Abstract
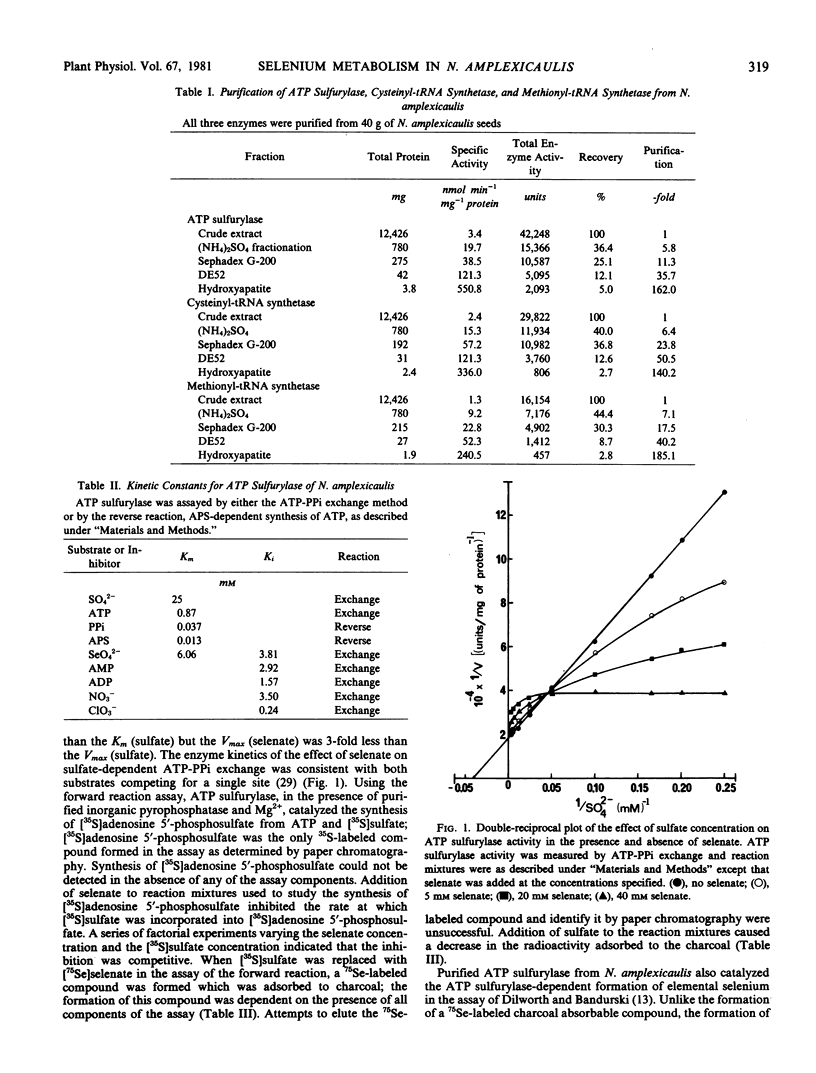
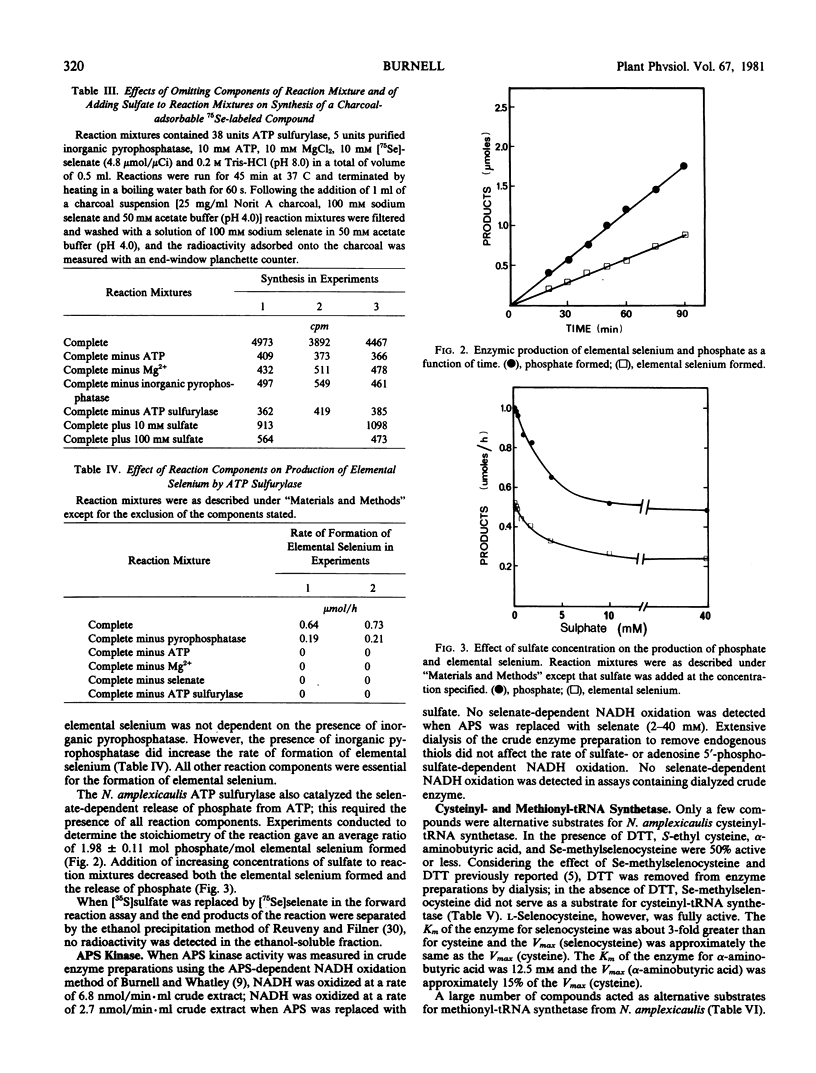
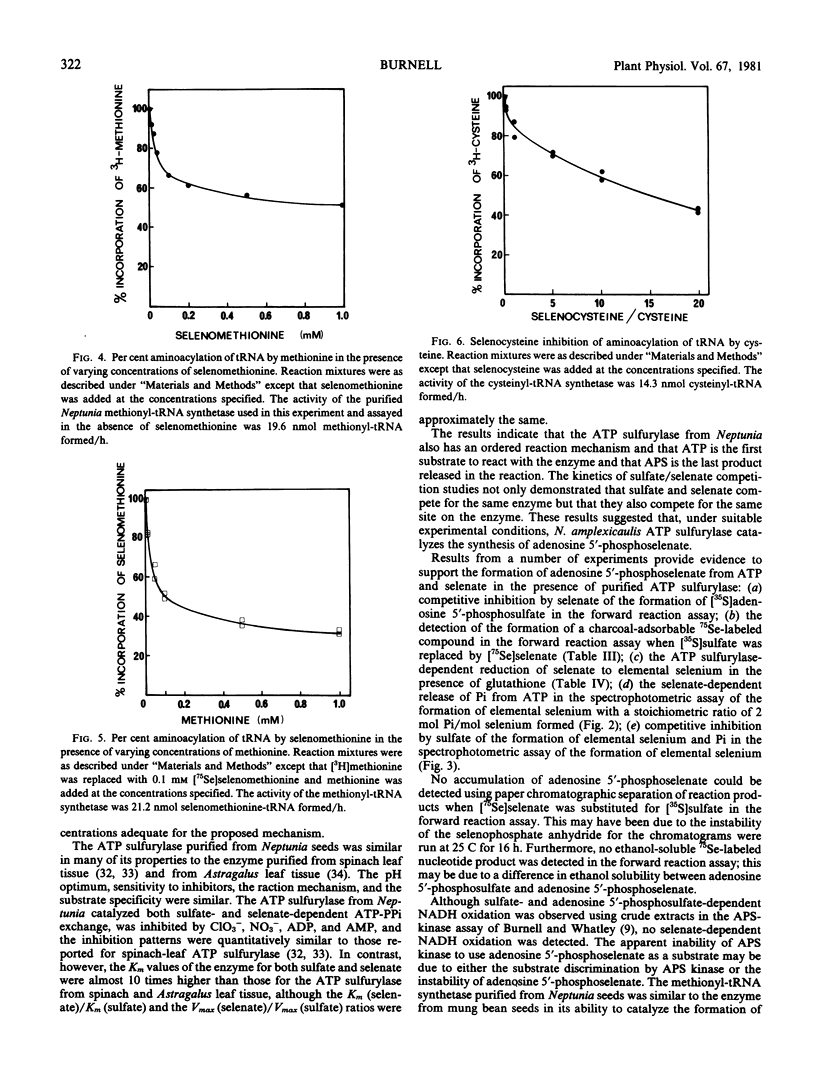
ATP sulfurylase (EC 2.7.7.4), cysteinyl-tRNA synthetase (EC 6.1.1.16), and methionyl-tRNA synthetase (EC 6.1.1.10) from Neptunia amplexicaulis have been purified approximately 162-, 140- and 185-fold, respectively. Purified ATP sulfurylase in the presence of purified inorganic pyrophosphatase catalyzed the incorporation of sulfate into adenosine 5′-phosphosulfate; evidence of an analogous reaction with selenate is presented. Crude extracts catalyzed both the sulfate- and the adenosine 5′-phosphosulfate-dependent NADH oxidation in the adenosine 5′-phosphosulfate kinase assay of Burnell and Whatley (1977 Biochim Biophys Acta 481: 266-278), but an analogous reaction with selenate could not be detected. Both purified cysteinyl-tRNA synthetase and methionyl-tRNA synthetase used selenium-containing analogs as substrates in both the ATP-pyrophosphate exchange and the aminoacylation assays.
