Abstract
Disruption of epigenetic gene control mechanisms in the brain causes significant cognitive impairment that is a debilitating hallmark of most neurodegenerative disorders, including Alzheimer’s disease (AD). Histone acetylation is one of the best characterized of these epigenetic mechanisms that is critical for regulating learning- and memory- associated gene expression profiles, yet the specific histone acetyltransferases (HATs) that mediate these effects have yet to be fully characterized. Here, we investigate an epigenetic role for the HAT Tip60 in learning and memory formation using the Drosophila CNS mushroom body (MB) as a well-characterized cognition model. We show that Tip60 is endogenously expressed in the Kenyon cells, the intrinsic neurons of the MB, and in the MB axonal lobes. Targeted loss of Tip60 HAT activity in the MB causes thinner and shorter axonal lobes while increasing Tip60 HAT levels cause no morphological defects. Functional consequences of both loss and gain of Tip60 HAT levels in the MB are evidenced by defects in immediate-recall memory. Our ChIP-Seq analysis reveals that Tip60 target genes are enriched for functions in cognitive processes, and, accordingly, key genes representing these pathways are misregulated in the Tip60 HAT mutant fly brain. Remarkably, we find that both learning and immediate-recall memory deficits that occur under AD-associated, amyloid precursor protein (APP)-induced neurodegenerative conditions can be effectively rescued by increasing Tip60 HAT levels specifically in the MB. Together, our findings uncover an epigenetic transcriptional regulatory role for Tip60 in cognitive function and highlight the potential of HAT activators as a therapeutic option for neurodegenerative disorders.
Keywords: Drosophila, Tip60, cognition, epigenetics, histone acetylation
THE ability of living organisms to respond to a constantly changing environment and fine-tune their complex behaviors accordingly is crucial for their adaptation and survival throughout development (Levenson and Sweatt 2005; Borrelli et al. 2008; West and Greenberg 2011; Al-Saigh et al. 2012). One of the most important of such experience-driven behavioral changes is learning and memory formation, as it directly impacts cognitive ability (Levenson and Sweatt 2005; Carulli et al. 2011; Nelson and Monteggia 2011; West and Greenberg 2011). In the brain, external stimuli are converted into intracellular signals that program coordinated expression of specific gene sets that promote sustained neuroplasticity and cognitive adaptation (Sweatt 2009; Riccio 2010; Carulli et al. 2011). Disruption of these response programs results in significant cognitive impairment disorders (West and Greenberg 2011; Ebert and Greenberg 2013; Pirooznia and Elefant 2013a). Epigenetic post-translational modifications (PTMs) of histone proteins that control nuclear chromatin packaging and gene expression profiles are emerging as a fundamental mechanism by which neurons adapt their transcriptional response to environmental cues (Feng et al. 2007; Sweatt 2009; Bousiges et al. 2010; Meaney and Ferguson-Smith 2010; Riccio 2010; Nelson and Monteggia 2011; Pirooznia and Elefant 2012). One of the best-characterized forms of epigenetic chromatin modification in the learning and memory field is histone acetylation (Peixoto and Abel 2013), which is regulated by the antagonistic activities of histone acetyltransferases (HATs) and histone deacetylases (HDACs) (Legube and Trouche 2003). Blocking histone acetylation has been reported to impair both long-lasting synaptic plasticity and behavioral performance (Korzus et al. 2004). Notably, inhibition of histone deacetylase activity reverses such deficits and improves memory formation (Korzus et al. 2004; Levenson et al. 2004), thus highlighting the importance of histone acetylation for memory formation.
