Abstract
Type III galactosemia is a metabolic disorder caused by reduced activity of UDP-galactose-4-epimerase, which participates in galactose metabolism and the generation of various UDP-sugar species. We characterized gale-1 in Caenorhabditis elegans and found that a complete loss-of-function mutation is lethal, as has been hypothesized for humans, whereas a nonlethal partial loss-of-function allele causes a variety of developmental abnormalities, likely resulting from the impairment of the glycosylation process. We also observed that gale-1 mutants are hypersensitive to galactose as well as to infections. Interestingly, we found interactions between gale-1 and the unfolded protein response.
Keywords: Caenorhabditis elegans, galactosemia type III, glycosylation, GALE, unfolded protein response
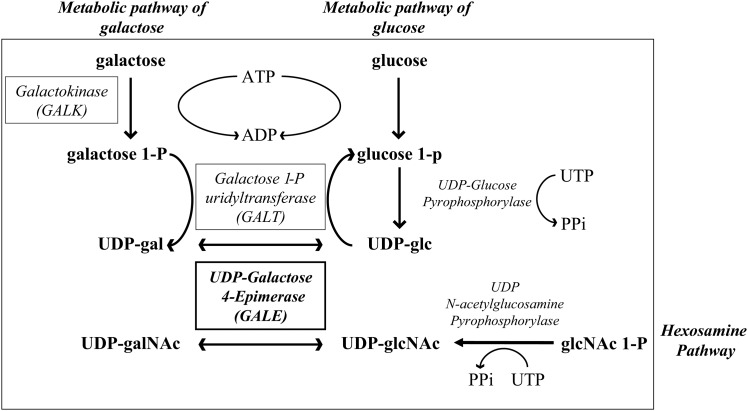
GALACTOSE is universally metabolized by three conserved enzymes that constitute the Leloir pathway (Figure 1) (Holden et al. 2003). UDP-galactose-4-epimerase (GALE) participates in the third step of the galactose metabolism pathway, catalyzing the interconversion of UDP-galactose (UDP-gal) and UDP-glucose (UDP-glc) and in some species, including humans, also the interconversion of UDP-N-acetylgalactosamine (UDP-galNAc) and UDP-N-acetylglucosamine (UDP-glcNAc) (Figure 1) (Maley and Maley 1959; Piller et al. 1983; Kingsley et al. 1986; Wohlers et al. 1999). Thus, GALE is essential not only to use galactose as an energy source but also to produce the different species of UDP sugars required for the glycosylation of proteins and lipids.
Figure 1.
The role of GALE in the biosynthesis of different UDP sugars. The enzymes in the Leloir pathway are boxed. GALE participates in the third step of the Leloir pathway and in the production of UDP-galNAc and UDP-glcNAc. Other metabolic pathways that participate in the production of UDP-glc and UDP-glcNAc are also represented.
In humans, mutations in GALE result in an autosomal recessive disorder known as type III galactosemia (OMIM #230350) (Fridovich-Keil 2006; Timson 2006), a rare disease characterized by the inability to metabolize galactose with symptoms that can include early onset cataracts, liver damage, deafness, and mental retardation (Walter et al. 1999). These symptoms are thought to occur as a result of the accumulation of intermediary galactose metabolites and the reduction of UDP-sugar species, which are needed for glycosylation. Other types of galactosemia are treated with a galactose-free diet. However, because of the dual role of GALE, patients with type III galactosemia are recommended to follow a galactose-restricted diet to avoid UDP-gal deficiency. This diet only partially alleviates the severe symptoms of galactosemia type III (Walter et al. 1999), and it has been proposed that patients receive UDP-galNAc as an additional treatment (Kingsley et al. 1986).
Traditional model systems to study type III galactosemia include yeast, which does not exhibit the interconversion of UDP-N acetylgalactosamine and UDP-N acetylglucosamine (Schulz et al. 2004), and cell lines, such as human fibroblasts and the ldlD cell line derived from Chinese hamster ovaries. The latter exhibits a complete loss of GALE activity, resulting in the abnormal processing of both N- and O-linked glycoproteins (Kingsley et al. 1986; Krieger et al. 1989; Slepak et al. 2007). Recently, the first multicellular model for this disease was generated in Drosophila, in which the deletion of GALE caused a lethal phenotype (Sanders et al. 2010). In the present study, we propose the use of Caenorhabditis elegans as a model to better understand the role of GALE in development and the consequences of its malfunctioning in multicellular organisms.
The free-living nematode C. elegans has been very useful for studies of development, disease, and other biological processes, including glycosylation (Culetto and Sattelle 2000; Kuwabara and O’Neil 2001; Berninsone and Hirschberg 2002; Berninsone 2006). Different mutations affecting enzymes involved in proteoglycan synthesis, N-glycosylation, O-glycosylation, and chitin and glycolipid synthesis have been isolated in this organism. Genetic and biochemical studies have demonstrated that most of the genes involved in glycosylation are conserved in humans (Schachter 2004; Berninsone 2006). Interestingly, most mutations affecting the glycosylation process show developmental defects. For example, most of the mutants defective in vulval epithelial invagination, the sqv mutant class, result from genes involved in the biosynthesis of the glycosaminoglycan chondroitin (Herman et al. 1999; Berninsone 2006), and mutations that affect N-glycosylation lead to the development of misshapen gonads. One of the genes involved in gonad development is MIG-17, a member of the ADAM (a disintegrin and metalloprotease) family. MIG-17 is a glycoprotein that is secreted by muscle cells and diffuses to the basement membrane of the gonad, where its function is required (Nishiwaki et al. 2000, 2004). Mutations that affect the MIG-17 glycosylation state prevent MIG-17 localization to the basement membrane and, as a consequence, exhibit a gonad migration defect similar to that of mig-17 mutants (Nishiwaki et al. 2000, 2004; Kubota et al. 2006). The analysis of mutants in which gonad migration is affected has enabled the identification of many different elements involved in the glycosylation process, indicating that C. elegans is an appropriate model for studying the mechanisms of glycosylation during development (Kubota and Nishiwaki 2006).
The process of protein glycosylation begins in the endoplasmic reticulum (ER), which is also responsible for protein folding, trafficking, quality control, degradation, and the coordinated response to the accumulation of unfolded proteins. Three proteins, conserved in all metazoans, are involved in sensing the stress caused by the accumulation of unfolded proteins (Bernales et al. 2006; Malhotra and Kaufman 2007): the protein kinase PERK (protein kinase RNA-like endoplasmatic reticulum kinase); the transcription factor ATF-6 (activating transcription factor 6); and XBP-1 (Xbox binding protein), a transcription factor that is activated after cleavage of the xbp-1 messenger RNA by IRE-1 (inositol-requiring enzyme 1; endonuclease resident in the ER). These sensors activate the unfolded protein response (UPR) pathway, initiating a complex response that involves the degradation of proteins, shuts off general translation, and enhances the expression of chaperones, such as the heat-shock proteins hsp-4 and hsp-3 in C. elegans (Lee 1992; Schroder and Kaufman 2005a,b; Shen et al. 2005).
Here, we characterize two different alleles of gale-1, the single homolog of the GALE gene in C. elegans that has 57% identity with the human GALE. The knockout allele is lethal, arresting development in the L2 larval stage, whereas a reduction-of-function allele is viable, although it exhibits severe developmental abnormalities consistent with impairment in protein glycosylation. This hypomorphic mutant is similar to the condition found in type III galactosemia patients and enables further characterization of gale-1 impairment. As described in humans and in model systems, this mutant strain is hypersensitive to a galactose-rich diet. Interestingly, it is also hypersensitive to human bacterial pathogens. We have also observed ER stress in the gale-1 reduction-of-function allele.
Materials and Methods
Strains and general methods
For the C. elegans strains, N2 and mutants were cultivated under standard conditions (Brenner 1974). The strains used are as follows: N2, GM107 [gale-1(pv18)], FX03267 gale-1(tm3267), MT7562 sqv-7(n2839), WT; Ex(mig-17::GFP), mig-23(k180); Ex(mig-17::GFP), GM238 gale-1(pv18); Ex[mig-17::GFP], GM328 sqv-7(n2839); Ex[mig-17::GFP], BC11076 C47B2.6::GFP, SJ4005 zcls4(hsp-4::gfp), GM266 gale-1(pv18); zcls4(hsp-4::gfp), GM308 atf-6(ok551); fer-15(b26), and GM 268 gale-1(pv18); pvIs1(C47B2.6 pgk10).
