and were inferior to Simplified Acute Physiology Score II (SAPS II) scores for the prediction of events during follow-up. hs-TnT dynamics did not discriminate between survivors and non-survivors or between a poor versus a favorable neurological outcome.
Conclusion
hs-TnT levels are elevated in critically ill patients with OHCA-VF/VT, but do not improve risk prediction.
Introduction
The long-term morbidity and mortality of successfully resuscitated patients following ventricular arrhythmia-induced out-of-hospital cardiac arrest (OHCA) are still high [1,2]. Standard treatment in patients with OHCA is targeted to support organ function and has included temperature control to alleviate cerebral injury [3], which is known to cause most deaths after OHCA [4-6]. However, as post-cardiac arrest shock affects two thirds of all OHCA patients [5] and contributes to mortality during follow up [4-6], cardiac biomarkers may provide additional prognostic information to established risk factors in OHCA [7]. Early myocardial stunning in post-cardiac arrest shock will also lead to systemic hypotension, which will limit the potential for brain recovery after OHCA [8].
Cardiac-specific troponin I and T are part of the contractile apparatus of cardiomyocytes, but leak into the circulation after cardiomyocyte injury [9]. Thus, symptoms suggestive of myocardial ischemia and dynamic troponin elevations (rise and/or fall) are required for the diagnosis of acute myocardial infarction (AMI) according to the third universal definition of myocardial infarction [10]. Chest pain patients with elevated troponin levels have a worse prognosis than patients with normal troponin levels, but this excessive risk can be reduced by early angiography and percutaneous coronary intervention [11]. Hence, in patients with chest pain troponins are useful for diagnostic and prognostic assessment, as well as for selecting patients who may benefit from invasive therapy. In contrast, whether troponins provide clinically relevant information in patients with ventricular fibrillation or tachycardia (VF/VT) and OHCA has not been established [7]. Previous studies, using sensitive or high-sensitivity (hs) troponin assays suggest that troponins have insufficient accuracy for diagnosing acute coronary artery occlusion in the setting of cardiac arrest [12-14]. However, troponins could still be of interest in OHCA by identifying the patient at highest risk of developing post-cardiac arrest syndrome [8]. To be clinically relevant, studies should be performed in OHCA patients receiving contemporary therapy, including therapeutic hypothermia (TH) [3,8]. Furthermore, as hs troponin assays have been found to be superior to previous generation troponin assays for prognostic assessment across the spectrum of cardiovascular disease [15-18], hs troponin assays should be tested for prognostic utility also in patients with OHCA. In cardiac arrest patients, prognosis differ markedly depending on the initially observed rhythm (shockable versus non-shockable) [19]. Thus, studies assessing the prognostic utility of troponins should preferably encompass a homogeneous patient population; that is, OHCA-VF/VT patients. Accordingly, in this study of OHCA patients with a shockable rhythm we wanted to (1) identify factors that influence hs-TnT levels and (2) assess whether hs-troponin T (hs-TnT) measurements improve prediction of morbidity and mortality, short- and long-term.
Methods
Study design
This is a substudy of FINNRESUSCI, an observational prospective multicenter study performed by the Finnish Intensive Care Consortium that comprised 21 of the 22 ICUs in Finland [20]. The study was conducted according to the Declaration of Helsinki and approved by the Ethics Committee of Helsinki University Hospital with written informed consent for blood sampling obtained from a legal representative. Inclusion criteria for the FINNRESUSCI Study were (1) OHCA, (2) successful resuscitation, (3) age >18 y, and (4) post-resuscitation care in a participating ICU.
The main study focused on post-resuscitation care for OHCA, and these results have previously been reported [20], including that 86% of unconscious patients with a shockable rhythm were treated with TH (33-34°C). In short, from 1 March 2010 to 28 February 2011 a total of 548 adult patients with OHCA admitted to an ICU were identified. A total of 311 patients had a shockable rhythm (OHCA-VF/VT), and blood samples (after an informed consent) were available within 6 h of ICU admission in 155 (50%) of these patients (Figure 1). No restrictions were implemented regarding techniques of induction or maintenance of TH in the FINNRESUSCI Study. However, the majority of Finnish ICUs use endovascular cooling devices. Patients with a non-shockable rhythm were excluded from this substudy because these patients have a clearly worse prognosis and are subjected to less standardized treatment than patients with a shockable rhythm [19,21].
Figure 1.
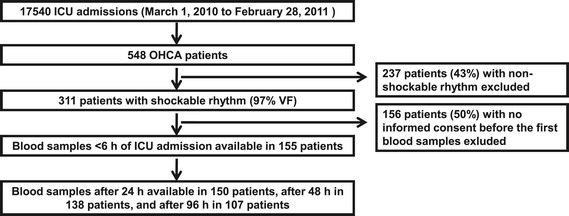
Flow chart of the study. OHCA, out-of-hospital cardiac arrest; VF, ventricular fibrillation.
We collected information on age, gender, weight and height, and previous medical history in an electronic case report form. Body mass index (BMI) was calculated by weight (kg)/(height (m))2. We collected information on the cardiac arrest episode, including the time to return of spontaneous circulation (ROSC) according to the Utstein style [22]. We also collected information on treatment during the ICU stay and whether the hospitalization was complicated by pneumonia or sepsis. Coronary angiography was performed at the discretion of the attending physicians at the different centers. We considered coronary artery intervention during the ICU stay as indicative of an acute coronary artery occlusion. The simplified acute physiology score (SAPS) II was calculated 24 h after ICU admission excluding points from temperature and negative effects of sedation on the Glasgow coma scale (GCS). Thus, the best GCS after resuscitation before start of sedation was recorded. We included endpoints of the different phases of the post-cardiac arrest period; that is, hospital mortality, the mortality after 1 year, and neurological status at 1 year after cardiac arrest according to recommendations [8]. Hospital mortality was chosen because SAPS II has been validated for this endpoint. Neurological status was assessed by a structured telephone interview with the patients categorized according to the Pittsburgh cerebral performance categories (CPC) [22]. Patients with CPC scores 1 to 2 were categorized as favorable and CPC scores 3 to 5 as having a poor neurological outcome. Hospital mortality was collected from the medical records and 1-year mortality was obtained from Statistics Finland.