It seems that selenium-containing amino acids are excluded from proteins by a mechanism(s) other than substrate discrimination at the amino acid activation stage of protein synthesis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. W., Rowan K. S. The extraction and assay of aminoacyl-transfer-ribonucleic acid synthetases of tobacco leaf. Biochem J. 1966 Oct;101(1):9–14. doi: 10.1042/bj1010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERG P. Acyl adenylates; the interaction of adenosine triphosphate and L-methionine. J Biol Chem. 1956 Oct;222(2):1025–1034. [PubMed] [Google Scholar]
- Burnell J. N., Anderson J. W. Adenosine 5'-sulphatophosphate kinase activity in spinach leaf tissue. Biochem J. 1973 Jun;134(2):565–579. doi: 10.1042/bj1340565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnell J. N. Cysteinyl-tRNA Synthetase from Astragalus Species. Plant Physiol. 1979 Jun;63(6):1095–1097. doi: 10.1104/pp.63.6.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnell J. N. Cysteinyl-tRNA Synthetase from Phaseolus aureus: Purification and Properties. Plant Physiol. 1977 Nov;60(5):670–674. doi: 10.1104/pp.60.5.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnell J. N., Karle J. A., Shrift A. Reduction of DL-selenocystine and isolation of L-seleoncysteine. J Inorg Biochem. 1980 Jul;12(4):343–351. doi: 10.1016/s0162-0134(00)80275-5. [DOI] [PubMed] [Google Scholar]
- Burnell J. N., Roy A. B. Purification and properties of the ATP sulphurylase of rat liver. Biochim Biophys Acta. 1978 Nov 10;527(1):239–248. doi: 10.1016/0005-2744(78)90273-5. [DOI] [PubMed] [Google Scholar]
- Burnell J. N., Whatley F. R. A new, rapid, and sensitive assay for adenosine 5'-phosphosulphate (APS) kinase. Anal Biochem. 1975 Sep;68(1):281–288. doi: 10.1016/0003-2697(75)90706-x. [DOI] [PubMed] [Google Scholar]
- Burnell J. N., Whatley F. R. Sulphur metabolism in Paracoccus denitrificans. Purification, properties and regulation of cysteinyl-and methionyl-tRNA synthetase. Biochim Biophys Acta. 1977 Mar 15;481(1):266–278. doi: 10.1016/0005-2744(77)90158-9. [DOI] [PubMed] [Google Scholar]
- Burnell J. N., Whatley F. R. Sulphur metabolism in Paracoccus denitrificans. Purification, properties and regulation of serine transacetylase, O-acetylserine sulphydrylase and beta-cystathionase. Biochim Biophys Acta. 1977 Mar 15;481(1):246–265. doi: 10.1016/0005-2744(77)90157-7. [DOI] [PubMed] [Google Scholar]
- Dilworth G. L., Bandurski R. S. Activation of selenate by adenosine 5'-triphosphate sulphurylase from Saccharomyces cerevisiae. Biochem J. 1977 Jun 1;163(3):521–529. doi: 10.1042/bj1630521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLAVIN M. Microbial transsulfuration: the mechanism of an enzymatic disulfide elimination reaction. J Biol Chem. 1962 Mar;237:768–777. [PubMed] [Google Scholar]
- HOLLEY R. W., APGAR J., DOCTOR B. P., FARROW J., MARINI M. A., MERRILL S. H. A simplified procedure for the preparation of tyrosine and valine-acceptor fractions of yeast "soluble ribonucleic acid". J Biol Chem. 1961 Jan;236:200–202. [PubMed] [Google Scholar]
- Hahn G. A., Brown J. W. Properties of a methionyl-tRNA synthetase from Sarcina lutea. Biochim Biophys Acta. 1967 Sep 12;146(1):264–271. doi: 10.1016/0005-2744(67)90093-9. [DOI] [PubMed] [Google Scholar]
- Hoffman J. L., McConnell K. P., Carpenter D. R. Aminoacylation of Escherichia coli methionine tRNA by selenomethionine. Biochim Biophys Acta. 1970 Feb 18;199(2):531–534. doi: 10.1016/0005-2787(70)90098-5. [DOI] [PubMed] [Google Scholar]