Bacterial strains included Escherichia coli OP50 and HT115, Enterococcus faecalis, Staphylococcus aureus, and Pseudomonas aeruginosa.
gale-1(pv18) mapping and identification
Genetic mapping using visible markers and nucleotide polymorphisms (Wicks et al. 2001) located the mutation in an interval of ∼60 kb on the right arm of chromosome I. Transgenic lines carrying cosmids mapped to this area revealed that the cosmid C47B2 complemented the mutant gale-1(pv18) phenotype. The five genes located in this cosmid were sequenced in the wild-type and in the pv18 mutant strain, revealing a mutation in the gale-1 gene, corresponding to the gene C47B2.6. Complementation of the temperature-sensitive lethal phenotype of gale-1(pv18) with a construct containing only the wild-type gale-1 gene driven by its own promoter demonstrated that pv18 is an allele of gale-1 UDP-galactose-4-epimerase.
This construct was generated by amplifying the gale-1 gene using the oligos 5′ TCTGACACCACGTGAAAACC 3′ and 5′ AGGTCCTACCGATGATGACG 3′ and by introducing the resulting PCR fragment into the pGEM-T Easy vector (Invitrogen). To generate the transgenic lines, we used the particle gun designed by Ralf Schnabel (http://www.ifg.tu-bs.de/Schnabel/) following the protocol described by Wilm et al. (1999). Transgenic lines were selected by screening for expression from the cotransfected plasmid pGK10 (sca-1::GFP) (Zwaal et al. 2001) using a Leica Fluo III stereoscope. In addition to the lethal temperature-sensitive phenotype, this construction fully rescues hypersensitivity to galactose, vulval, and gonad morphogenesis and hypersensibility to infection and rescues partially the synergistic lethal effect on xbp-1 RNA interference (RNAi).
Bacterial pathogen assay
Wild-type and gale-1(pv18) mutant worms (10 L4 per plate) were grown in the presence of pathogenic bacteria as the sole food source, and the numbers of survivors were counted every 2 days. E. faecalis strain OG1RF was grown in brain heart infusion with 40 μg/ml of gentamycin. S. aureus strain NTCT8325 was grown in Trypticase Soy Broth with 10 μg/ml of nalidixic acid.
Treatment with sugars
Eggs were incubated on NGM plates containing 0, 0.5, 1, 2, or 3% D-galactose (Sigma, G0625), D-glucose (Panreac 131341.0914), D-mannose (Fluka 63580), or L-glucose (G5500). Animals were scored after 4 days at 20°. Experiments with UV-killed bacteria were performed as described by Gems and Riddle (2000).
RNAi assays
RNAi feeding was performed as described in Kamath et al. (2003). L4 hermaphrodites were transferred to empty plasmid control pL4440 or to the appropriate RNAi plates at 20°, and the subsequent generation was analyzed.
Microscopy
L4 worms were mounted in M9 buffer between a coverslip and a 2% agarose pad. Images of the whole worm of SJ4005 and GM266 strains were captured using a Zeiss Axio Imager M2 microscope with N-Achroplan 10× and Plan Apochromat 10× objectives and a GFP-FITC-A488 filter. For MIG-17::GFP, we used an EC-Plan Neofluar ×40 objective. All pictures were acquired under the same conditions and with identical exposure time. For eggs, gonads, and vulva, we used this objective with Nomarski optics.
For coelomocytes, transmitted light and epifluorescence were recorded with a Nikon-A1R confocal microscope through a 60×/1.4 objective, captured using integral Nikon software.
HPLC
Whole extracts from 1000 young wild-type and gale-1(pv18) hermaphrodites were analyzed. Animals were collected in M9 buffer, incubated for 30 min at room temperature, washed three times in 3 ml of H2O, resuspended in 50 μl H2O, freeze-thawed three times on methanol/dry ice, and stored at −80°. For cell disruption, samples were thawed rapidly, and 500 μl of glass beads (0.2–1.5 g glass beads/ml, 0.1 mm diameter; Cole Parmer) was added at 4°. Bead-vortexing was performed at 4° on a Hybaid Ribolyser Mixer at 3000 × g for 1 min. After disruption, samples were centrifuged at top speed at 4° for 15 min. The supernatant was filtered and injected onto an reversed phase (RP) HPLC column (Supelcosil LC-18-T HPLC column, 5-μm particle size, length × i.d. of 25 cm × 4.6 mm). Detection was conducted by ultraviolet absorption at 262 nm (absorbance detector, Waters). The mobile phase was conducted with 150 mM KH2PO4 containing 2 mM tetrabutylammonium phosphate monobasic (Aldrich), pH 6.75, at a flow of 0.5 ml/min. Before and after each set of runs, standards of all four UDP sugars were injected and quantified. The UDP sugars of interest (UDP-gal, UDP-glc, UDP-galNAc, and UDP-glcNAc) demonstrated baseline separation.
Results
gale-1 mutations
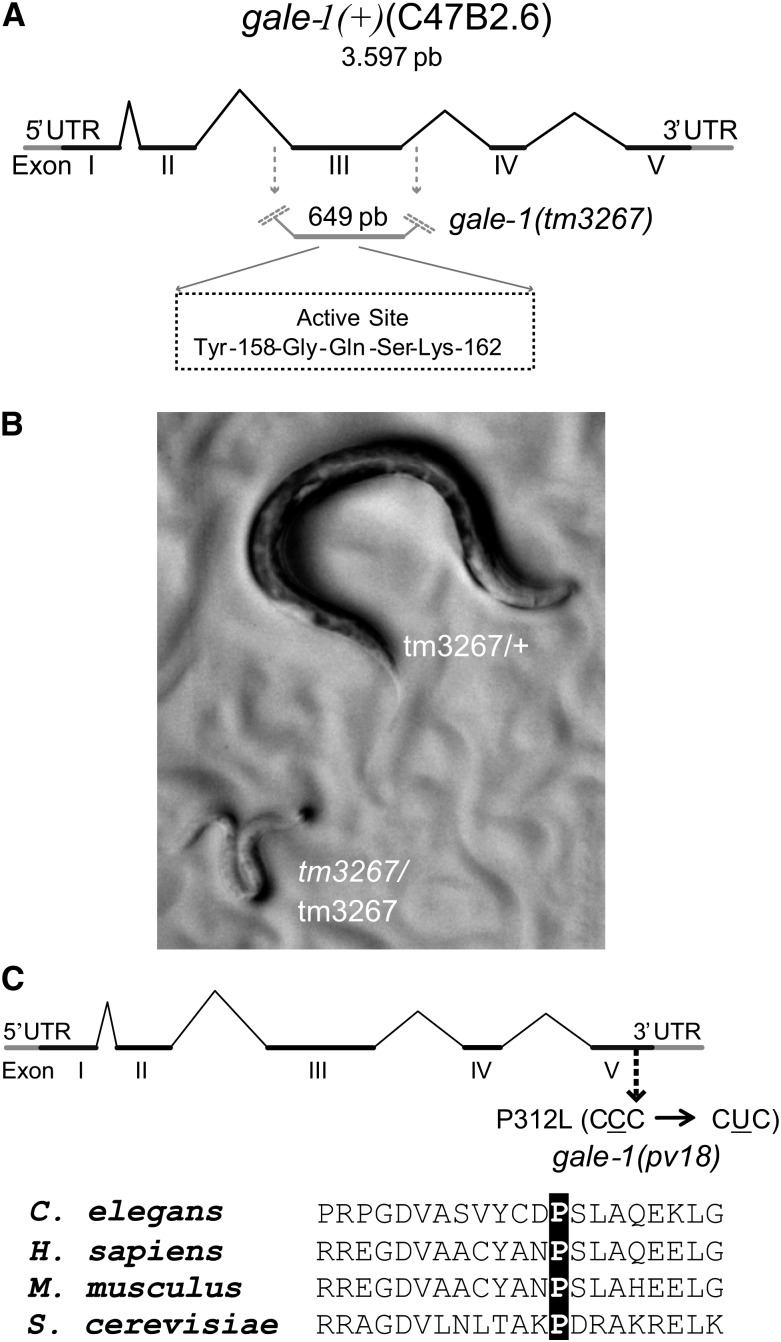
We characterized two different gale-1 alleles: gale-1(tm3267) and gale-1(pv18). In gale-1(tm3267), 649 bp encompassing the third exon in addition to the flanking intronic sequences have been replaced by an insertion of 6 bp, removing the active site of the GALE-1 protein (Thoden and Holden 1998; Thoden et al. 2001), which indicates that gale-1(tm3267) is a knockout allele (Figure 2A). Homozygous animals carrying this allele arrested in the L2 larval stage and exhibited a lethargic phenotype and pale body color (Figure 2B). This lethal phenotype complicated further characterization and is distinct from the condition of humans with type III galactosemia, who exhibit a reduction of GALE activity that generates a variety of disorders; no patients have been found to completely lack GALE function (Timson 2006).