Biochemical analysis
We measured troponin T in serum samples obtained within 6 h, 24 h, 48 h, and 96 h after cardiac arrest by the Elecsys TNT hs STAT assay (Roche Diagnostics, Penzberg, Germany). The hs-TnT assay has an analytical measurement range of 3 to 10,000 ng/L and the 99th percentile in the healthy population is 14 ng/L. Samples with concentrations above the upper limit were diluted before they were reanalyzed. The analytical characteristics of the hs-TnT assay have previously been reported for our laboratory [23]. Creatinine was measured by routine methods, and we calculated the estimated creatinine clearance by the Cockcroft-Gault formula [24].
Statistics
We present data as median (quartile (Q) 1 to 3) or absolute numbers and percentages. Continuous variables demonstrated a non-normal distribution, as assessed by the Kolmogorov-Smirnov test, and group differences were explored by the Mann-Whitney U-test. Categorical variables were assessed by the Chi-square test or the Fisher exact test. Variables associated with hs-TnT levels on ICU admission were assessed by linear regression analysis and the analysis included age, gender, BMI, estimated creatinine clearance, comorbidities, information on the cardiac arrest, and evidence of acute coronary occlusion. The ability of hs-TnT to predict endpoints during follow up was explored by univariate and multivariate logistic regression analyses. The odds ratios (OR) are presented with 95% CI. Variables significantly associated with the endpoints in univariate analyses were included in the multivariate models (forward selection). hs-TnT levels were transformed by the natural logarithm prior to regression analyses due to a right-skewed distribution. We also assessed prognostic accuracy of hs-TnT levels by receiver operating statistics (ROC) with area under the curve (AUC) presented with 95% CI. We used the Wilcoxon matched-pairs signed-rank test to assess changes in hs-TnT levels (∆) from the initial to later time points. A P-value <0.05 was considered statistically significant. Statistical analyses were performed with SPSS for Windows version 19.0 (SPSS, Chicago, IL, USA) and MedCalc for Windows, version 12.1.4.0 (MedCalc Software, Mariakerke, Belgium).
Results
Patient characteristics
Characteristics of the patients in this substudy (n =155) were comparable to all patients with OHCA and a shockable rhythm in the main study (n =311) (Table 1). In total, 152 of 155 patients (98%) had ventricular fibrillation as the initial rhythm. The median age was 63 (Q1 to Q3 56 to 72) years, 132 (85%) were male, most patients experienced witnessed cardiac arrest and received bystander cardiopulmonary resuscitation, and 134 (87%) were treated with TH (Table 2).
Table 1.
Patient characteristics in the biomarker substudy and the patients with out-of-hospital cardiac arrest (OHCA) and a shockable rhythm in the main FINNRESUSCI Study
| Biomarker substudy | Main study | |
|---|---|---|
| (n =155) | (n =311) | |
| Age | 63 (56 to 72) | 63 (56 to 72) |
| Male sex, n (%) | 132 (85%) | 253 (81%) |
| Coronary artery disease, n (%) | 50 (32%) | 113 (36%) |
| Diabetes mellitus, n (%) | 33 (21%) | 59 (19%) |
| Hypertension, n (%) | 70 (45%) | 140 (45%) |
| Heart failure, n (%) | 23 (15%) | 45 (15%) |
| SAPS II score | 58 (40 to 69) | 54 (39 to 66) |
| Resuscitation | ||
| Witnessed cardiac arrest, n (%) | 143 (92%) | 288 (93%) |
| Bystander CPR, n (%) | 105 (68%) | 195 (63%) |
| Time to ROSC, minutes | 20 (14 to 29) | 20 (14 to 27) |
| Treatment during ICU stay | ||
| Awake on ICU admission, n (%) | 9 (6%) | 30 (10%) |
| Therapeutic hypothermia, n (%) | 134 (87%) | 241 (78%) |
| Coronary angiography, n (%) | 34 (22%) | 72 (23%) |
| PCI, n (%) | 15 (10%) | 38 (12%) |
| Infection | ||
| Pneumonia, n (%) | 64 (41%) | 111 (36%) |
| Sepsis, n (%) | 9 (6%) | 14 (5%) |
| Outcome | ||
| Mechanical ventilation, h | 50 (40 to 79) | 46 (35 to 72) |
| ICU LOS, days | 3.2 (2.2 to 5.0) | 2.9 (1.9 to 4.8) |
| Hospital mortality, n (%) | 45 (29%) | 96 (31%) |
| Mortality after 1 year, n (%) | 59 (38%) | 124 (40%) |
| CPC 3 to 5 after 1 year, n (%) | 65 (42%) | 136 (44%) |
n, number of patients; hs-TnT, high-sensitivity troponin T; SAPS II, simplified acute physiology score II; CPR, cardiopulmonary resuscitation; ROSC, return of spontaneous circulation; PCI, percutaneous coronary intervention; LOS, length of stay; CPC, cerebral performance categories. Continuous data are presented as median (quartile 1 to 3).
Table 2.