Disruption of epigenetic gene control mechanisms in the brain causes significant cognitive impairment that is a debilitating hallmark of most neurodegenerative disorders, including Alzheimer’s disease (AD). Sporadic cases of reduced histone acetylation levels are found in the brains of animal models for multiple types of neurodegenerative diseases that include AD. These changes have been shown to cause an epigenetic blockade of transcription that results in cognitive impairment. (Graff et al. 2012a). Pharmacological treatments aimed at increasing histone acetylation levels by inhibiting histone deacetylase action in these models have shown promising effects in reversing cognitive deficits (Kazantsev and Thompson 2008). However, little is known about HATs that modify the neural epigenome by laying down specific epigenetic marks required for proper cognition and thus likely serve as causative agents of memory-impairing histone acetylation changes. A promising candidate is the HAT, Tip60 (Tat interactive protein), which has been implicated in AD owing to its role in epigenetically regulating gene expression via complex formation with the amyloid precursor protein (APP) intracellular domain (AICD) (Cao and Sudhof 2001, 2004).
Tip60 is a multifunctional HAT that has been shown by others and us to epigenetically regulate genes essential for neurogenesis (Lorbeck et al. 2011; Pirooznia et al. 2012b). Such an effect is thought to be mediated through recruitment of Tip60-containing protein complexes to target gene promoters in the nervous system that are then epigenetically modified via site-specific acetylation and accordingly activated or repressed. We have recently reported that the histone acetylase function of Tip60 promotes neuronal and organismal survival in a Drosophila model for AD by activating pro-survival factors while concomitantly repressing activators of cell death (Pirooznia et al. 2012b). Overexpression of Tip60 also promotes axonal growth of the Drosophila circadian neurons, the small ventrolateral neurons (sLNvs), and rescues axonal outgrowth and transport as well as associated behavioral phenotypes such as sleep and locomotion under APP-induced neurodegenerative conditions (Pirooznia et al. 2012a). While these effects support a neuroprotective role for Tip60 under degenerative conditions such as those induced by neuronal overexpression of APP, an epigenetic role for Tip60 in mediating gene expression changes that underlie memory formation remains to be elucidated.
Drosophila is an attractive model for studies focused on molecular dissection of components of memory formation due to the availability of reproducible memory assays and genetic tools that enable restricting gene expression manipulation to specific subregions of the brain (Siwicki and Ladewski 2003; Fiala 2007; Kahsai and Zars 2011). The Drosophila mushroom body (MB) is known to regulate behavioral and physiological functions that range from olfactory learning and courtship conditioning to decision making under uncertain conditions (Heisenberg 2003; Margulies et al. 2005; Akalai et al. 2006; Farris 2013; Guven-Ozkan and Davis 2014). Courtship conditioning in Drosophila is a complex behavioral learning paradigm that requires multimodal sensory input involving chemosensory, mechanosensory, visual, and olfactory pathways and is thus well suited to study experience-dependent synaptic plasticity (Dubnau and Tully 2001; Mehren et al. 2004; Keene and Waddell 2007; Busto et al. 2010).
In this study, we focus on the Drosophila MB as a well-characterized cognition model to investigate an epigenetic role for Tip60 HAT action in learning and memory formation. We find that Tip60 is robustly produced in the MB of the adult fly brain. Targeted loss of Tip60 HAT activity in the MB causes abnormal development of the MB axonal lobes, while increasing Tip60 HAT levels causes no morphological defects. Furthermore, we show that misregulation of Tip60 HAT activity in the MB leads to immediate-recall memory deficits in adult flies. Our ChIP-Seq analysis reveals that Tip60 target genes are enriched for functions in cognitive processes, and, accordingly, key genes representing these pathways are misregulated in the Tip60 HAT mutant fly brain. Importantly, we show that both learning and memory defects in Drosophila that occur under AD-associated, APP-induced neurodegenerative conditions can be effectively rescued by increasing Tip60 HAT levels in the Drosophila MB. Together, our studies uncover an epigenetic transcriptional regulatory role for Tip60 HAT action in cognitive function and highlight the therapeutic potential of utilizing specific HAT activators for treatment of cognitive deficits in neurodegenerative disorders.