Figure 2.
Locations of the gale-1(tm3267) and gale-1(pv18) mutations. (A) The gale-1(tm267) allele lacks a central region of the protein that contains the active site (YXXXK). (B) gale-1(tm267) homozygous mutants arrest during development at L2 stage with reduced movement and pale body color. The genotypes of these animals were confirmed by PCR. (C) gale-1(pv18) is a missense mutation that generates the substitution of a proline for a leucine residue in a conserved region.
Using the selection protocol for thermotolerant L1 larvae described in Munoz and Riddle (2003), we isolated the viable gale-1(pv18) mutant, which carries a leucine substitution for a conserved proline at position 312, which is close to the N-acetyl group interaction site (Figure 2C) (Thoden et al. 2001; Thoden et al. 2002) (see Materials and Methods for gene identification). Phenotypes of the maternal-effect mutant gale-1(pv18) described below and the lethal phenotype of gale-1(tm3267) were rescued with a transgene carrying the gale-1 wild-type allele driven by 1.2 kb upstream of the ORF, indicating that they are caused by the mutations in the gale-1 gene.
gale-1(pv18) is impaired in UDP-sugar levels
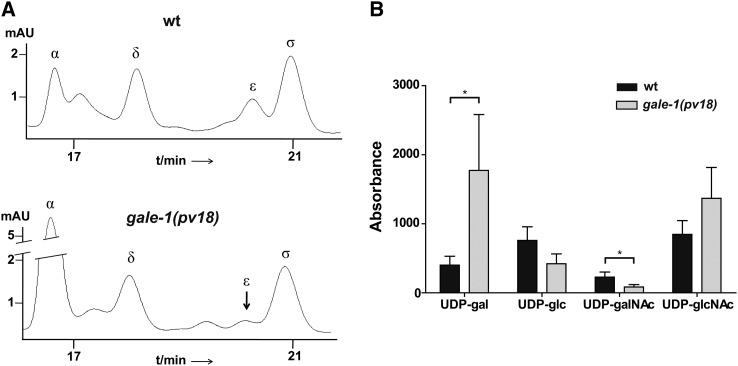
Using RP-HPLC, we compared the profile of the UDP-sugars synthesized by GALE-1 in the wild-type and gale-1(pv18) strains and found that mutant worms fed the standard E. coli strain OP50 accumulate UDP-gal and exhibit diminished levels of UDP-galNAc (Figure 3, A and B), as expected in response to a general reduction of the function of the gale-1 gene. Changes in UDP-glc and UDP-glcNAc are not significant. Interestingly, increased UDP-gal levels have been also observed in human patients (Walter et al. 1999) as in the yeast model for galactosemia type III (Quimby et al. 1997; Ross et al. 2004; Wasilenko et al. 2005; Chhay et al. 2008).
Figure 3.
RP-HPLC profile of the UDP-sugars synthesized by gale-1 in the gale-1(pv18) mutant. (A, Top) A representative profile from an extract of 1000 wild-type young adult worms. (Bottom) A representative profile from an extract of 1000 gale-1(pv18) young adult worms grown under the same conditions. α, UDP-gal; δ, UDP-glc; ε, UDP-galNAc; σ, UDP-glcNAc. (B) Mean and standard deviation of the absorbance area of the four sugars in three different repetitions for each strain. Two tailed t-test shows that only the differences in the UDP-gal and UDP-GalNAc levels are statistically significant. *P < 0.05.
gale-1(pv18) confers hypersensitivity to galactose
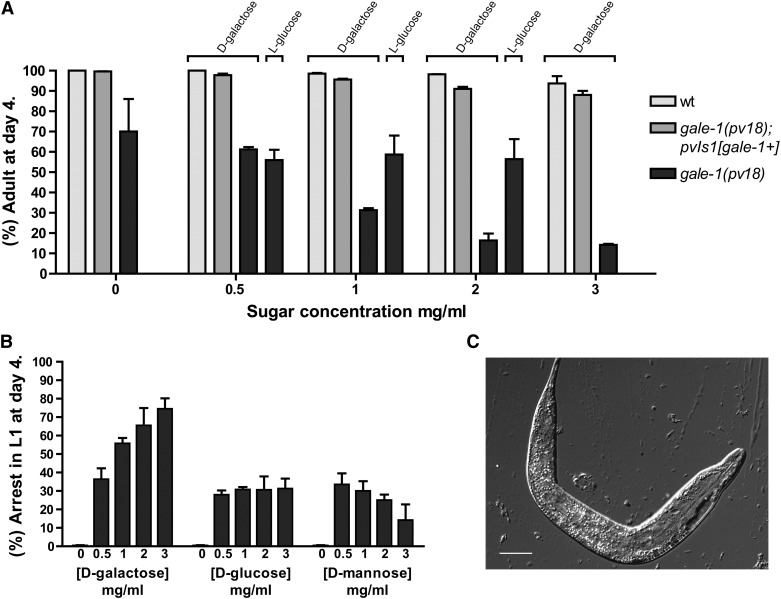
One of the symptoms of galactosemia is intolerance to a galactose-rich diet, which has been attributed to the accumulation of toxic galactose metabolism intermediates (Walter et al. 1999; Ross et al. 2004; Sanders et al. 2010; McCorvie et al. 2011). We observed that gale-1(pv18) worms cultivated in a high concentration of galactose develop slowly, indicating a hypersensitivity to galactose, whereas wild-type worms or gale-1(pv18) carrying a construction with gale-1(+) are not affected (Figure 4A). Interestingly, a percentage of animals cultivated in galactose were arrested at the L1 stage of development in a dosage-dependent manner, which was not observed in the wild-type strain. These animals showed a swollen and dark body (Figure 4, B and C). To test if this effect is specific to galactose, we also assayed different sugars in UV-killed bacteria to avoid interference with the bacterial metabolism, and, surprisingly, we found that treatment with glucose or mannose also generates a small percentage of L1 arrest. Interestingly, the percentage of arrested animals does not increase with the concentration of the sugar as happens with galactose (Figure 4B), indicating that galactose or metabolites derived from galactose have a more prominent effect on the L1 arrest phenotype.
Figure 4.
Galactose sensitivity of gale-1(pv18). (A) The percentage (%) of animals reaching adulthood after 4 days of incubation at different concentrations of D-galactose. Dramatically fewer gale-1(pv18) animals reached adulthood at the highest concentration of D-galactose, whereas wild-type or gale-1(pv18) transgenic with gale-1(+) animals were hardly affected. The nonmetabolizable sugar L-glucose does not affect development of gale-1(pv18) at any concentration assayed. There are no significant differences between wild type at 0 and any galactose concentration. There are significant differences between gale-1(pv18) at 0 and 1–3 mg/ml of galactose. P < 0.1. (B) The percentage (%) of animals arrested in L1 at different concentrations of galactose, glucose, or mannose. Treatment with 0.5 mg/ml of any of the sugars generates a percentage of arrested animals. This percentage does not increase in higher concentrations of glucose or mannose (P > 0.1). At higher concentrations of galactose, the percentage of arrested animals increases (P < 0.05 for 0.5 mg/ml vs. any other concentration). (C) A representative L1-arrested animal in the presence of galactose. Bar, 15 μm. Data are from two different experiments with n ≥ 200 in each.
gale-1(pv18) affects organ morphogenesis
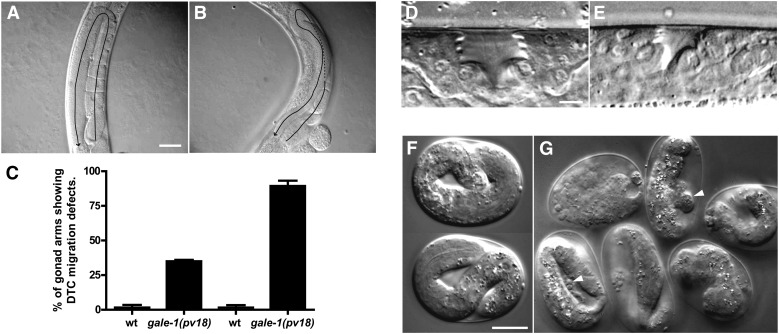
gale-1(pv18), but not gale-1(pv18) rescued with gale-1(+), worms exhibit multiple developmental defects. The animals develop more slowly at 16° and 20° than their wild-type counterparts (Supporting Information, Table S1), and their gonads are misshapen as a result of a migration defect (Figure 5, A and B). This last phenotype resembles those of mutants affected by alterations in the N-glycosylation process, such as mig-17, mig-23, and cogc-1–8 (Nishiwaki et al. 2004; Kubota et al. 2006). As with the gonads in those mutants, we observed that the posterior gonad in gale-1(pv18) mutants is more prone to this defect (Figure 5C).