Patient characteristics according to hs-TnT levels on inclusion in the study
| All patients | Quartile (Q) 1 to 2 | Quartile (Q) 3 to 4 | P -value | |
|---|---|---|---|---|
| (n =155) | (n =78) | (n =77) | (Q 1 to 2 versus Q 3 to 4) | |
| hs-TnT, ng/L, range | 18-17837 | 18-415 | 418-17837 | - |
| Age | 63 (56 to 72) | 63 (56 to 70) | 64 (57 to 72) | 0.25 |
| Male sex | 132 (85%) | 67 (86%) | 65 (84%) | 0.80 |
| Body mass index | 26 (24 to 29) | 27 (25 to 29) | 26 (24 to 28) | 0.054 |
| Creatinine clearance, mL/minute | 94 (72 to 126) | 100 (77 to 129) | 88 (67 to 123) | 0.13 |
| Coronary artery disease, n (%) | 50 (32%) | 26 (33%) | 24 (31%) | 0.77 |
| Diabetes mellitus, n (%) | 33 (21%) | 22 (28%) | 11 (14%) | 0.034 |
| Hypertension, n (%) | 70 (45%) | 35 (45%) | 35 (46%) | 0.94 |
| Heart failure, n (%) | 23 (15%) | 16 (21%) | 7 (9%) | 0.045 |
| SAPS II score | 58 (40 to 69) | 54 (37 to 66) | 62 (44 to 69) | 0.034 |
| Resuscitation | ||||
| Witnessed cardiac arrest, n (%) | 143 (92%) | 73 (94%) | 70 (91%) | 0.53 |
| Bystander CPR, n (%) | 105 (68%) | 55 (71%) | 50 (65%) | 0.46 |
| Time to ROSC, minutes | 20 (14 to 29) | 17 (11 to 21) | 26 (18 to 32) | <0.001 |
| Treatment during ICU stay | ||||
| Therapeutic hypothermia | 134 (87%) | 67 (86%) | 67 (87%) | 0.84 |
| Coronary angiography | 34 (22%) | 14 (18%) | 20 (26%) | 0.23 |
| PCI | 15 (10%) | 2 (3%) | 13 (17%) | 0.003 |
| Infection | ||||
| Pneumonia | 64 (41%) | 34 (44%) | 30 (39%) | 0.56 |
| Sepsis | 9 (6%) | 5 (6%) | 4 (5%) | 1.00 |
| Outcome | ||||
| Mechanical ventilation, h | 50 (40 to 79) | 49 (40 to 73) | 57 (43 to 89) | 0.24 |
| ICU LOS, days | 3.2 (2.2 to 5.0) | 3.1 (2.1 to 5.2) | 3.6 (2.3 to 4.9) | 0.51 |
| Hospital mortality | 45 (29%) | 18 (23%) | 27 (35%) | 0.10 |
| Mortality after 1 year | 59 (38%) | 22 (28%) | 37 (48%) | 0.011 |
| CPC 3 to 5 after 1 year | 65 (42%) | 24 (31%) | 41 (53%) | 0.005 |
n, number of patients; hs-TnT, high-sensitivity troponin T; SAPS II, simplified acute physiology score II; SOFA, sequential organ failure assessment score CPR, cardiopulmonary resuscitation; ROSC, return of spontaneous circulation; PCI, percutaneous coronary intervention; LOS, length of stay; CPC, cerebral performance categories. Continuous data are presented as median (quartile 1 to 3).
Admission hs-TnT levels
The range of hs-TnT levels within 6 h of admission for OHCA-VF/VT was 18 to 17,837 ng/L with median level 415 ng/L (Q1 to Q3 199 to 916 ng/L). The prevalence of diabetes mellitus and heart failure was higher among patients with hs-TnT levels below the median, while time to ROSC was longer in patients with supramedian hs-TnT levels (Table 2). The median hs-TnT level in patients with acute coronary artery occlusions (n =15) was 1,497 ng/L (Q1 to Q3 753 to 8,875 ng/L) compared to median 387 ng/L (182 to 815 ng/L) for the other patients (P <0.001). Admission hs-TnT levels were not associated with the probability of receiving TH (Table 2).
Admission hs-TnT levels correlated positively with time to ROSC (r =0.47, P <0.001), SAPS II score (r =0.16, P =0.045), and duration of mechanical ventilation (r =0.17, P =0.038), and were inversely correlated to BMI and estimated creatinine clearance (both r = -0.17, P =0.032). Evidence of acute coronary artery occlusion (β =0.39, P <0.001), time to ROSC (β =0.35, P <0.001), history of heart failure (β = -0.22, P =0.001), and estimated creatinine clearance (β = -0.17, P =0.013) were associated with admission hs-TnT levels in multivariate linear regression analysis (r2 = 0.37). We found close correlations between admission hs-TnT levels and hs-TnT levels after 24 h (r =0.78); after 48 h (r =0.71); and after 96 h (r =0.63) (P <0.001 for all).
hs-TnT levels and hospital and 1-year mortality/poor neurological outcome (CPC 3 to 5)
Of the 155 patients, 45 patients (29%) died during the hospitalization. One year after cardiac arrest 59 patients (38%) had died and 65 patients (42%) had a poor neurological outcome (CPC 3 to 5). Admission hs-TnT levels were not statistically different between hospital non-survivors and hospital survivors: 792 ng/L (191 to 1224 ng/L) versus 387 ng/L (195 to 756 ng/L), respectively, P =0.08. In contrast, admission hs-TnT levels were higher in 1-year non-survivors versus survivors (747 ng/L (206 to 1061 ng/L) versus 345 ng/L (184 to 740 ng/L) P =0.023) and in patients with a poor neurological outcome compared to a favorable neurological outcome (739 ng/L (191 to 1061 ng/L) versus 334 ng/L (195 to 716 ng/L), P =0.028). As assessed by ROC curve analysis, admission hs-TnT levels separated between patients that were dead or categorized as having an unfavorable neurological outcome after 1 year, but did not discriminate regarding hospital mortality (Table 3). Similar results were also found for hs-TnT measurements after 24 h, but the accuracy of hs-TnT levels after 24 h were inferior to SAPS II score for all endpoints (Table 4).
Table 3.
Prognostic value of high-sensitivity troponin T (hs-TnT) measured ≤24 h after ICU admission as assessed by receiver operating characteristic curve analysis
| Hospital mortality | Mortality after 1 year | CPC 3 to 5 after 1 year | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AUC | 95% CI | P -value | AUC | 95% CI | P -value | AUC | 95% CI | P -value | |
| Admission (n =155) | 0.59 | 0.51, 0.68 | 0.10 | 0.61 | 0.52, 0.68 | 0.03 | 0.60 | 0.52, 0.68 | 0.03 |
| 24 h (n =150) | 0.60 | 0.51, 0.68 | 0.05 | 0.62 | 0.54, 0.70 | 0.009 | 0.62 | 0.54, 0.70 | 0.01 |
| ∆ hs-TnT (n =150) | 0.50 | 0.42, 0.58 | 0.99 | 0.51 | 0.43, 0.59 | 0.85 | 0.51 | 0.42, 0.59 | 0.92 |
CPC, cerebral performance categories; AUC, area under the curve; ∆ (delta), change in hs-TnT levels from admission to 24 h.
Table 4.