- James H. L., Bucovaz E. T. Purification and properties of the L-cysteinyl ribonucleic acid synthetase of bakers' yeast. J Biol Chem. 1969 Jun 25;244(12):3210–3216. [PubMed] [Google Scholar]
- Jáuregui-Adell J. Selenium derivatives in proteins. Adv Protein Chem. 1966;21:387–415. doi: 10.1016/s0065-3233(08)60130-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- NISSEN P., BENSON A. A. ABSENCE OF SELENATE ESTERS AND "SELENOLIPID" IN PLANTS. Biochim Biophys Acta. 1964 Feb 10;82:400–402. doi: 10.1016/0304-4165(64)90313-7. [DOI] [PubMed] [Google Scholar]
- Old J. M., Jones D. S. The aminoacylation of transfer ribonucleic acid. Recognition of methionine by Escherichia coli methionyl-transfer ribonucleic acid synthetase. Biochem J. 1977 Aug 1;165(2):367–373. doi: 10.1042/bj1650367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSON P. J., BUTLER G. W. Paper chromatographic and electrophoretic systems for the identification of sulphur and selenium amino acids. J Chromatogr. 1962 May;8:70–74. doi: 10.1016/s0021-9673(01)99231-3. [DOI] [PubMed] [Google Scholar]
- Peterson P. J., Butler G. W. The occurrence of selenocystathionine in Morinda reticulata Benth., a toxic seleniferous plant. Aust J Biol Sci. 1971 Feb;24(1):175–177. doi: 10.1071/bi9710175. [DOI] [PubMed] [Google Scholar]
- Pocklington T., Jeffery J. Competition of two substrates for a single enzyme. A simple kinetic theorem exemplified by a hydroxy steroid dehydrogenase reaction. Biochem J. 1969 Apr;112(3):331–334. doi: 10.1042/bj1120331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveny Z., Filner P. A new assay for ATP sulfurylase based on differential solubility of the sodium salts of adenosine 5'-phosphosulfate and sulfate. Anal Biochem. 1976 Oct;75(2):410–428. doi: 10.1016/0003-2697(76)90095-6. [DOI] [PubMed] [Google Scholar]
- Shaw W. H., Anderson J. W. Assay of adenosine 5-triphosphate sulfurylase by pyrophosphate exchange. Plant Physiol. 1971 Jan;47(1):114–118. doi: 10.1104/pp.47.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. H., Anderson J. W. Comparative enzymology of the adenosine triphosphate sulphurylases from leaf tissue of selenium-accumulator and non-accumulator plants. Biochem J. 1974 Apr;139(1):37–42. doi: 10.1042/bj1390037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. H., Anderson J. W. Purification, properties and substrate specificity of adenosine triphosphate sulphurylase from spinach leaf tissue. Biochem J. 1972 Mar;127(1):237–247. doi: 10.1042/bj1270237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. H., Anderson J. W. The enzymology of adenosine triphosphate sulphurylase from spinach leaf tissue. Biochem J. 1974 Apr;139(1):27–35. doi: 10.1042/bj1390027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrift A., Bechard D., Harcup C. Utilization of Selenocysteine by a Cysteinyl-tRNA Synthetase from Phaseolus aureus. Plant Physiol. 1976 Sep;58(3):248–252. doi: 10.1104/pp.58.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. K. Assay of proteins by Lowry's method in samples containing 2-mercaptoethanol. Anal Biochem. 1978 May;86(1):327–331. doi: 10.1016/0003-2697(78)90351-2. [DOI] [PubMed] [Google Scholar]
- Virupaksha T. K., Shrift A. Biochemical differences between selenium accumulator and non-accumulator Astragalus species. Biochim Biophys Acta. 1965 Aug 24;107(1):69–80. doi: 10.1016/0304-4165(65)90389-2. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P. A., Kaiser I. I. Aminoacylation of Escherichia coli cysteine tRNA by selenocysteine. Arch Biochem Biophys. 1975 Dec;171(2):483–489. doi: 10.1016/0003-9861(75)90057-0. [DOI] [PubMed] [Google Scholar]