Figure 5.
Developmental defect phenotypes of gale-1(pv18). (A) Wild-type gonad, which exhibits a U-shape. (B) gale-1(pv18) gonad. In this mutant, the gonads exhibit an irregular shape and are not symmetrical as indicated by the arrow. (C) Percentage of abnormal gonads in wild-type (N2) and gale-1 mutant. n > 20. P < 0.05. Animals were grown at 20°. (D) Wild-type vulva at the L4 stage. (E) Vulva of an L4 gale-1(pv18) animal. Note the reduced size (see also Figure S1). Animals were grown at 20°. (F) Wild-type embryos incubated at 25°. (G) gale-1(pv18) embryos incubated at 25°. Note the bubbles of embryonic material and vacuoles in the mutant indicated by arrowheads (see also File S1). Bars, 40 μm (A and B); 2.5 μm (D and E); and 20 μm (F and G).
gale-1(pv18) worms also showed a small vulval lumen with a reduction of >50% in size at the L4 stage (Figure 5, D and E, and Figure S1). This phenotype is similar to that of the squashed-vulva mutant class “sqv,” which primarily represents mutants in which the synthesis of the proteoglycan chondroitin is affected (Berninsone and Hirschberg 2002; Bulik and Robbins 2002; Hwang et al. 2003; Suzuki et al. 2006). Chondroitin is formed by repeated units of galNAc and glucuronic acid; therefore, the reduction of UDP-galNAc found in the gale-1(pv18) mutant is consistent with the impairment in the synthesis of this proteoglycan.
In addition, when gale-1(pv18) worms are reared at 25°, most embryos die before hatching and survivors arrest as L1 larvae. A closer inspection of the embryonic development showed frequent cell–cell adhesion defects, which caused embryos to disintegrate at an advanced stage of development. Additionally, some embryos failed to hatch even when they successfully completed morphogenesis (Figure 5, F and G, and File S1). The phenotypes described here indicate that the reduction of gale-1 activity affects multiple aspects of development, likely as a result of a general defect in the glycosylation process.
To investigate the expression pattern of gale-1, we used the BC11076 strain, in which expression of GFP is driven by 2927 bp upstream of the ATG of gale-1. Previous reports indicate that gale-1 is expressed mainly in the gonads, vulva, intestine, and nervous system in L4/adult-stage worms (WormBase: http://www.wormbase.org). We observed that gale-1 is also expressed in muscle tissue. Especially high and broad gale-1 expression was detected in embryos and in the L1 stage (Figure S2).
MIG-17 is mislocalized in the gale-1(pv18) mutant
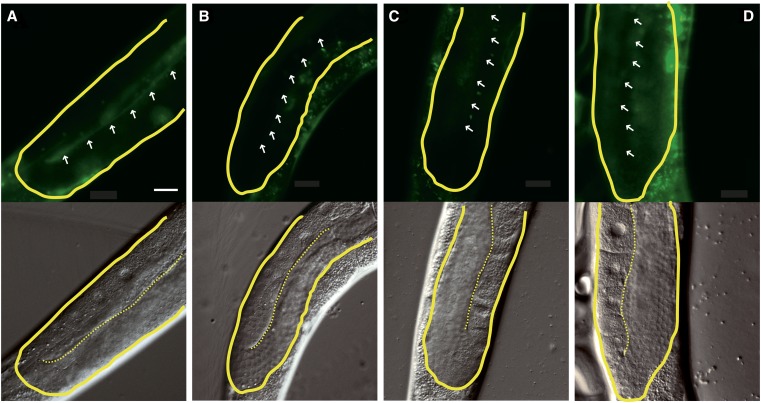
To identify a possible mechanism for the gonad migration defects of gale-1(pv18), we investigated the location of the metalloprotease MIG-17. To carry out its normal functions, N-glycosylated MIG-17 is secreted by muscle cells and localizes to the basement membrane of the gonad (Nishiwaki et al. 2004). Mutations in mig-17 or in genes involved in MIG-17 glycosylation cause gonad migration defects similar to that described in the gale-1(pv18) mutant. We observed that MIG-17 localization to the gonad basement membrane is reduced in the gale-1(pv18) mutant (Figure 6, A and B), similarly to the mig-23 mutant, which affects MIG-17 glycosylation (Figure 6D), and to mutations at MIG-17 glycosylation sites (Nishiwaki et al. 2004). This suggests that the impairment of MIG-17 glycosylation may be responsible for the gonad-migration phenotype observed in the gale-1(pv18) mutant.
Figure 6.
MIG-17 location is altered in gale-1 and sqv-7 mutant backgrounds. (Top) GFP fluorescence. (Bottom) Nomarski. White arrows indicate the location of the basement membrane. (A) MIG-17::GFP is located in the basement membrane of the gonad in a wild-type background; 32 ± 10.6% of increase of fluorescence level in the basement membrane relative to the central region of the gonad. n = 8. (B) In a gale-1(pv18) mutant background, the location of MIG-17::GFP in the basement membrane is reduced relative to wild-type background; 10.2 ± 3.9% increase of fluorescence level in the basement membrane relative to the central region of the gonad. n = 8. The differences with the wild-type background are significant; P < 0.001.Two tailed t-tests were used for comparison. (C) Similarly, in an sqv-7(n2839) background, MIG-17::GFP is also mislocalized. (D) mig-23(k180), in which MIG-17::GFP has been reported to be mislocalized, was used as control. Bar, 20 μm.
sqv-7 encodes a protein that transports UDP-glucuronic acid, UDP-gal, and UDP-galNAc to the Golgi apparatus. The sqv-7(n2839) mutant exhibited both an sqv phenotype (Herman et al. 1999; Berninsone et al. 2001; Bulik and Robbins 2002; Hwang and Horvitz 2002) and, as we observed, a gonad migration defect similar to that of the gale-1(pv18) mutant (Figure S3). Interestingly, we found that MIG-17 is also mislocalized in an sqv-7 mutant background (Figure 6C), suggesting that a decrease of UDP-sugar in the cytosol as a result of the gale-1 mutation or in the Golgi as a result of impairment of SQV-7 activity generates improper glycosylation, incorrect localization, and the malfunctioning of MIG-17. Because UDP-galNAc is affected in both gale-1 and sqv-7 mutants, these data highlight UDP-galNAc as an important molecule in the sqv and mig phenotypes.
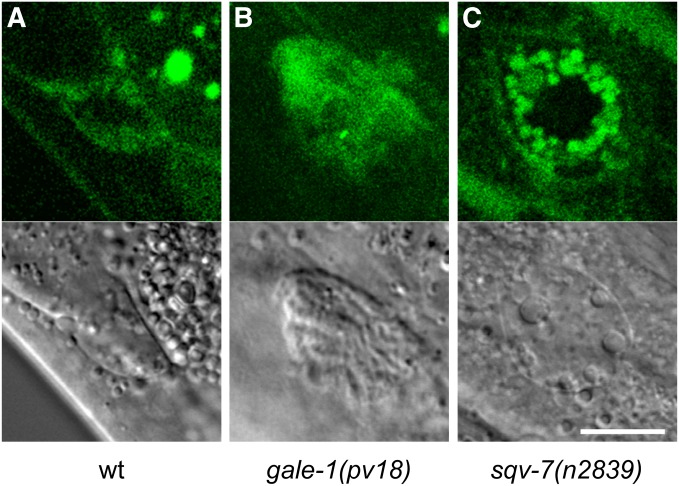
The phenotype of MIG-17 mislocalization could be due either to a defect in the secretion from the muscle cells or to the loss of affinity to the basal membrane of the gonad. To discriminate between these possibilities, we have studied the accumulation of MIG-17, labeled with GFP, in the coelomocyte cells. Those cells endocytose the pseudocoelom fluid and are useful to test if proteins are secreted from other cell types (Kubota et al. 2006). We have observed the presence of MIG-17-GFP in coelomocytes of gale-1(pv18) and sqv-7(n2839) mutants (Figure 7), indicating that MIG-17 is secreted from the muscle cells in both genetic backgrounds.