Comparison of high-sensitivity troponin T (hs-TnT) measured ≥24 h after ICU admission and SAPS II score as assessed by receiver operating characteristics curve analysis
| Hospital mortality | Mortality after 1 year | CPC 3 to 5 after 1 year | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AUC | 95% CI | P -value | AUC | 95% CI | P -value | AUC | 95% CI | P -value | |
| After 24 h(n =150) | |||||||||
| SAPS II score | 0.76 | 0.69, 0.83 | Reference | 0.76 | 0.69, 0.83 | Reference | 0.78 | 0.70, 0.84 | Reference |
| hs-TnT | 0.60 | 0.51, 0.68 | 0.005 | 0.62 | 0.54, 0.70 | 0.02 | 0.62 | 0.54, 0.70 | 0.004 |
| ∆ hs-TnT | 0.50 | 0.42, 0.58 | <0.001 | 0.51 | 0.43, 0.59 | <0.001 | 0.51 | 0.42, 0.59 | <0.001 |
| hs-TnT, 48 h (n =138) | |||||||||
| SAPS II score | 0.76 | 0.68, 0.83 | Reference | 0.77 | 0.69, 0.83 | Reference | 0.78 | 0.70, 0.85 | Reference |
| hs-TnT | 0.63 | 0.54, 0.71 | 0.03 | 0.65 | 0.56, 0.73 | 0.04 | 0.66 | 0.57, 0.74 | 0.03 |
| ∆ hs-TnT | 0.54 | 0.45, 0.62 | 0.006 | 0.53 | 0.44, 0.61 | <0.001 | 0.51 | 0.42, 0.59 | <0.001 |
| hs-TnT, 96 h (n =107) | |||||||||
| SAPS II score | n.a. | 0.75 | 0.68, 0.82 | - | 0.79 | 0.70, 0.86 | Reference | ||
| hs-TnT | n.a. | 0.62 | 0.54, 0.70 | 0.02 | 0.60 | 0.50, 0.69 | 0.005 | ||
| ∆ hs-TnT | n.a. | 0.51 | 0.43, 0.59 | <0.001 | 0.53 | 0.43, 0.63 | 0.001 | ||
P-values are for simplified acute physiology score (SAPS) II versus high-sensitivity troponin T (hs-TnT) levels. CPC, cerebral performance categories; We did not calculate results for hospital mortality for measurements after 96 h due to the low number of events in this group (n =17) (total hospital mortality in the FINNRESUSCI laboratory substudy was 45 patients). AUC, area under the curve; ∆ (delta), change in hs-TnT levels from admission to later timepoints; n.a., not applicable.
Most patients demonstrated a reduction in hs-TnT levels from admission to 24 h (∆ hs-TnT levels), but no significant differences were found for ∆ hs-TnT levels relating to hospital mortality (median 10 versus 51 ng/L reduction for non-survivors versus survivors); 1-year mortality (68 versus 46 ng/L reduction); nor neurological outcome (median 46 versus 49 ng/L reduction for poor versus favorable outcome) (Figure 2). In most patients, hs-TnT levels further decreased 48 h and 96 h after ICU admission with no differences in hs-TnT dynamics according to mortality or morbidity during follow up (Table 4 and Figure 3). By logistic regression analyses, admission hs-TnT levels (logarithmically transformed) were neither associated with hospital nor 1-year mortality, nor with 1-year neurological outcome according to CPC score (Table 5). In contrast, we found several established risk variables in OHCA-VF/VT associated with mortality and morbidity in our cohort (Table 5).
Figure 2.
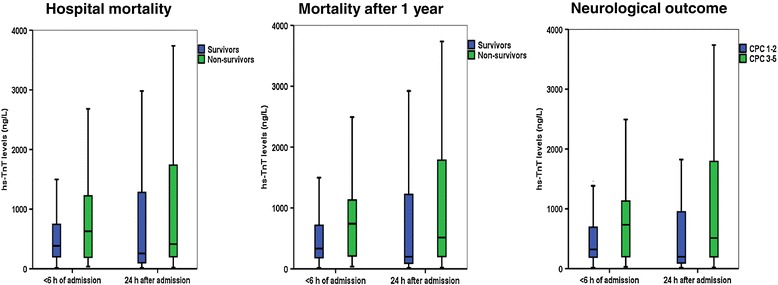
hs-TnT levels on admission and after 24 h in patients with OHCA-VF/VT divided according to mortality and neurological outcome. The horizontal line within the box represents the median concentration, the boundaries of the box quartiles 1-3, and the whiskers range (maximum value restricted to 1.5 x interquartile range from the median).
Figure 3.
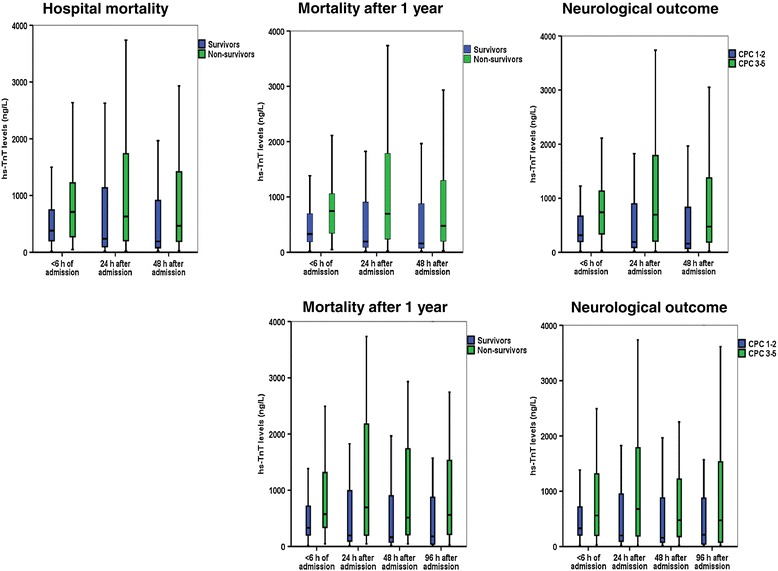
High-sensitivity troponin T (hs-TnT) levels in the patients with blood samples available also after 48 h (upper panel) and 96 h (lower panel). The horizontal line within the box represents the median concentration, the boundaries of the box quartiles 1 to 3, and the whiskers range (maximum value restricted to 1.5 × interquartile range from the median).
Table 5.
