Abstract
Various properties of phosphoenolpyruvate carboxylases were compared in leaf preparations from C3-C4 intermediate, C3, and C4Panicum species. Values of Vmax in micromoles per milligram chlorophyll per hour at pH 8.3 were 57 to 75 for the enzyme from Panicum milioides, Panicum schenckii, and Panicum decipiens (all C3-C4). The values for Panicum laxum (C3) and Panicum prionitis (C4) were 20 to 40 and 952 to 1374, respectively. The Vmax values did not change at pH 7.3 except for the C4 value, which increased about 24%. At pH 8.3, the phosphoenolpyruvate carboxylases from C3 and C3-C4 species had slightly higher Km HCO3− and lower K′ phosphoenolpyruvate values than did the C4 enzyme. With each species at pH 7.3, all K′ phosphoenolpyruvate values were 2- to 4-fold greater.
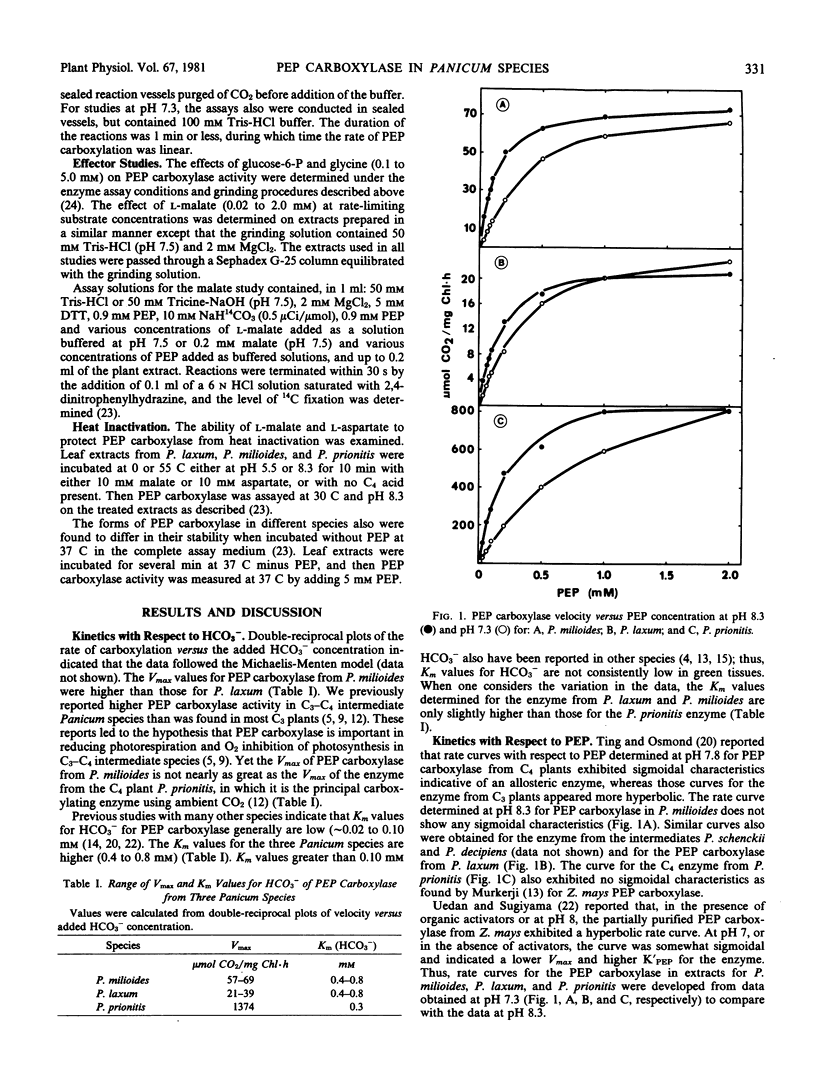
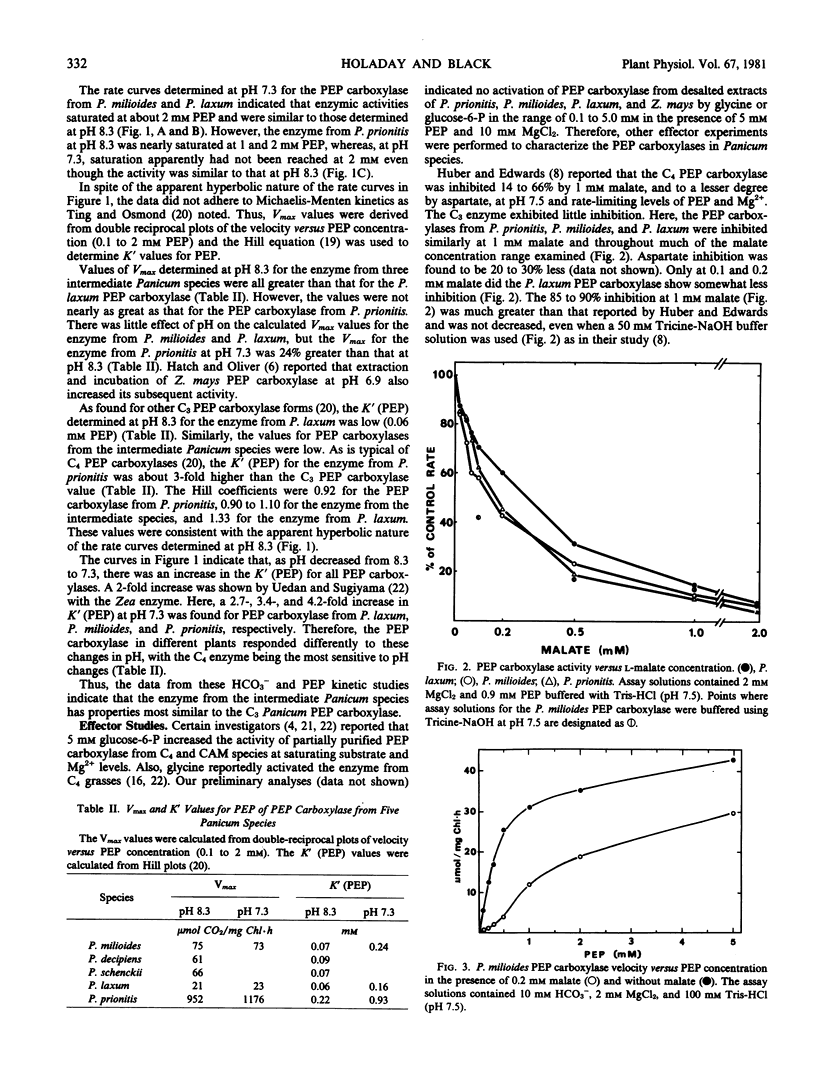
The enzyme from all species was inhibited 85 to 90% by 1 millimolar malate at rate-limiting phosphoenolpyruvate and Mg2+ levels. With low levels of malate, 0.2 millimolar, the rate curve with respect to phosphoenolpyruvate was distinctly sigmoidal, and the inhibition was not eliminated at 5 millimolar phosphoenolpyruvate.
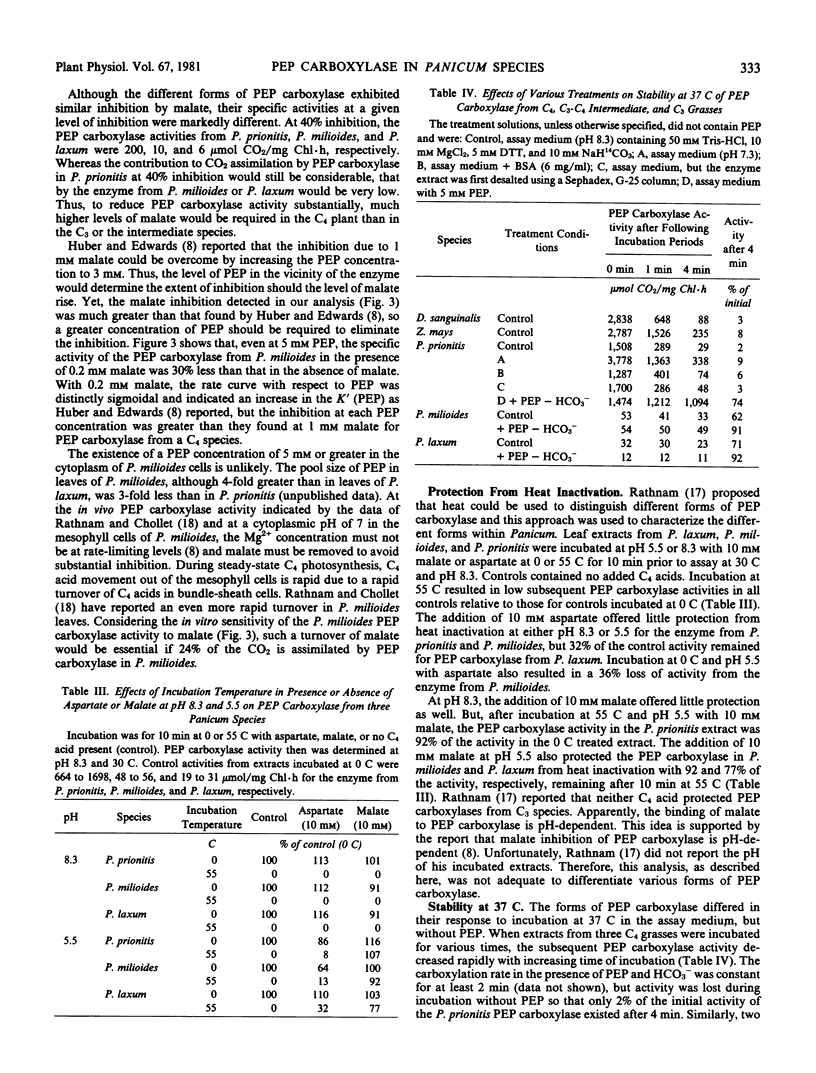