Figure 7.
Uptake of MIG-17::GFP by coelomocytes. (Top) GFP fluorescence. (Bottom) Nomarski of the same coleomocytes. (A) MIG-17::GFP located in the coleomocytes in a wild-type background, (B) in a gale-1(pv18) background, and (C) in a sqv-7(n2839) mutant background.
gale-1(pv18) activates the unfolded protein response pathway
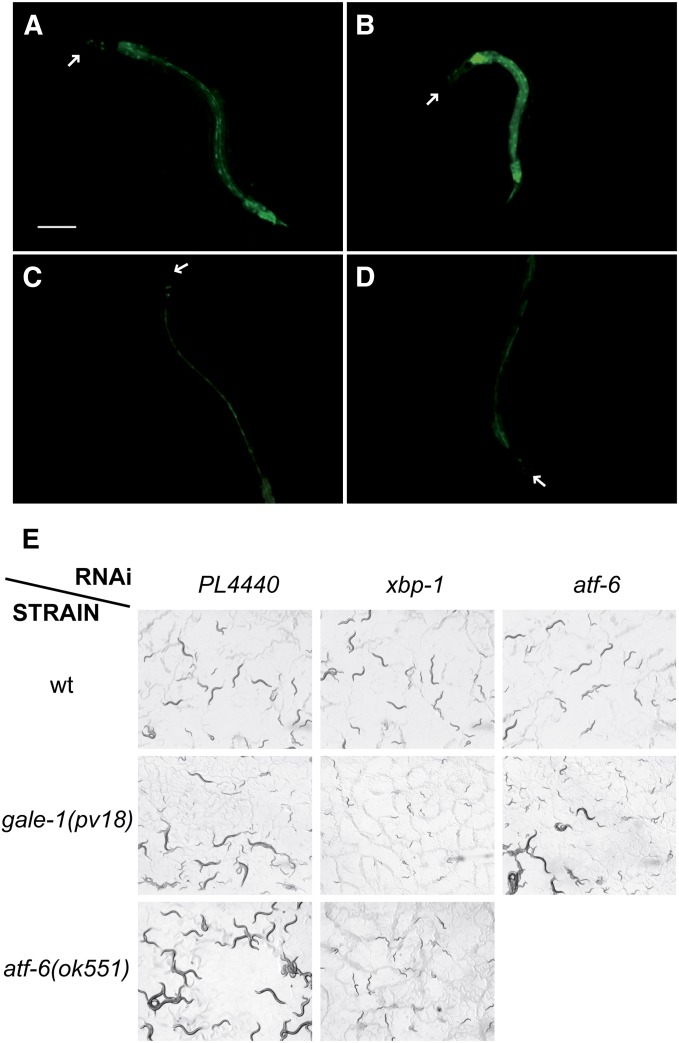
Because the N-glycosylation process begins in the ER, we hypothesized that the altered UDP-sugar levels observed in the gale-1(pv18) mutant may affect the activity of this organelle and may activate the unfolded protein response pathway. One of the genes involved in this pathway is xbp-1, which produces an inactive transcript that is activated by the ire-1 endonuclease upon ER stress (Tirasophon et al. 1998; Wang et al. 1998). The transcription factor XBP-1 promotes the expression of numerous genes involved in the stress pathway, among them hsp-4 (Calfon et al. 2002; Shen et al. 2005). We therefore tested the expression of hsp-4, and, as shown in Figure 8, A and B, the expression was ∼2.5 times higher in gale-1(pv18) than in the wild type (Figure S4). A high level of hsp-4 expression is also observed in a atf-6(ok551) mutant background (Figure S5). The reduction of XBP-1 or IRE-1 activity by RNAi completely suppresses hsp-4 expression in both mutants (Figure 8, C and D, Figure S5, and data not shown). These results suggest that the expression of hsp-4 in atf-6(ok551) and in gale-1(pv18) is caused by the activation of xbp-1.
Figure 8.
Interaction of gale-1(pv18) with the UPR pathway. (A) hsp-4::gfp expression in a wild-type background. (B) The expression of hsp-4 is induced in a gale-1(pv18) mutant background, indicating that the ER is stressed (see also Figure S4). (C) hsp-4::gfp expression in animals treated with xbp-1 RNAi is absent in a wild-type background as well as (D) in a gale-1(pv18) mutant background. Arrows indicate the head of the animals. Bar, 100 μm. Animals shown are representative of the population. (E) L4 animals were transferred to RNAi, and the next generation was analyzed. Wild-type animals (top) treated with control pL4440 (empty plasmid), RNAi of xbp-1, and atf-6 reach adulthood. gale-1(pv18) (middle) treated with control pL4440 (empty plasmid), RNAi of xbp-1, and atf-6. Note that gale-1(pv18) animals treated with xbp-1 RNAi arrest at L1 and L2 stages but those treated with atf-6 RNAi reach adulthood. atf-6(ok551) (bottom) treated with control pL4440 (empty plasmid) and RNAi of xbp-1. atf-6(ok551) similarly to gale-1(pv18) animals treated with xbp-1 RNAi arrested at L2 and L1 stages. atf-6(ok551) is in a fer-15(b26) background that does not affect this experiment.
xbp-1 participates together with another stress sensor, atf-6, in C. elegans development. Reduction of either of those genes independently does not affect development, but animals arrest in the L2 stage when both are simultaneously eliminated (Shen et al. 2005). We observed that gale-1(pv18) can develop to adulthood under atf-6 RNAi treatment but arrest in the L2 stage under reduction of xbp-1 activity by RNAi, similarly to xbp-1 RNAi treatment of atf-6 mutants (Figure 8E). This result suggests that gale-1 and atf-6 are involved in the same genetic pathway to affect development. Heteroallelic tm3267/pv18 animals also arrest development when subjected to xbp-1 RNAi (data not shown). tm3267; xbp-1(RNAi) animals are fully rescued by the gale-1+ transgene (data not shown) while pv18; xbp-1(RNAi) animals are not fully rescued to viability by this transgene; these animals, instead of arresting at the L2 stage as their not-transgenic siblings do, continue development to L4, but they do not reach adulthood. This result suggests a semidominant effect of pv18 for this phenotype.
The expression of hsp-4 and the arrest in development with the xbp-1 RNAi treatment in gale-1(pv18) suggest that the endoplasmic reticulum is compromised in animals lacking normal GALE-1 activity.
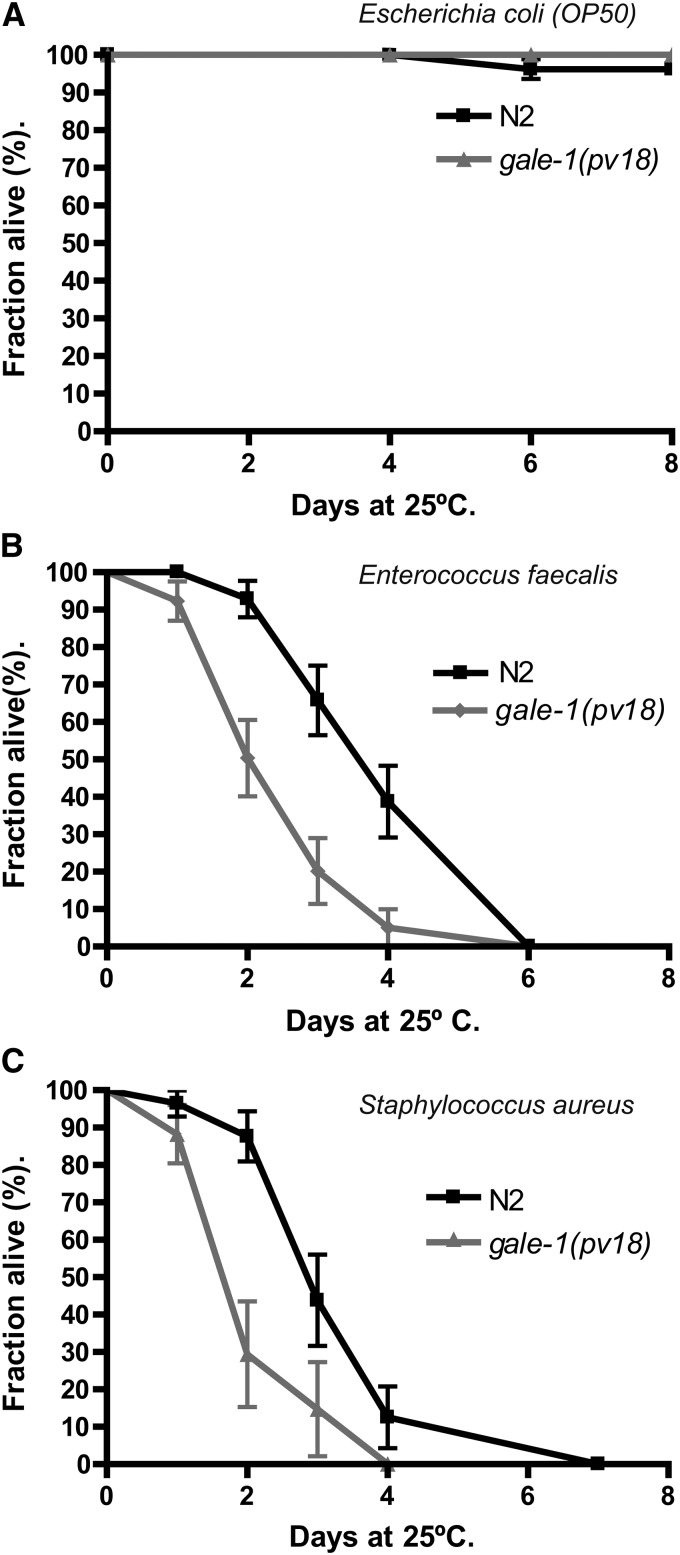
gale-1(pv18) is hypersensitive to infection
Patients affected with type I galactosemia have been described as suffering an increased risk of infections (Levy et al. 1977; Litchfield and Wells 1978; Waggoner et al. 1990; Rathi and Rathi 2011). C. elegans is a widely used model to understand host–pathogen interaction, and many human bacterial pathogen have been described to affect this nematode (Sifri et al. 2005).
