Abstract
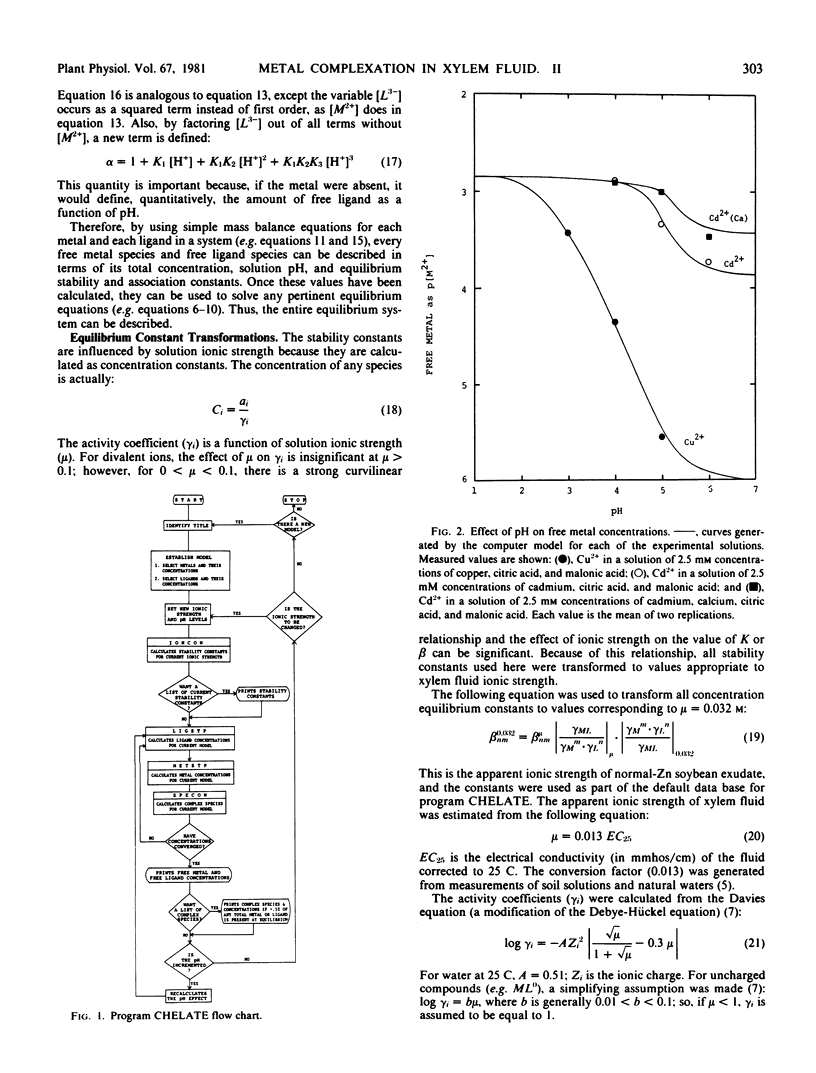
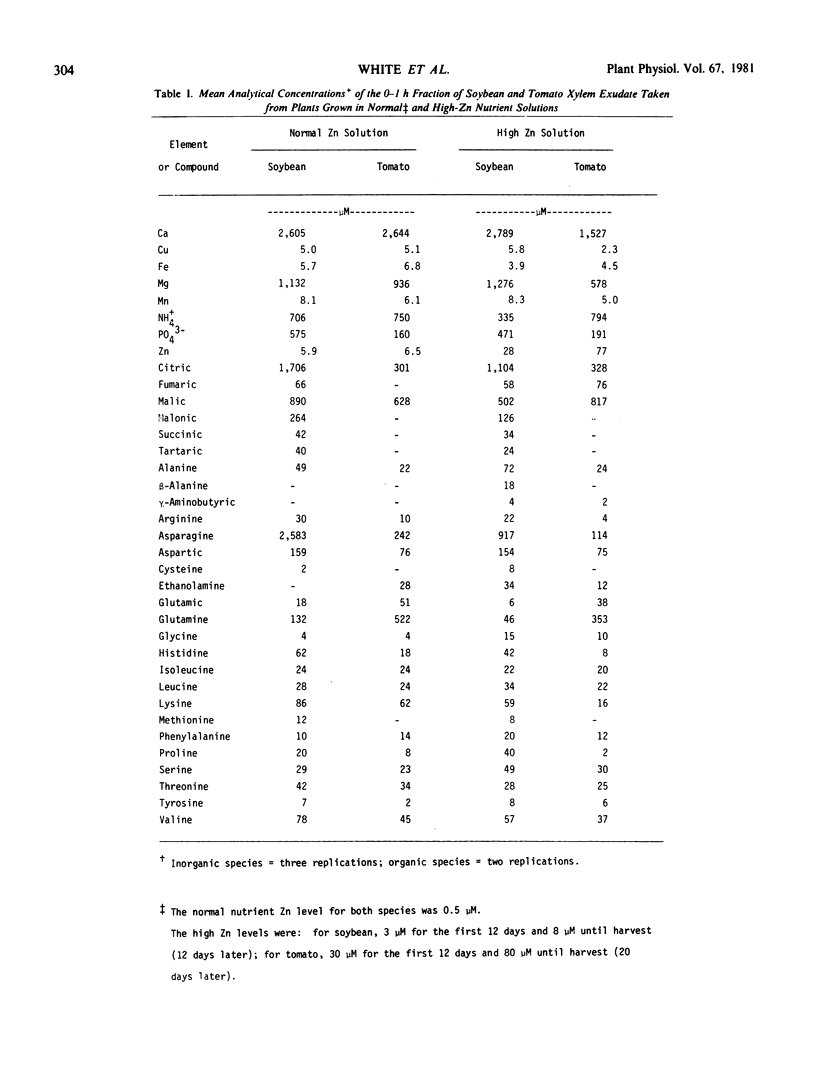
Theoretical considerations of metal complex formation in aqueous solutions were used to develop a computer program (CHELATE) to calculate all equilibrium species (free metal ions, metal complexes, etc.) in any user-defined system, such as xylem fluid. Mass-balance equations were established to describe each free metal ion and each free ligand concentration as a function of solution pH, total metal or total ligand, hydrogen-association constants, and the stability constants of known metal complexes. A default data base can be altered by the user to define any desired system covered by the stored equilibrium data. The program can currently handle nine metal ions, 35 ligands, and 500 complex species. The validity of the program was confirmed by using experimental test systems in which free-metal ion activity measurements were made with ion-selective electrodes.
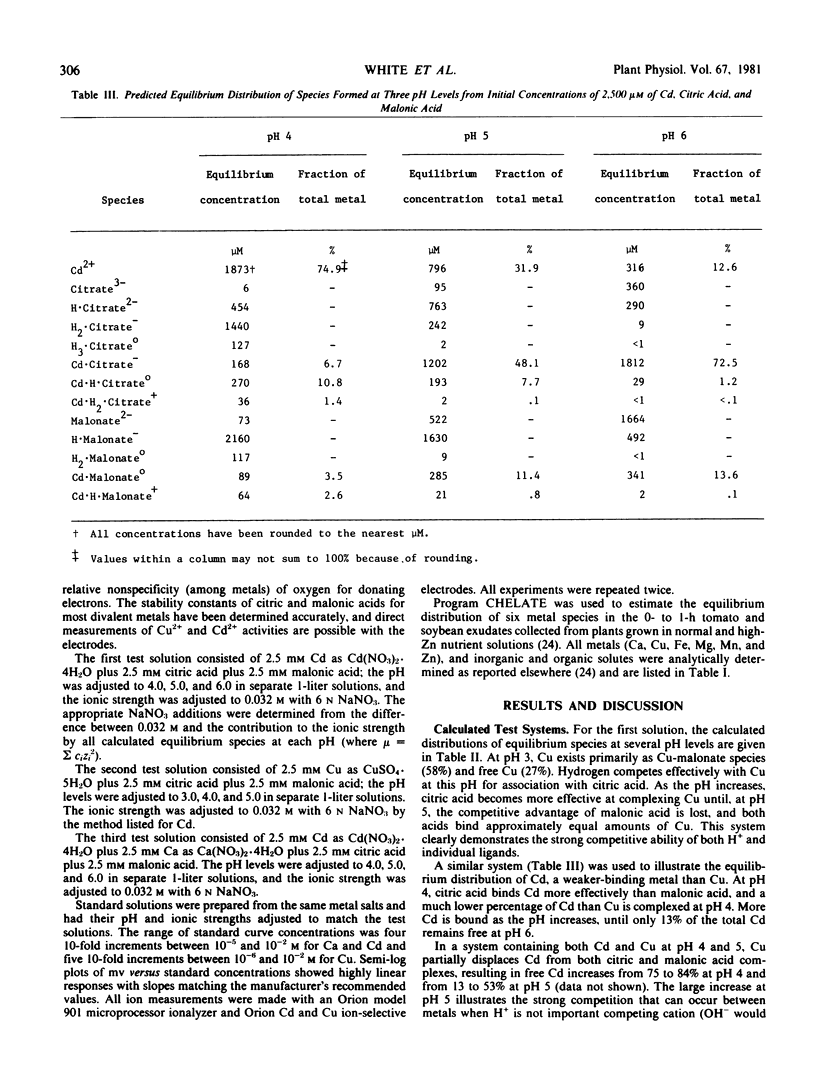