Associations between variables available <24 h after admission for OHCA-VF/VT and hospital mortality and 1 year outcomes
| Hospital mortality | Mortality after 1 year | CPC 3 to 5 after 1 year | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P -value | Odds ratio | 95% CI | P -value | Odds ratio | 95% CI | P -value | |
| Univariate analysis | |||||||||
| Age | 1.05 | 1.02, 1.09 | 0.003 | 1.05 | 1.02, 1.08 | 0.002 | 1.05 | 1.02, 1.08 | 0.001 |
| Male | 1.19 | 0.44, 3.24 | 0.74 | 1.90 | 0.70, 5.13 | 0.21 | 1.79 | 0.69, 4.64 | 0.23 |
| Body mass index | 1.006 | 0.93, 1.09 | 0.89 | 1.004 | 0.94, 1.08 | 0.91 | 0.99 | 0.93, 1.07 | 0.87 |
| Creatinine clearance | 0.99 | 0.98, 0.99 | 0.011 | 0.99 | 0.98, 1.00 | 0.052 | 0.99 | 0.98, 1.00 | 0.062 |
| History of coronary artery disease | 2.80 | 1.36, 5.78 | 0.005 | 2.68 | 1.34, 5.36 | 0.005 | 2.65 | 1.33, 5.28 | 0.006 |
| History of diabetes mellitus | 2.56 | 1.15, 5.69 | 0.021 | 1.48 | 0.68, 3.22 | 0.33 | 1.20 | 0.55, 2.60 | 0.64 |
| History of hypertension | 1.40 | 0.70, 2.81 | 0.34 | 1.16 | 0.61, 2.23 | 0.65 | 1.07 | 0.56, 2.03 | 0.83 |
| History of heart failure | 3.27 | 1.32, 8.12 | 0.010 | 3.01 | 1.21, 7.48 | 0.018 | 3.08 | 1.22, 7.77 | 0.018 |
| Witnessed cardiac arrest | 0.38 | 0.11, 1.23 | 0.11 | 0.28 | 0.08, 0.97 | 0.044 | 0.22 | 0.06, 0.83 | 0.025 |
| Bystander CPR | 0.93 | 0.45, 1.95 | 0.86 | 0.89 | 0.44, 1.77 | 0.73 | 0.78 | 0.40, 1.54 | 0.48 |
| Time to ROSC | 1.07 | 1.03, 1.10 | <0.001 | 1.09 | 1.05, 1.13 | <0.001 | 1.09 | 1.05, 1.13 | <0.001 |
| Therapeutic hypothermia | 0.79 | 0.30, 2.11 | 0.64 | 1.27 | 0.48, 3.35 | 0.63 | 1.20 | 0.47, 3.10 | 0.70 |
| Coronary angiography | 0.26 | 0.09, 0.79 | 0.017 | 0.28 | 0.11, 0.71 | 0.008 | 0.28 | 0.11, 0.70 | 0.006 |
| PCI | 0.16 | 0.02, 1.22 | 0.077 | 0.10 | 0.01, 0.79 | 0.029 | 0.09 | 0.01, 0.66 | 0.019 |
| hs-TnT <6 h | 1.25 | 0.95, 1.64 | 0.12 | 1.26 | 0.97, 1.63 | 0.09 | 1.26 | 0.97, 1.63 | 0.08 |
| Multivariate analysis | |||||||||
| Age | 1.07 | 1.03, 1.11 | 0.001 | 1.05 | 1.02, 1.09 | 0.004 | 1.06 | 1.02, 1.09 | 0.003 |
| Time to ROSC | 1.07 | 1.03, 1.11 | <0.001 | 1.11 | 1.06, 1.16 | <0.001 | 1.11 | 1.07, 1.16 | <0.001 |
| Diabetes mellitus | 2.87 | 1.19, 6.95 | 0.019 | n.s. | n.s. | ||||
| PCI | n.s. | 0.09 | 0.01, 0.79 | 0.030 | 0.07 | 0.007, 0.61 | 0.017 | ||
OHCA, out-of-hospital cardiac arrest; VF, ventricular fibrillation; VT, ventricular tachycardia; CPR, cardiopulmonary resuscitation; hs-TnT, high-sensitivity troponin T ROSC, return of spontaneous circulation; PCI, percutaneous coronary intervention; n.s., non-significant.
Discussion
The main finding of this study was that hs-TnT levels were above the 99th percentile in all critically ill patients successfully resuscitated from OHCA after VF or VT. However, hs-TnT measurements on admission or later during the ICU stay did not provide incremental prognostic information about hospital mortality or 1-year neurological outcome. hs-TnT dynamics during the ICU stay also failed to provide prognostic information, and the lack of prognostic information obtained from hs-TnT measurement was evident for both hospital and 1-year mortality.
Several factors may explain the limited prognostic information by measuring hs-TnT levels in patients with OHCA-VF/VT. First, myocardial cell necrosis is not the main determinant of outcome after cardiac arrest [4-6]. Hence, in patients with OHCA-VF/VT other factors such as brain injury and the systemic ischemia/reperfusion syndrome will be of greater importance for short- and long-term outcome [7]. Deaths during follow up may also relate to the initial brain injury, for example, patients with poor cognitive function after OHCA-VF/VT will be susceptible to dying from pneumonia or other infections during follow up. Thus, unlike the situation in chest-pain patients, pathology not reflected by hs-TnT levels is likely to determine survival after cardiac arrest.
Second, the influence of several factors besides acute coronary artery occlusion to hs-TnT levels may attenuate the prognostic value of hs-TnT in OHCA-VF/VT patients. The duration of cardiopulmonary resuscitation and number of defibrillation shocks have previously been reported to increase troponin levels [25-27], and we also found the time to ROSC to be closely associated with admission hs-TnT levels. Impaired renal function was also associated with increased hs-TnT levels in OHCA-VF/VT patients and this association is well-known from previous studies [16-18,23]. In contrast, it is surprising that a previous diagnosis of heart failure was associated with lower hs-TnT levels in our study as patients with heart failure in general will have high troponin levels [28]. A possible explanation for this result could be less concomitant acute coronary artery occlusion in heart failure patients with OHCA-VF/VT, thus, heart failure patients with OHCA-VF/VT will have elevated hs-TnT levels compared to the general population, but still lower levels compared to other patients with OHCA-VF/VT. The very high hs-TnT levels observed in this study support this model. Previous studies using older troponin assays have also found troponin levels to exceed the upper reference limit in the majority of OHCA patients [29-31].