We tested the survival of gale-1(pv18) mutants grown in the presence of different species of human pathogenic bacteria that have been previously shown to infect C. elegans (Garsin et al. 2003). We observed that gale-1(pv18) animals incubated with E. faecalis or S. aureus (Figure 9) exhibited lower survival than wild-type worms but not when complemented with the transgene containing gale-1(+) (Figure S6), indicating that they are hypersensitive to infection. This result, if conserved in humans, may have medical implications for the management of patients with type III galactosemia.
Figure 9.
Sensitivity to infection with human bacterial pathogens in gale-1(pv18) mutant and wild-type animals. (A) gale-1(pv18) survival in the E. coli strain OP50 is not affected. (B) gale-1(pv18) is hypersensitive to E. faecalis. (C) gale-1(pv18) is hypersensitive to S. aureus. n > 30. Two different trials were assayed with similar results. The Kaplan–Meier statistical analysis was used. In A, no differences between the wild-type and the gale-1(pv18) mutant were observed; P > 0.1. In B and C, differences between the wild-type and the gale-1(pv18) mutant were significant; P < 0.005.
Discussion
In this study, we have reported the characterization of two mutations in the C. elegans GALE ortholog, a knockout allele, and a reduction-of-function allele. We found that the loss of function of gale-1 in C. elegans is lethal. The same lethal effect has also been shown in Drosophila and has been hypothesized for humans (Kalckar 1965; Sanders et al. 2010). This condition complicates phenotypic analysis and is dissimilar to the condition found in type III galactosemia in humans, in which patients exhibit a reduction of the GALE activity but never a complete lack of activity. We studied the reduction-of-function allele gale-1(pv18) in detail because this allele enables physiologic and genetic studies. We observed that the changes in the UDP-sugar profile found in gale-1(pv18) are consistent with a reduction of activity of GALE. The increased level of UDP-gal can be explained by the inability to metabolize dietary galactose, and the strong reduction of UDP-galNAc levels can be attributed to the fact that GALE is the primary enzyme responsible for UDP-galNAc biosynthesis. In contrast, UDP-glc and UDP-glcNAc are synthesized also by GALE-independent pathways (Figure 1), which may explain why the changes observed in the levels of these sugars are not statistically significant (Schulz et al. 2005; Johnston et al. 2006). These data, together with the recessive nature of gale-1(pv18), strongly suggest that this is a viable reduction-of-function allele similar to the situation described in patients with type III galactosemia (Timson 2006). While all GALE homologs are able to metabolize UDP-gal and UDP-glc, not all the species are able to interconvert UDP-glcNAc and UDP-galNAc. The strong reduction of UDP-galNAc observed in the gale-1(pv18) mutant indicates that C. elegans GALE-1 can accomplish this reaction, similarly to human GALE.
Patients affected by type III galactosemia are sensitive to dietary galactose, likely as a result of the accumulation of toxic intermediary galactose metabolites. As expected, gale-1(pv18) animals are hypersensitive to a galactose-rich diet, which has been reported not only for humans but also for Drosophila and Saccharomyces cerevisiae (Walter et al. 1999; Ross et al. 2004; Sanders et al. 2010; McCorvie et al. 2011). The toxic effects of elevated galactose concentrations observed in C. elegans include developmental delay and arrest in L1 stages. Surprisingly, this effect is produced by adding not only galactose, but also other sugars, although only with galactose is a dose-dependent effect observed, which suggests a more direct interaction with this sugar or with derived metabolites of galactose.
Strikingly, in the wild-type strain, the ER stressor tunicamycin also generates a developmental arrest at or prior to L3 stage (Shen et al. 2001), similarly to ire-1pek-1, atf-6xbp-1, or gale-1xbp-1 double mutants. Those data suggest that strong ER stress could cause an arrest of development. It is possible that, in the gale-1(pv18) mutant, the alteration of the UDP-glucose/galactose rate in a galactose-rich diet and the strong demand of protein folding during development could generate this collapse. Alternatively, accumulation of other metabolites derived specifically from galactose could be responsible for this arrest.
In addition, our analysis strongly suggests that the gale-1(pv18) mutant has defects in the glycosylation process; mutant worms exhibit sqv and mig phenotypes, which are often observed in glycosylation mutants, as well as temperature-sensitive lethality with reduced cellular cohesion. In addition, the MIG-17 metalloprotease, which must be glycosylated to be anchored in the basement membrane, requires GALE-1 for proper localization; although MIG-17 is secreted from the muscle cells in the gale-1(pv18) mutant, it fails to accumulate in the gonadal basement membrane. Interestingly, sqv-7, which encodes a UDP-glucuronic acid, UDP-gal, and a UDP-GalNAc Golgi transporter, shows a similar phenotype not only in the formation of the vulval lumen (Berninsone et al. 2001) but also, as we observed, in gonad migration and MIG-17 localization. These data suggest that the sqv and mig phenotypes in the gale-1 and sqv-7 mutants may result from a decrease in the availability of UDP-galNAc, which is the only sugar defective in both mutants.
UDP-galNAc does not belong to the core branch of N-glycosylation in C. elegans and is usually added at the end of the sugar chain (Paschinger et al. 2008). Therefore, if MIG-17 is affected by the alteration of UDP-galNAc level, probably the changes in MIG-17 glycosylation will not be profound, but sufficient to impair attachment to the basement membrane of the gonad. Alternatively, the reduction of UDP-galNAc may not affect MIG-17 glycosylation at all. Instead, the alteration of the extracellular matrix, composed of proteoglycans, may impair the migration of MIG-17 from muscle to gonad. In support of this, mutations in the sqv-5 or in its cofactor mig-22 genes, which are involved in the formation of chondroitin, also have-gonad migration-defect phenotypes (Suzuki et al. 2006).
In this work, we have also observed that a decrease in GALE-1 function activates the unfolded protein response pathway, possibly by generating chronic ER stress. The sugar reduced in gale-1(pv18), UDP-galNAc, does not participate in the glycosylation carried out in the ER (Berninsone 2006), suggesting that the stress observed in this organelle may be caused not by a reduction of this substrate in the ER but by the incorrect glycosylation of proteins involved in ER functions or perhaps by alterations of the UDP-glucose/galactose ratio. Indeed, it has been shown that increased galactose or galactose-derived metabolites induces the activation of the ER stress response by an unknown mechanism in a human cell model of galactosemia (Slepak et al. 2005, 2007; Mulhern et al. 2006).
gale-1(pv18) animals are hypersensitive to human pathogens. Although we do not know the nature of this sensitivity, research suggests that ER stress is implicated. This may be due to a possible impairment in secretion of proteins, among them antimicrobial proteins, or to the collapse of ER by the increase of ER stress upon bacterial infection.