Materials and Methods
Fly stocks and maintenance
Flies were reared on standard medium (cornmeal/sugar/yeast) at 25° with a 12-hr light/dark (LD) cycle. Canton-S flies were used as wild-type controls. OK107-GAL4 and UAS-GFP stocks were obtained from the Bloomington Drosophila Stock Center (Indiana University). The generation and characterization of UAS-dTip60E431Q and UAS-dTip60WT flies are described in Lorbeck et al. (2011). Generation and characterization of the double-transgenic UAS-Tip60;APP fly lines are described in Pirooznia et al. (2012b). Double-transgenic lines carrying the UAS-mCD8-GFP and either UAS-dTip60E431Q or UAS-dTip60WT constructs were generated according to standard procedures. All of the UAS-dTip60 fly lines described here are contained within a w1118 genetic background. Additionally, for all experiments, transgene expression levels for each of the UAS fly lines were assessed as described (Lorbeck et al. 2011; Pirooznia et al. 2012a,b; Johnson et al. 2013) to ensure that the different transgenic lines used for phenotype comparisons show equivalent dTip60E431Q, dTip60WT, APP, or APP-CT expression levels.
Courtship suppression assay
Assays were performed as described in McBride et al. (2005) and Melicharek et al. (2010). Briefly, virgin males of the appropriate genotype were collected within 6 hr of eclosion and reared in individual food vials at 25° in 12:12 LD for 5 days prior to behavioral training and testing. Virgin wild-type Canton-S females were collected and kept in groups in food vials. Mated Canton-S females used for training were 5 days old and observed to have mated with a Canton-S male the evening prior to training. Virgin Canton-S females used for testing were 5 days old. All experiments were conducted during light phase. All behavior was digitally recorded using a Sony DCR-SR47 Handycam with Carl Zeiss optics. The total time that a male performed courtship activity was subsequently measured and scored. The courtship index was calculated as the total time observed performing courting behavior divided by the total time assayed.
On the day of training (day 5), male flies were assigned to random groups, and the assay set up with the experimenter blind to the genotype of the test males. Male flies were transferred without anesthesia to one half of a partitioned mating chamber from Aktogen (http://www.aktogen.com) that contained a previously mated Canton-S female in the other partitioned half. Males were allowed to acclimate for 1 min, and then the partition between the male and female was removed. Male flies were then trained for 60 min. After 60 min, male flies were transferred within 2 min without anesthesia to one half of a clean partitioned mating chamber that contained a virgin Canton-S female in the other partitioned half. The partition was removed, and behavior of the flies was recorded for 10 min. During the testing phase, untrained males of the appropriate genotype were assayed alongside the trained males to serve as controls. To determine the significance between different measures of the same genotype, a two-tailed paired Student’s t-test was performed. Significance was determined at the 95% confidence interval.
Immunohistochemistry and antibodies
Third instar larvae or adult brains were dissected in PBS, fixed in 4% paraformaldehyde in PBS, washed three times in PBS containing 0.1% Triton X-100, blocked for 1 hr at room temperature (RT) in PBT containing 5% normal goat serum, and incubated with primary antibodies in blocking solution overnight at 4°. Anti-Tip60 (1:400) was generated by Open Biosystems (Rockford, IL), and anti-Fasciclin (mAb1D4; 1:10), anti-Trio (mAb9.4A; 1:4), anti-ELAV (1:400) were obtained from the Developmental Studies Hybridoma Bank (University of Iowa). Anti-GFP (1:100) was obtained from Millipore. Samples were washed three times in PBT at RT, and secondary antibodies (Jackson Immunoresearch) were applied in blocking solution for 2 hr at RT. After washing three times in PBS, samples were mounted in Vectashield (Vector Laboratories).
Imaging and quantification
Larval and adult brain preparations were imaged using the appropriate secondary antibodies. Anti-GFP and anti-Tip60 immunostaining were visualized using Alexa-Fluor 488 and Alexa-Fluor 647, respectively. Anti-Elav, anti-Fasciclin, anti-Trio were visualized using Alexa-Fluor 568. Confocal microscopy was performed using an Olympus microscope with fluoview acquisition software (Olympus, Center Valley, PA). Images were displayed as projections of 1-μM serial Z-sections. Consecutive subsets of the Z-stacks were utilized for the final projection. Images were adjusted for brightness and contrast using the ImageJ program to more clearly define MBs. Projections from unprocessed images are found in Supporting Information, Figure S1. Area of the MB lobes in the different genotypes was measured using National Institutes of Health ImageJ software.