Malate at 10 millimolar protected all phosphoenolpyruvate carboxylases from inactivation at 55 C at pH 5.5, but not at pH 8.3. Aspartate did not protect well. When incubated at 37 C at pH 8.3 without phosphoenolpyruvate, but with HCO3−, the enzyme from several C4 grasses lost 92 to 98% of the initial activity after 4 minutes, whereas the enzymes from C3 and C3-C4Panicum species retained 60 to 70% of their activities. In contrast, 5 millimolar phosphoenolpyruvate stabilized the enzyme at 37 C in all plant extracts.
The phosphoenolpyruvate carboxylase from C3-C4 intermediate Panicum species has properties most similar to the enzyme from C3Panicum species. The higher leaf activity of the enzyme from the intermediate plants than from C3 species is not due to any unusual property assayed other than a higher Vmax.
Full text
PDF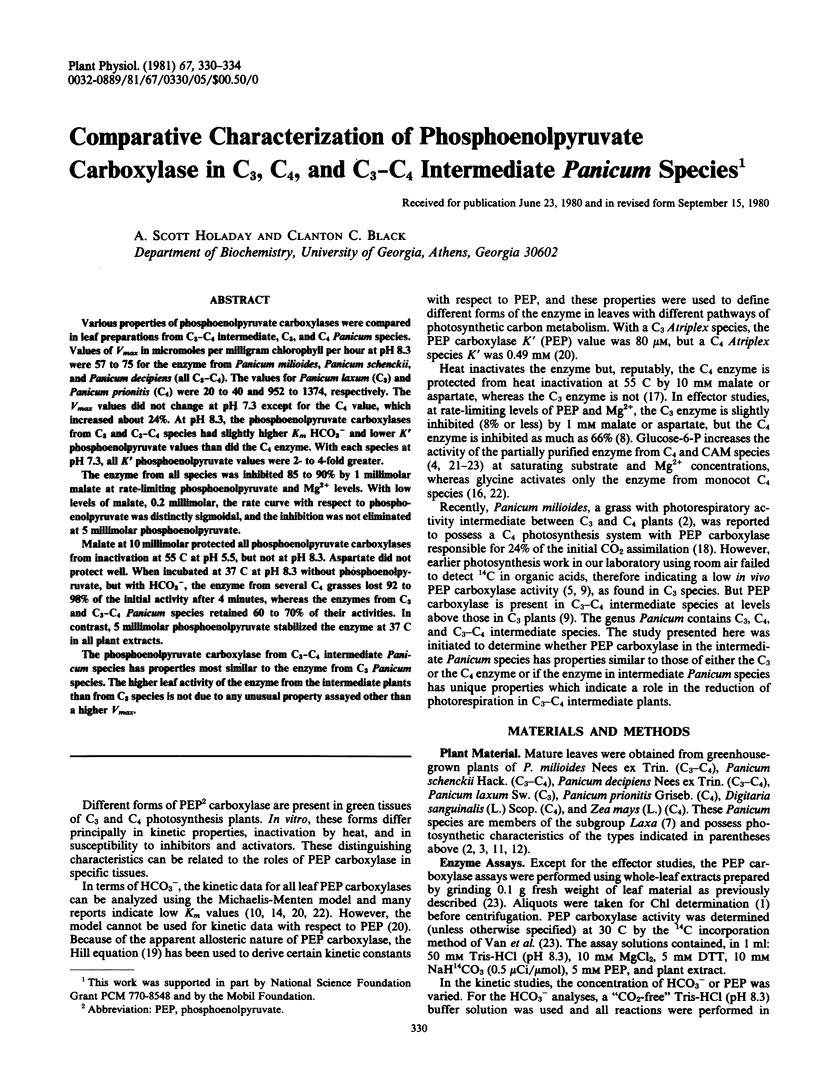

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. H. Photosynthesis of Grass Species Differing in Carbon Dioxide Fixation Pathways: IV. ANALYSIS OF REDUCED OXYGEN RESPONSE IN PANICUM MILIOIDES AND PANICUM SCHENCKII. Plant Physiol. 1980 Feb;65(2):346–349. doi: 10.1104/pp.65.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestler D. P., Mayne B. C., Ray T. B., Goldstein L. D., Brown R. H., Black C. C. Biochemical components of the photosynthetic CO2 compensation point of higher plants. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1439–1446. doi: 10.1016/0006-291x(75)90520-3. [DOI] [PubMed] [Google Scholar]