Interestingly, hypersensitivity to pathogens has also been described for patients with type I galactosemia (Levy et al. 1977; Litchfield and Wells 1978; Waggoner et al. 1990; Rathi and Rathi 2011). This phenotype that we observed in the C. elegans model for type III galactosemia may be conserved in humans and could have medical implications in the management of patients with type III galactosemia.
In summary, we have characterized two mutations in the gene affected in type III galactosemia, a knockout allele that presents a lethal phenotype and a reduction-of-function allele that is more similar to the mutations found in human patients. The genetic studies using the reduction-of-function allele have generated findings that may have medical implications: hypersensitivity to human pathogens and elevated ER stress. In addition, we observed strong developmental defects in gonad migration and in vulva formation. This model organism and the mutants described here will be useful not only for learning about this disease but also for conducting complementation tests of different human alleles, drug efficacy tests, or large-scale drug screens and thus may be of special interest for designing treatments for type III galactosemia.
Supplementary Material
Acknowledgments
We thank the Caenorhabditis Genetics Center, Frederick Ausubel, David Baillie, Kijochi Nishiwaki, and the lab of Shohei Mitani through the National Bio-Resource Project of Ministry of Education, Culture, Sports, Science, and Technology, Japan, for providing strains; Peter Askjaer for help with the DeltaVision microscope and critical reading of this manuscript; and Alejandra Cano, Victor Carranco, Valle Rubio, Katherina García, and Ana Isabel López for their excellent technical assistance. The authors declare no competing financial interests. This work was supported by the Junta de Andalucía (P07-CVI-02697) and the Spanish Ministry of Science and Innovation (BFU2006-07391/BMC) and Ministry of Economy and Competitiveness (BFU2013-46923-P). A.M.B.-L. was supported by a Plan Propio de Investigación fellowship from the Universidad Pablo de Olavide. J.M.M. was supported by the Formación del personal Universitario program of the Spanish Ministry of Science and Innovation.
Footnotes
Supporting information is available online at http://www.genetics.org/lookup/suppl/doi:10.1534/genetics.114.170084/-/DC1.
Communicating editor: M. V. Sundaram
Literature Cited
- Bernales S., Papa F. R., Walter P., 2006. Intracellular signaling by the unfolded protein response. Annu. Rev. Cell Dev. Biol. 22: 487–508. [DOI] [PubMed] [Google Scholar]
- Berninsone P. M., 2006. Carbohydrates and glycosylation. (December 18, 2006), WormBook, ed. The C. elegans Research Community, WormBook, /10.1895/wormbook.1.125.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berninsone P. M., Hirschberg C. B., 2002. The nematode Caenorhabditis elegans as a model to study the roles of proteoglycans. Glycoconj. J. 19: 325–330. [DOI] [PubMed] [Google Scholar]
- Berninsone P., Hwang H. Y., Zemtseva I., Horvitz H. R., Hirschberg C. B., 2001. SQV-7, a protein involved in Caenorhabditis elegans epithelial invagination and early embryogenesis, transports UDP-glucuronic acid, UDP-N- acetylgalactosamine, and UDP-galactose. Proc. Natl. Acad. Sci. USA 98: 3738–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik D. A., Robbins P. W., 2002. The Caenorhabditis elegans sqv genes and functions of proteoglycans in development. Biochim. Biophys. Acta 1573: 247–257. [DOI] [PubMed] [Google Scholar]
- Calfon M., Zeng H., Urano F., Till J. H., Hubbard S. R., et al. , 2002. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415: 92–96. [DOI] [PubMed] [Google Scholar]
- Chhay J. S., Vargas C. A., McCorvie T. J., Fridovich-Keil J. L., Timson D. J., 2008. Analysis of UDP-galactose 4′-epimerase mutations associated with the intermediate form of type III galactosaemia. J. Inherit. Metab. Dis. 31: 108–116. [DOI] [PubMed] [Google Scholar]
- Culetto E., Sattelle D. B., 2000. A role for Caenorhabditis elegans in understanding the function and interactions of human disease genes. Hum. Mol. Genet. 9: 869–877. [DOI] [PubMed] [Google Scholar]
- Fridovich-Keil J. L., 2006. Galactosemia: the good, the bad, and the unknown. J. Cell. Physiol. 209: 701–705. [DOI] [PubMed] [Google Scholar]
- Garsin D. A., Villanueva J. M., Begun J., Kim D. H., Sifri C. D., et al. , 2003. Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science 300: 1921. [DOI] [PubMed] [Google Scholar]
- Gems D., Riddle D. L., 2000. Genetic, behavioral and environmental determinants of male longevity in Caenorhabditis elegans. Genetics 154: 1597–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman T., Hartwieg E., Horvitz H. R., 1999. sqv mutants of Caenorhabditis elegans are defective in vulval epithelial invagination. Proc. Natl. Acad. Sci. USA 96: 968–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden H. M., Rayment I., Thoden J. B., 2003. Structure and function of enzymes of the Leloir pathway for galactose metabolism. J. Biol. Chem. 278: 43885–43888. [DOI] [PubMed] [Google Scholar]
- Hwang H. Y., Horvitz H. R., 2002. The SQV-1 UDP-glucuronic acid decarboxylase and the SQV-7 nucleotide-sugar transporter may act in the Golgi apparatus to affect Caenorhabditis elegans vulval morphogenesis and embryonic development. Proc. Natl. Acad. Sci. USA 99: 14218–14223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang H. Y., Olson S. K., Esko J. D., Horvitz H. R., 2003. Caenorhabditis elegans early embryogenesis and vulval morphogenesis require chondroitin biosynthesis. Nature 423: 439–443. [DOI] [PubMed] [Google Scholar]
- Johnston W. L., Krizus A., Dennis J. W., 2006. The eggshell is required for meiotic fidelity, polar-body extrusion and polarization of the C. elegans embryo. BMC Biol. 4: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalckar H. M., 1965. Galactose metabolism and cell “sociology.” Science 150: 305–313. [DOI] [PubMed] [Google Scholar]
- Kamath R. S., Fraser A. G., Dong Y., Poulin G., Durbin R., et al. , 2003. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421: 231–237. [DOI] [PubMed] [Google Scholar]
- Kingsley D. M., Kozarsky K. F., Hobbie L., Krieger M., 1986. Reversible defects in O-linked glycosylation and LDL receptor expression in a UDP-Gal/UDP-GalNAc 4-epimerase deficient mutant. Cell 44: 749–759. [DOI] [PubMed] [Google Scholar]
- Krieger M., Reddy P., Kozarsky K., Kingsley D., Hobbie L., et al. , 1989. Analysis of the synthesis, intracellular sorting, and function of glycoproteins using a mammalian cell mutant with reversible glycosylation defects. Methods Cell Biol. 32: 57–84. [DOI] [PubMed] [Google Scholar]
- Kubota Y., Nishiwaki K., 2006. C. elegans as a model system to study the function of the COG complex in animal development. Biol. Chem. 387: 1031–1035. [DOI] [PubMed] [Google Scholar]
- Kubota Y., Sano M., Goda S., Suzuki N., Nishiwaki K., 2006. The conserved oligomeric Golgi complex acts in organ morphogenesis via glycosylation of an ADAM protease in C. elegans. Development 133: 263–273. [DOI] [PubMed] [Google Scholar]
- Kuwabara P. E., O’Neil N., 2001. The use of functional genomics in C. elegans for studying human development and disease. J. Inherit. Metab. Dis. 24: 127–138. [DOI] [PubMed] [Google Scholar]
- Lee A. S., 1992. Mammalian stress response: induction of the glucose-regulated protein family. Curr. Opin. Cell Biol. 4: 267–273. [DOI] [PubMed] [Google Scholar]
- Levy H. L., Sepe S. J., Shih V. E., Vawter G. F., Klein J. O., 1977. Sepsis due to Escherichia coli in neonates with galactosemia. N. Engl. J. Med. 297: 823–825. [DOI] [PubMed] [Google Scholar]
- Litchfield W. J., Wells W. W., 1978. Effect of galactose on free radical reactions of polymorphonuclear leukocytes. Arch. Biochem. Biophys. 188: 26–30. [DOI] [PubMed] [Google Scholar]