Program CHELATE was used to calculate the distribution of six metals in 0- to 1-hour exudate from soybean (Glycine max L. Merr.) and tomato (Lycopersicon esculentum Mill.) plants grown in normal and Zn-phytotoxic nutrient solutions. The results indicated that Fe is bound by citric acid, and Cu is bound by several amino acids in the normal-Zn exudate. Most of the Cu in soybean exudate is bound to asparagine and histidine. In tomato, Cu is bound to histidine, glutamine, and asparagine. Zinc, Mn, Ca, and Mg are bound primarily by citric acid and malic acid in both species; the per cent bound for these metals is lower than that for Fe and Cu. Zinc phytotoxicity caused equilibrium concentration shifts and resulted in the formation of several additional metal complexes not found in the normal-Zn exudate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cataldo D. A., Garland T. R., Wildung R. E. Nickel in Plants: II. Distribution and Chemical Form in Soybean Plants. Plant Physiol. 1978 Oct;62(4):566–570. doi: 10.1104/pp.62.4.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman H. C., Martin R. P. Potentiometric study of equilibria in aqueous solution between copper (II) ions, L (or D)-histidine and L-threonine and their mixtures. J Biol Chem. 1969 Sep 25;244(18):4823–4830. [PubMed] [Google Scholar]
- Hallman P. S., Perrin D. D., Watt A. E. The computed distribution of copper(II) and zinc(II) ions among seventeen amino acids present in human blood plasma. Biochem J. 1971 Feb;121(3):549–555. doi: 10.1042/bj1210549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A. S., Oberleas D. Binding of zinc to amino acids and serum proteins in vitro. J Lab Clin Med. 1970 Sep;76(3):416–425. [PubMed] [Google Scholar]
- Sarkar B. State of iron(3) in normal human serum: low molecular weight and protein ligands besides transferrin. Can J Biochem. 1970 Dec;48(12):1339–1350. doi: 10.1139/o70-208. [DOI] [PubMed] [Google Scholar]
- Tiffin L. O. Iron Translocation II. Citrate/Iron Ratios in Plant Stem Exudates. Plant Physiol. 1966 Mar;41(3):515–518. doi: 10.1104/pp.41.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffin L. O. Translocation of iron citrate and phosphorus in xylem exudate of soybean. Plant Physiol. 1970 Mar;45(3):280–283. doi: 10.1104/pp.45.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]