- Miziorko H. M., Nowak T., Mildvan A. S. Spinach leaf phosphoenolpyruvate carboxylase: purification, properties, and kinetic studies. Arch Biochem Biophys. 1974 Jul;163(1):378–389. doi: 10.1016/0003-9861(74)90489-5. [DOI] [PubMed] [Google Scholar]
- Morgan J. A., Brown R. H. Photosynthesis in Grass Species Differing in Carbon Dioxide Fixation Pathways: II. A Search for Species with Intermediate Gas Exchange and Anatomical Characteristics. Plant Physiol. 1979 Aug;64(2):257–262. doi: 10.1104/pp.64.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. A., Brown R. H. Photosynthesis in Grass Species Differing in Carbon Dioxide Fixation Pathways: III. OXYGEN RESPONSE AND ENZYME ACTIVITIES OF SPECIES IN THE LAXA GROUP OF PANICUM. Plant Physiol. 1980 Jan;65(1):156–159. doi: 10.1104/pp.65.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukerji S. K. Corn leaf phosphoenolpyruvate carboxylases. Purification and properties of two isoenzymes. Arch Biochem Biophys. 1977 Jul;182(1):343–351. doi: 10.1016/0003-9861(77)90315-0. [DOI] [PubMed] [Google Scholar]
- Mukerji S. K., Ting I. P. Phosphoenolpyruvate carboxylase isoenzymes: separation and properties of three forms from cotton leaf tissue. Arch Biochem Biophys. 1971 Mar;143(1):297–317. doi: 10.1016/0003-9861(71)90212-8. [DOI] [PubMed] [Google Scholar]