We now support and extend these observations by reporting hs-TnT levels above the 99th percentile of the healthy population in all patients with OHCA-VF/VT. The high troponin levels in OHCA-VF/VT patients are likely a result of prolonged cardiopulmonary resuscitation, multiple defibrillation shocks, and a proportion of the patients having acute coronary artery occlusion as the cause of OHCA-VF/VT. Of note, the results for hs-TnT in OHCA-VF/VT are analogous to the situation for hs-TnT measurements in other cohorts of critically ill patients, including in severe sepsis where hs-TnT levels are elevated in the majority of patients, but fail to improve risk assessment beyond established risk indices [32].
Our study has several strengths. First, an obvious strength is that the OHCA-VF/VT patients in this biomarker substudy were comparable to the OHCA-VF/VT patients in the large observational FINNRESUSCI study and should therefore be representative of the general OHCA-VF/VT cohort. In addition, a high proportion of patients (86%) were treated with TH according to the current guidelines. These two issues increase the external validity of our findings. We also measured troponin T levels at several time points by a high-sensitivity assay, which allowed us to assess the relevance of troponin dynamics. However, any inferences based on the findings of this study are subject to some obvious limitations. First, we were not able to study consecutive patients due to the requirement of written informed consent for blood sampling. Second, the majority of our patients were not examined by angiography, although the proportion in our study were comparable to the proportion examined by angiography in the main FINNRESUSCI Study [20] and in other studies [29,31]. Our study was not designed to assess the potential of hs-TnT to diagnose acute coronary occlusion and hs-TnT levels could still be of value for selecting patients who may benefit from coronary revascularization in OHCA-VF/VT. Furthermore, other cardiac biomarkers like the B-type natriuretic peptides reflect additional pathophysiology and may provide stronger prognostic information in OHCA-VF/VT than hs-TnT levels. Finally, the study patients were treated according to the current guidelines proposing TH (33 to 34°C), and the results may be different in future populations if milder hypothermia (36°C) will be recommended [33].
Conclusion
We found hs-TnT levels to exceed the 99th percentile in successfully resuscitated OHCA-VF/VT patients admitted to the ICU. However, our data do not support the use of hs-TnT measurements for risk stratification in critically ill OHCA-VF/VT patients.
Key messages
hs-TnT levels were elevated in all patients with OHCA-VF/VT
Admission hs-TnT levels were higher in 1-year non-survivors and patients with an unfavorable neurological outcome after 1 year
hs-TnT measurements did not add to current risk variables in OHCA-VF/VT.
Acknowledgements
We would like to acknowledge the contribution by all personnel involved in the FINNRESUSCI Laboratory Study and the expert contribution by Susann C Brunell, BSc, Department of Clinical Biochemistry, Akershus University Hospital to the hs-TnT analysis. A complete list of the investigators who participated in the study is presented: the FINNRESUSCI Laboratory Study Group: participating hospitals, investigators (Inv) and study nurses (SN) in the FINNRESUSCI Study: Satakunta Central Hospital, Dr Vesa Lund (Inv), Päivi Tuominen, Satu Johansson, Pauliina Perkola, Elina Kumpulainen (SN); East Savo Central Hospital, Dr Markku Suvela (Inv), Sari Hirvonen, Sirpa Kauppinen (SN); Central Finland Central Hospital, Dr Raili Laru-Sompa (Inv), Mikko Reilama (SN); South Savo Central Hospital, Dr Heikki Laine (Inv), Pekka Kettunen, Iina Smolander (SN); North Carelia Central Hospital, Dr Matti Reinikainen (Inv),Tero Surakka (SN); Seinäjoki Central Hospital, Dr Kari Saarinen (Inv), Pauliina Lähdeaho, Johanna Soini (SN); South Carelia Central Hospital, Dr Seppo Hovilehto (Inv); Kanta-Häme Central Hospital, Dr Ari Alaspää (Inv), Tarja Heikkilä (SN); Lappi Central Hospital, Dr Outi Kiviniemi (Inv), Esa Lintula (SN); Keski-Pohjanmaa Central Hospital, Dr Tadeusz Kaminski (Inv), Jane Roiko (SN); Kymenlaakso Central Hospital, Dr Seija Alila, Dr Jussi Pentti (Inv), Reija Koskinen (SN); Länsi-Pohja’s Central Hospital, Dr Jorma Heikkinen (Inv) Helsinki University Hospital, Jorvi Hospital, Dr Jukka Vaahersalo, Dr Tuomas Oksanen, Dr Tero Varpula (Inv), Anne Eronen, Teemu Hult, Taina Nieminen (SN); Meilahti Hospital Medical ICU, Dr Tom Bäcklund (Inv), Leevi Kauhanen (SN); Meilahti Hospital ICU, Dr Kirsi-Maija Kaukonen, Dr Ville Pettilä (Inv), Leena Pettilä, Sari Sutinen (SN); Tampere University Hospital, Dr Sanna Hoppu, Dr Jyrki Tenhunen, Dr Sari Karlsson (Inv), Atte Kukkurainen, Simo Varila, Samuli Kortelainen, Minna-Liisa Peltola (SN); Kuopio University Hospital, Dr Pamela Hiltunen, Dr Jouni Kurola, Dr Esko Ruokonen (Inv), Elina Halonen, Saija Rissanen, Sari Rahikainen (SN); Oulu University Hospital, Dr Risto Ahola, Dr Tero Ala-Kokko (Inv), Sinikka Sälkiö (SN).
Abbreviations
- AMI
acute myocardial infarction
- AUC
area under the curve
- BMI
body mass index
- CPC
cerebral performance categories
- GCS
Glasgow coma scale
- hs-TnT
high-sensitivity troponin T levels
- OHCA
out-of-hospital cardiac arrest
- OR
odds ratio
- PCP
Pittsburgh cerebral performance categories
- Q
quartile
- ROC
receiver operating characteristic statistics
- ROSC
return of spontaneous circulation
- SAPS II
simplified acute physiology score II
- TH
therapeutic hypothermia
- VF
ventricular fibrillation
- VT
ventricular tachycardia
Footnotes
Competing interests
The FINNRESUSCI Study was funded by grants from Helsinki University Hospital and Kuopio University Hospital. Roche Diagnostics supported the study by providing reagents at a reduced prize to Akershus University Hospital (TAH). The sponsors played no role in any of the following: design and conduct of the study, collection, management, analysis and interpretation of the data, or preparation, review and approval of the manuscript. Professor Omland has received speaker’s honoraria from Abbott Diagnostics, Siemens Healthcare Diagnostics, and Roche Diagnostics; and a research grant support from Abbott Diagnostics and Roche Diagnostics through Akershus University Hospital. The other authors have no disclosures relating to this work.