- Maley F., Maley G. F., 1959. The enzymic conversion of glucosamine to galactosamine. Biochim. Biophys. Acta 31: 577–578. [DOI] [PubMed] [Google Scholar]
- Malhotra J. D., Kaufman R. J., 2007. The endoplasmic reticulum and the unfolded protein response. Semin. Cell Dev. Biol. 18: 716–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCorvie T. J., Wasilenko J., Liu Y., Fridovich-Keil J. L., Timson D. J., 2011. In vivo and in vitro function of human UDP-galactose 4′-epimerase variants. Biochimie 93: 1747–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulhern M. L., Madson C. J., Danford A., Ikesugi K., Kador P. F., et al. , 2006. The unfolded protein response in lens epithelial cells from galactosemic rat lenses. Invest. Ophthalmol. Vis. Sci. 47: 3951–3959. [DOI] [PubMed] [Google Scholar]
- Munoz M. J., Riddle D. L., 2003. Positive selection of Caenorhabditis elegans mutants with increased stress resistance and longevity. Genetics 163: 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiwaki K., Hisamoto N., Matsumoto K., 2000. A metalloprotease disintegrin that controls cell migration in Caenorhabditis elegans. Science 288: 2205–2208. [DOI] [PubMed] [Google Scholar]
- Nishiwaki K., Kubota Y., Chigira Y., Roy S. K., Suzuki M., et al. , 2004. An NDPase links ADAM protease glycosylation with organ morphogenesis in C. elegans. Nat. Cell Biol. 6: 31–37. [DOI] [PubMed] [Google Scholar]
- Paschinger K., Gutternigg M., Rendic D., Wilson I. B., 2008. The N-glycosylation pattern of Caenorhabditis elegans. Carbohydr. Res. 343: 2041–2049. [DOI] [PubMed] [Google Scholar]
- Piller F., Hanlon M. H., Hill R. L., 1983. Co-purification and characterization of UDP-glucose 4-epimerase and UDP-N-acetylglucosamine 4-epimerase from porcine submaxillary glands. J. Biol. Chem. 258: 10774–10778. [PubMed] [Google Scholar]
- Quimby B. B., Alano A., Almashanu S., DeSandro A. M., Cowan T. M., et al. , 1997. Characterization of two mutations associated with epimerase-deficiency galactosemia, by use of a yeast expression system for human UDP-galactose-4-epimerase. Am. J. Hum. Genet. 61: 590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathi N., Rathi A., 2011. Galactosemia presenting as recurrent sepsis. J. Trop. Pediatr. 57: 487–489. [DOI] [PubMed] [Google Scholar]
- Ross K. L., Davis C. N., Fridovich-Keil J. L., 2004. Differential roles of the Leloir pathway enzymes and metabolites in defining galactose sensitivity in yeast. Mol. Genet. Metab. 83: 103–116. [DOI] [PubMed] [Google Scholar]
- Sanders R. D., Sefton J. M., Moberg K. H., Fridovich-Keil J. L., 2010. UDP-galactose 4′ epimerase (GALE) is essential for development of Drosophila melanogaster. Dis. Model. Mech. 3: 628–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter H., 2004. Protein glycosylation lessons from Caenorhabditis elegans. Curr. Opin. Struct. Biol. 14: 607–616. [DOI] [PubMed] [Google Scholar]
- Schroder M., Kaufman R. J., 2005a ER stress and the unfolded protein response. Mutat. Res. 569: 29–63. [DOI] [PubMed] [Google Scholar]
- Schroder M., Kaufman R. J., 2005b The mammalian unfolded protein response. Annu. Rev. Biochem. 74: 739–789. [DOI] [PubMed] [Google Scholar]
- Schulz J. M., Watson A. L., Sanders R., Ross K. L., Thoden J. B., et al. , 2004. Determinants of function and substrate specificity in human UDP-galactose 4′-epimerase. J. Biol. Chem. 279: 32796–32803. [DOI] [PubMed] [Google Scholar]
- Schulz J. M., Ross K. L., Malmstrom K., Krieger M., Fridovich-Keil J. L., 2005. Mediators of galactose sensitivity in UDP-galactose 4′-epimerase-impaired mammalian cells. J. Biol. Chem. 280: 13493–13502. [DOI] [PubMed] [Google Scholar]
- Shen X., Ellis R. E., Lee K., Liu C. Y., Yang K., et al. , 2001. Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell 107: 893–903. [DOI] [PubMed] [Google Scholar]
- Shen X., Ellis R. E., Sakaki K., Kaufman R. J., 2005. Genetic interactions due to constitutive and inducible gene regulation mediated by the unfolded protein response in C. elegans. PLoS Genet. 1: e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifri C. D., Begun J., Ausubel F. M., 2005. The worm has turned: microbial virulence modeled in Caenorhabditis elegans. Trends Microbiol. 13: 119–127. [DOI] [PubMed] [Google Scholar]
- Slepak T., Tang M., Addo F., Lai K., 2005. Intracellular galactose-1-phosphate accumulation leads to environmental stress response in yeast model. Mol. Genet. Metab. 86: 360–371. [DOI] [PubMed] [Google Scholar]
- Slepak T. I., Tang M., Slepak V. Z., Lai K., 2007. Involvement of endoplasmic reticulum stress in a novel classic galactosemia model. Mol. Genet. Metab. 92: 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N., Toyoda H., Sano M., Nishiwaki K., 2006. Chondroitin acts in the guidance of gonadal distal tip cells in C. elegans. Dev. Biol. 300: 635–646. [DOI] [PubMed] [Google Scholar]
- Thoden J. B., Holden H. M., 1998. Dramatic differences in the binding of UDP-galactose and UDP-glucose to UDP-galactose 4-epimerase from Escherichia coli. Biochemistry 37: 11469–11477. [DOI] [PubMed] [Google Scholar]
- Thoden J. B., Wohlers T. M., Fridovich-Keil J. L., Holden H. M., 2001. Human UDP-galactose 4-epimerase. Accommodation of UDP-N-acetylglucosamine within the active site. J. Biol. Chem. 276: 15131–15136. [DOI] [PubMed] [Google Scholar]
- Thoden J. B., Henderson J. M., Fridovich-Keil J. L., Holden H. M., 2002. Structural analysis of the Y299C mutant of Escherichia coli UDP-galactose 4-epimerase. Teaching an old dog new tricks. J. Biol. Chem. 277: 27528–27534. [DOI] [PubMed] [Google Scholar]
- Timson D. J., 2006. The structural and molecular biology of type III galactosemia. IUBMB Life 58: 83–89. [DOI] [PubMed] [Google Scholar]
- Tirasophon W., Welihinda A. A., Kaufman R. J., 1998. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev. 12: 1812–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner D. D., Buist N. R., Donnell G. N., 1990. Long-term prognosis in galactosaemia: results of a survey of 350 cases. J. Inherit. Metab. Dis. 13: 802–818. [DOI] [PubMed] [Google Scholar]
- Walter J. H., Roberts R. E., Besley G. T., Wraith J. E., Cleary M. A., et al. , 1999. Generalised uridine diphosphate galactose-4-epimerase deficiency. Arch. Dis. Child. 80: 374–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Z., Harding H. P., Zhang Y., Jolicoeur E. M., Kuroda M., et al. , 1998. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 17: 5708–5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasilenko J., Lucas M. E., Thoden J. B., Holden H. M., Fridovich-Keil J. L., 2005. Functional characterization of the K257R and G319E-hGALE alleles found in patients with ostensibly peripheral epimerase deficiency galactosemia. Mol. Genet. Metab. 84: 32–38. [DOI] [PubMed] [Google Scholar]
- Wicks S. R., Yeh R. T., Gish W. R., Waterston R. H., Plasterk R. H., 2001. Rapid gene mapping in Caenorhabditis elegans using a high density polymorphism map. Nat. Genet. 28: 160–164. [DOI] [PubMed] [Google Scholar]
- Wilm T., Demel P., Koop H. U., Schnabel H., Schnabel R., 1999. Ballistic transformation of Caenorhabditis elegans. Gene 229: 31–35. [DOI] [PubMed] [Google Scholar]
- Wohlers T. M., Christacos N. C., Harreman M. T., Fridovich-Keil J. L., 1999. Identification and characterization of a mutation, in the human UDP-galactose-4-epimerase gene, associated with generalized epimerase-deficiency galactosemia. Am. J. Hum. Genet. 64: 462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaal R. R., Van Baelen K., Groenen J. T., van Geel A., Rottiers V., et al. , 2001. The sarco-endoplasmic reticulum Ca2+ ATPase is required for development and muscle function in Caenorhabditis elegans. J. Biol. Chem. 276: 43557–43563. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.