- Mukerji S. K., Yang S. F. Phosphoenolpyruvate carboxylase from spinach leaf tissue: inhibition by sulfite ion. Plant Physiol. 1974 Jun;53(6):829–834. doi: 10.1104/pp.53.6.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikido T., Takanashi H. Glycine activation of PEP carboxylase from monocotyledoneous C4 plants. Biochem Biophys Res Commun. 1973 Jul 2;53(1):126–133. doi: 10.1016/0006-291x(73)91410-1. [DOI] [PubMed] [Google Scholar]
- Rathnam C. K., Chollet R. Photosynthetic carbon metabolism in Panicum milioides, a C3-C4 intermediate species: evidence for a limited C4 dicarboxylic acid pathway of photosynthesis. Biochim Biophys Acta. 1979 Dec 6;548(3):500–519. doi: 10.1016/0005-2728(79)90061-6. [DOI] [PubMed] [Google Scholar]
- Ting I. P., Osmond C. B. Photosynthetic phosphoenolpyruvate carboxylases: characteristics of alloenzymes from leaves of c(3) and c(1) plants. Plant Physiol. 1973 Mar;51(3):439–447. doi: 10.1104/pp.51.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uedan K., Sugiyama T. Purification and characterization of phosphoenolpyruvate carboxylase from maize leaves. Plant Physiol. 1976 Jun;57(6):906–910. doi: 10.1104/pp.57.6.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van T. K., Haller W. T., Bowes G. Comparison of the photosynthetic characteristics of three submersed aquatic plants. Plant Physiol. 1976 Dec;58(6):761–768. doi: 10.1104/pp.58.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]