Real-time PCR analysis
Total RNA was isolated from adult fly heads using the RNeasy Plus Mini Kit (QIAGEN). Complementary DNA (cDNA) was prepared using the SuperScript II reverse transcriptase kit (Invitrogen) according to the manufacturer’s instructions with 1 μg total RNA and 0.2 μg/ml random hexamer primers (Roche Applied Science). PCRs were performed in a 20-μl reaction volume containing cDNA, 1 μΜ Power SYBR Green PCR Master Mix (Applied Biosystems), and 10 μM of both forward and reverse primers (primer pairs available upon request). PCR was performed using an ABI 7500 Real-Time PCR system (Applied Biosystems) following the manufacturer’s instructions. Fold change in messenger RNA expression was determined by the ΔΔCt method.
Cell culture
Drosophila S2 cells (Invitrogen) were grown at 22° in Schneider’s Drosophila Medium (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (SAFC Biosciences, Lenexa, KS) and penicillin–streptomycin (Invitrogen)
ChIP-Seq
Chromatin from S2 cells was prepared as follows. Protein from 1–5 × 107 cells was cross-linked to DNA using 1% formaldehyde for 10 min at room temperature. Cross-linking was quenched by adding 2.5 M glycine to a final concentration of 0.125 M. Quenching was performed at RT for 10 min with constant agitation. The cells were then pelleted by centrifugation for 10 min at 2500 × g at 4°. The cells were washed with 1 ml of 1× PBS and pelleted by centrifugation for 10 min at 2500 × g at 4°. The pellet was then resuspended in 1 ml of cell lysis buffer supplemented with 5 μl each of protease inhibitor cocktail (PIC) and PMSF. The cells were transferred to an ice-cold douncer and dounced on ice to aid in release of nuclei. Lysed cells were transferred to a 1.7-ml centrifuge tube and centrifuged for 10 min at 5000 × g at 4° to pellet the nuclei. The supernatant was removed and the pelleted nuclei were resuspended in 350 μl of Shearing Buffer supplemented with 1.75 μl each of PIC and PMSF. The nuclei were sonicated at 30% output using a sonic dismembrator (Fischer Scientific, Pittsburgh) on ice for 40 sec. Sonication was carried out for a total of three times with 2-min intervals on ice yielding sheared chromatin fragments ranging from 200 to 500 bp.
Chromatin precipitation assays were performed using a ChIP-IT Express Kit (Active Motif), following the manufacturer’s instructions. Briefly, chromatin immunoprecipitation (ChIP) was carried out with 50 μg of sheared chromatin using three different antibodies: (i) 10 μg of RNA Pol II antibody (Abcam, Cambridge, MA); (ii) 10 μg of Tip60 antibody that targets residues 450–513 in the C terminus of Tip60 (Abcam); and (iii) 10 μg of Tip60 antibody that targets residues 500–513 in the C terminus of Tip60 (Thermo Fisher Scientific, Huntsville, AL). A mock reaction containing all reagents except the antibody was also set up as a control. The chromatin was immunoprecipated using the ChIP IT Express kit (Active Motif) exactly following the manufacturer’s specifications. The eluted material from the immunoprecipitation was then purified using a QIAquick PCR purification kit (Qiagen) and was directly used for real-time PCR.
DNA library preparation and sequencing of ChIP DNA samples was carried out by the Jefferson Kimmel Cancer Center, Cancer Genomics Shared Resource. Sequencing of ChIP DNA samples was performed using the Illumina HiSeq2000 platform.
Bioinformatic analysis
Peak calling:
MACS v1.4 peak-calling algorithm was used to find overrepresented sequence regions (peaks) representing likely in vivo binding sites for Tip60 or RNA Pol II, and the final set of significant peaks was produced in interval (BED) format. Prior to peak calling, BAM files of biological replicates for each antibody or input were merged together. Peak calling with MACS was done using the online Galaxy platform with the following parameters: mfold = 5, tag size = 35 nt, P-value cutoff = 1e-05, genome size = 130e+06, and FutureFDR = true.