Authors’ contributions
HR conceived and designed this study, analyzed the data, wrote the paper, and critically revised the manuscript. JV contributed to data acquisition, analyzed the data, wrote the paper, and critically revised the manuscript. TAH contributed to data acquisition and critically revised the manuscript. VP conceived and designed this study, contributed to data acquisition, and critically revised the manuscript. JK contributed to data acquisition and critically revised the manuscript. TO conceived and designed this study, analyzed the data, wrote the paper, and critically revised the manuscript. All authors read and approved the final version of the manuscript.
Contributor Information
Helge Røsjø, Email: helge.rosjo@medisin.uio.no.
Jukka Vaahersalo, Email: jukka.vaahersalo@hus.fi.
Tor-Arne Hagve, Email: t.a.hagve@medisin.uio.no.
Ville Pettilä, Email: Ville.Pettila@hus.fi.
Jouni Kurola, Email: Jouni.Kurola@kuh.fi.
Torbjørn Omland, Email: torbjorn.omland@medisin.uio.no.
References
- 1.Stiell IG, Wells GA, Field B, Spaite DW, Nesbitt LP, De Maio VJ, Nichol G, Cousineau D, Blackburn J, Munkley D, Luinstra-Toohey L, Campeau T, Dagnone E, Lyver M, Ontario Prehospital Advanced Life Support Study Group Advanced cardiac life support in out-of-hospital cardiac arrest. N Engl J Med. 2004;351:647–656. doi: 10.1056/NEJMoa040325. [DOI] [PubMed] [Google Scholar]
- 2.Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, Rea T, Lowe R, Brown T, Dreyer J, Davis D, Idris A, Stiell I, Resuscitation Outcomes Consortium Investigators Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nolan JP, Soar J, Zideman DA, Biarent D, Bossaert LL, Deakin C, Koster RW, Wyllie J, Böttiger B, ERC Guidelines Writing Group European Resuscitation Council Guidelines for Resuscitation 2010 Section 1. Executive summary. Resuscitation. 2010;81:1219–1276. doi: 10.1016/j.resuscitation.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 4.Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30:2126–2128. doi: 10.1007/s00134-004-2425-z. [DOI] [PubMed] [Google Scholar]
- 5.Lemiale V, Dumas F, Mongardon N, Giovanetti O, Charpentier J, Chiche JD, Carli P, Mira JP, Nolan J, Cariou A. Intensive care unit mortality after cardiac arrest: the relative contribution of shock and brain injury in a large cohort. Intensive Care Med. 2013;39:1972–1980. doi: 10.1007/s00134-013-3043-4. [DOI] [PubMed] [Google Scholar]
- 6.Dragancea I, Rundgren M, Englund E, Friberg H, Cronberg T. The influence of induced hypothermia and delayed prognostication on the mode of death after cardiac arrest. Resuscitation. 2013;84:337–342. doi: 10.1016/j.resuscitation.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Scolletta S, Donadello K, Santonocito C, Franchi F, Taccone FS. Biomarkers as predictors of outcome after cardiac arrest. Expert Rev Clin Pharmacol. 2012;5:687–699. doi: 10.1586/ecp.12.64. [DOI] [PubMed] [Google Scholar]
- 8.Nolan JP, Neumar RW, Adrie C, Aibiki M, Berg RA, Böttiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC, Kern KB, Laurent I, Longstreth WT, Merchant RM, Morley P, Morrison LJ, Nadkarni V, Peberdy MA, Rivers EP, Rodriguez-Nunez A, Sellke FW, Spaulding C, Sunde K, Hoek TV. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation. 2008;79:350–379. doi: 10.1016/j.resuscitation.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Omland T. New features of troponin testing in different clinical settings. J Intern Med. 2010;268:207–217. doi: 10.1111/j.1365-2796.2010.02253.x. [DOI] [PubMed] [Google Scholar]
- 10.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, the Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 11.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Jneid H, Ettinger SM, Ganiats TG, Lincoff AM, Philippides GJ, Zidar JP, American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e663–e828. doi: 10.1161/CIR.0b013e31828478ac. [DOI] [PubMed] [Google Scholar]
- 12.Dumas F, Manzo-Silberman S, Fichet J, Mami Z, Zuber B, Vivien B, Chenevier-Gobeaux C, Varenne O, Empana JP, Pène F, Spaulding C, Cariou A. Can early cardiac troponin I measurement help to predict recent coronary artery occlusion in out-of-hospital cardiac arrest survivors? Crit Care Med. 2012;40:1777–1784. doi: 10.1097/CCM.0b013e3182474d5e. [DOI] [PubMed] [Google Scholar]
- 13.Geri G, Mongardon N, Dumas F, Chenevier-Gobeaux C, Varenne O, Jouven X, Vivien B, Mira JP, Empana JP, Spaulding C, Cariou A. Diagnosis performance of high sensitivity troponin assay in out-of-hospital cardiac arrest patients. Int J Cardiol. 2013;169:449–454. doi: 10.1016/j.ijcard.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Kruse JM, Enghard P, Schröder T, Hasper D, Kühnle Y, Jörres A, Storm C. Weak diagnostic performance of troponin, creatinine kinase and creatinine kinase-MB to diagnose or exclude myocardial infarction after successful resuscitation. Int J Cardiol. 2014;173:216–221. doi: 10.1016/j.ijcard.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 15.Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, Biedert S, Schaub N, Buerge C, Potocki M, Noveanu M, Breidthardt T, Twerenbold R, Winkler K, Bingisser R, Mueller C. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858–867. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 16.Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, Tjora S, Domanski MJ, Gersh BJ, Rouleau JL, Pfeffer MA, Braunwald E, Prevention of Events with Angiotensin Converting Enzyme Inhibition (PEACE) Trial Investigators A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361:2538–2547. doi: 10.1056/NEJMoa0805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, McGuire DK. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Røsjø H, Andreassen J, Edvardsen T, Omland T. Prognostic usefulness of circulating high-sensitivity troponin T in aortic stenosis and relation to echocardiographic indexes of cardiac function and anatomy. Am J Cardiol. 2011;108:88–91. doi: 10.1016/j.amjcard.2011.02.346. [DOI] [PubMed] [Google Scholar]