Gene annotation of ChIP-Seq peaks:
ChIP-Seq peaks in the form of genomic intervals (BED file) were intersected with genomic coordinates of genes in the Drosophila melanogaster genome annotation release R5.56 (ftp://ftp.flybase.net/releases/FB2014_02/) using the Galaxy platform (http://usegalaxy.org/), and gene identifiers were confirmed and converted where necessary for further analysis using the University of California at Santa Cruz (UCSC) table browser (http://genome.ucsc.edu/).
Functional annotation and cluster enrichment:
Functional annotation and enrichment cluster analysis of the Tip60-associated gene list was performed using the Database for Annotation, Visualization and Integrated Discovery (DAVID) v6.7 and Gene Annotation Co-occurrence Discovery (GeneCodis) v3 (http://genecodis.cnb.csic.es/). P-values for significant enrichment were obtained with a hypergeometric test and corrected for multiple comparisons by FDR. Pathway enrichment was determined using the pathway enrichment module of the FlyMine integrated genomics database platform.
Tissue-specific expression profiles:
Tissue-specific expression data for the 321 Tip60-associated genes were obtained from FlyAtlas using the FlyMine integrated genomics database.
Transcription factor motif enrichment:
Transcription factor (TF) motif enrichment analysis was performed on Tip60-associated neuronal genes using the MEME-ChIP suite, RSAT (Thomas-Chollier et al. 2011, 2012), and Motif Enrichment Tool. TF motif databases utilized for comparison with discovered motifs included JASPAR Core Insects, FlyFactorSurvey, DMMPMM, and IDMMPMM Drosophila databases. As each tool employs a different algorithm for determining significance of motif enrichment and database match, where database matches are identified by more than one tool, significance values reported in this work represent the most conservative significance value found.
Results
Tip60 is expressed throughout the adult fly brain, including the mushroom body
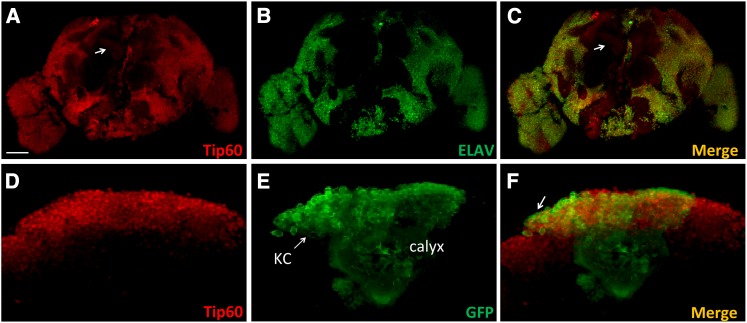
Given the importance of brain-specific histone acetylation profiles in cognitive function, we wanted to examine whether the HAT Tip60 was produced in the adult fly brain. Tip60 production in the adult fly brain was characterized by immunohistochemistry on whole-mount Canton-S adult brains using an anti-Drosophila Tip60 antibody. We found that Tip60 was robustly and widely produced throughout the adult brain in a pattern similar to the pan-neuronal ELAV protein including the MB lobes (Figure 1, A–C). To examine Tip60 concentration in the MB, immunohistochemistry for Tip60 was performed on brains expressing mCD8-GFP under control of the MB-specific driver OK107-GAL4 that marks MB structure. In the MB neurons, called Kenyon cells, mCD8-GFP was observed in the cytoplasm surrounding the Tip60-positive nuclei and Tip60 was detected in all cells that expressed mCD8-GFP (Figure 1, D–F).
Figure 1.