- 19.Sasson C, Rogers MAM, Dahl J, Kellermann AL. Predictors of survival from out-of-hospital cardiac arrest: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2010;3:63–81. doi: 10.1161/CIRCOUTCOMES.109.889576. [DOI] [PubMed] [Google Scholar]
- 20.Vaahersalo J, Hiltunen P, Tiainen M, Oksanen T, Kaukonen KM, Kurola J, Ruokonen E, Tenhunen J, Ala-Kokko T, Lund V, Reinikainen M, Kiviniemi O, Silfvast T, Kuisma M, Varpula T, Pettilä V, FINNRESUSCI Study Group Therapeutic hypothermia after out-of-hospital cardiac arrest in Finnish intensive care units: the FINNRESUSCI study. Intensive Care Med. 2013;39:826–837. doi: 10.1007/s00134-013-2868-1. [DOI] [PubMed] [Google Scholar]
- 21.Berdowski J, Berg RA, Tijssen JGP, Koster RW. Global incidences of out-of-hospital cardiac arrest and survival rates: Systematic review of 67 prospective studies. Resuscitation. 2010;81:1479–1487. doi: 10.1016/j.resuscitation.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Cummins RO, Chamberlain DA, Abramson NS, Allen M, Baskett PJ, Becker L, Bossaert L, Delooz HH, Dick WF, Eisenberg MS. Recommended guidelines for uniform reporting of data from out-of-hospital cardiac arrest: the Utstein Style. A statement for health professionals from a task force of the American Heart Association, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, and the Australian Resuscitation Council. Circulation. 1991;84:960–975. doi: 10.1161/01.CIR.84.2.960. [DOI] [PubMed] [Google Scholar]
- 23.Røsjø H, Kravdal G, Høiseth AD, Jørgensen M, Badr P, Røysland R, Omland T. Troponin I measured by a high-sensitivity assay in patients with suspected reversible myocardial ischemia: data from the Akershus Cardiac Examination (ACE) 1 study. Clin Chem. 2012;58:1565–1573. doi: 10.1373/clinchem.2012.190868. [DOI] [PubMed] [Google Scholar]
- 24.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 25.Grubb NR, Fox KAAA, Cawood P. Resuscitation from out-of-hospital cardiac arrest: implication for cardiac enzyme estimation. Resuscitation. 1996;33:35–41. doi: 10.1016/S0300-9572(96)00971-9. [DOI] [PubMed] [Google Scholar]
- 26.Muellner M, Oschatz E, Stertz F, Pirich C, Exner M, Schörkhuber W, Laggner AN, Hirschl MM. The influence of chest compressions and external defibrillation on the release of creatinine kinase-MB and cardiac troponin T in patients resuscitated from out-of-hospital cardiac arrest. Resuscitation. 1998;38:99–105. doi: 10.1016/S0300-9572(98)00087-2. [DOI] [PubMed] [Google Scholar]
- 27.Lin CC, Chiu TF, Fang JY, Kuan JT, Chen JC. The influence of cardiopulmonary resuscitation without defibrilliation on serum levels of cardiac enzymes: A time course study of out-of-hospital cardiac arrest survivors. Resuscitation. 2006;68:343–349. doi: 10.1016/j.resuscitation.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 28.Masson S, Anand I, Favero C, Barlera S, Vago T, Bertocchi F, Maggioni AP, Tavazzi L, Tognoni G, Cohn JN, Latini R, Valsartan Heart Failure Trial (Val-HeFT) and Gruppo Italiano per lo Studio della Sopravvivenza nell’Insufficienza Cardiaca–Heart Failure (GISSI-HF) Investigators Serial measurement of cardiac troponin T using a highly sensitive assay in patients with chronic heart failure: data from 2 large randomized clinical trials. Circulation. 2012;125:280–288. doi: 10.1161/CIRCULATIONAHA.111.044149. [DOI] [PubMed] [Google Scholar]
- 29.Oh SH, Kim YM, Kim HJ, Youn CS, Choi SP, Wee JH, Kim SH, Jeong WJ, Park KN. Implication of cardiac marker elevation in patients who resuscitated from out-of-hospital cardiac arrest. Am J Emerg Med. 2012;30:464–471. doi: 10.1016/j.ajem.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 30.Voicu S, Sideris G, Deye N, Dillinger JG, Logeart D, Broche C, Vivien B, Brun PY, Capan DD, Manzo-Silberman S, Megarbane B, Baud FJ, Henry P. Role of cardiac troponin in the diagnosis of acute myocardial infarction in comatose patients resuscitated from out-of-hospital cardiac arrest. Resuscitation. 2012;83:452–458. doi: 10.1016/j.resuscitation.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Kontos MC, Ornato JP, Kurz MC, Roberts CS, Gossip M, Dhindsa HS, Reid RD, Peberdy MA. Prevalence of troponin elevations in patients with cardiac arrest and implications for assessing quality of care in hypothermia centers. Am J Cardiol. 2013;112:933–937. doi: 10.1016/j.amjcard.2013.05.024. [DOI] [PubMed] [Google Scholar]
- 32.Røsjø H, Varpula M, Hagve T-A, Karlsson S, Ruokonen E, Pettilä V, Omland T, FINNSEPSIS Study Group Circulating high sensitivity troponin T in severe sepsis and septic shock: distribution, associated factors, and relation to outcome. Intensive Care Med. 2011;37:77–85. doi: 10.1007/s00134-010-2051-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M, Pellis T, Stammet P, Wanscher M, Wise MP, Aneman A, Al-Subaie N, Boesgaard S, Bro-Jeppesen J, Brunetti I, Bugge JF, Hingston CD, Juffermans NP, Koopmans M, Køber L, Langørgen J, Lilja G, Møller JE, Rundgren M, Rylander C, TTM Trial Investigators et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369:2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]