Tip60 expression in the adult Drosophila brain. Frontal view of a wild-type (Canton S) adult Drosophila brain stained with an antibody to Tip60 (red) and counterstained with anti-ELAV antibody (green). Tip60 is widely expressed in the adult fly brain (A) including the MB lobes (A, arrow), with an expression pattern similar to the pan-neuronal ELAV protein (B and C). (A–C) Whole-brain reconstruction of individual confocal image slices. Bar, 100 μM. A single confocal plane through the MB at the level of the calyx (approximately the center of the Z-stack) in flies that express mCD8-GFP under the control of OK107-GAL4 driver shows Tip60 expression in Kenyon cell (KC) nuclei with a halo of GFP expression in the cell membrane and calyx (dendritic processes) (D–F).
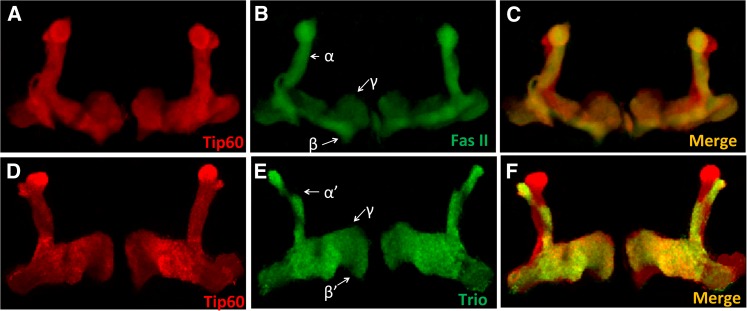
During development, the Kenyon cells of the MB undergo an ordered differentiation process into three types of neurons, namely, the α/α′ neurons, β/β′ neurons, and γ neurons (Lee et al. 1999). Each neuron projects dendrites that contribute to a large dendritic field in the calyx and an axon that travels anteroventrally, forming a tightly bundled peduncle before branching dorsally to form the α/α′ lobes and medially to form the β/β′ and γ lobes. In addition to the Kenyon cells, Tip60 was also detected in the α/α′, β/β′, and γ lobes (Figure 2, A and D). Specific MB lobes were unambiguously identified immunohistochemically by costaining with markers specific for each of the lobes. Fasciclin II (Fas II) is a cell adhesion molecule that participates in axonal pathfinding (Fushima and Tsujimura 2007) and is expressed strongly in the α/β lobes (Figure 2B) (Crittenden et al. 1998). Drosophila Trio is a Dbl family protein that participates in patterning of axons by regulating their directional extension and is expressed strongly in the α′/β′ lobes (Figure 2E) (Awasaki et al. 2000). Both markers are produced weakly in the γ lobe as well (Figure 2, B and E) (Bates et al. 2010). Tip60 production pattern in the α/β and α′/β′ lobes followed the pattern of Fas-II and Trio, respectively (Figure 2, C and F).
Figure 2.
Tip60 is expressed in the MB lobes. Representative confocal images of adult MB lobes in wild-type (Canton S) Drosophila brain stained with Tip60 antibody (A and D) and costained with antibodies to either Fasciclin II (Fas II) (B) or Trio (E) antibodies. Fas II is a cell adhesion molecule that is expressed strongly in the MB α/β lobes and weakly in the γ lobe. Trio is a Dbl family protein that activates Rho family GTPases and is expressed strongly in the α′/β′ lobes and weakly in the γ lobe. Tip60 is expressed in all the lobes of the MB and colocalizes with Fas II and Trio in the α/β (C) and α′/β′ (F) lobes, respectively. Tip60 also colocalizes with Fas II and Trio in the γ lobes (C and F).
Appropriate Tip60 HAT levels are required for immediate-recall memory
Our finding that Tip60 is endogenously and robustly produced in the adult MB prompted us to ask whether Tip60 epigenetically regulates memory formation. To address this question, we used the conditioned courtship suppression assay (Siegel and Hall 1979). This assay is an associative conditioning procedure that measures both learning and memory in individual flies (Broughton et al. 2003). The conditioning aspect of the assay is based on the observation that male courtship behavior is modified by exposure to a previously mated female that is unreceptive to courting (Siegel and Hall 1979; Siwicki et al. 2005). Thus, after a 1-hr training session with a mated female, wild-type males suppress their courtship behavior even toward subsequent receptive virgin females, an effect that decays after 1–3 hr (Siegel and Hall 1979)
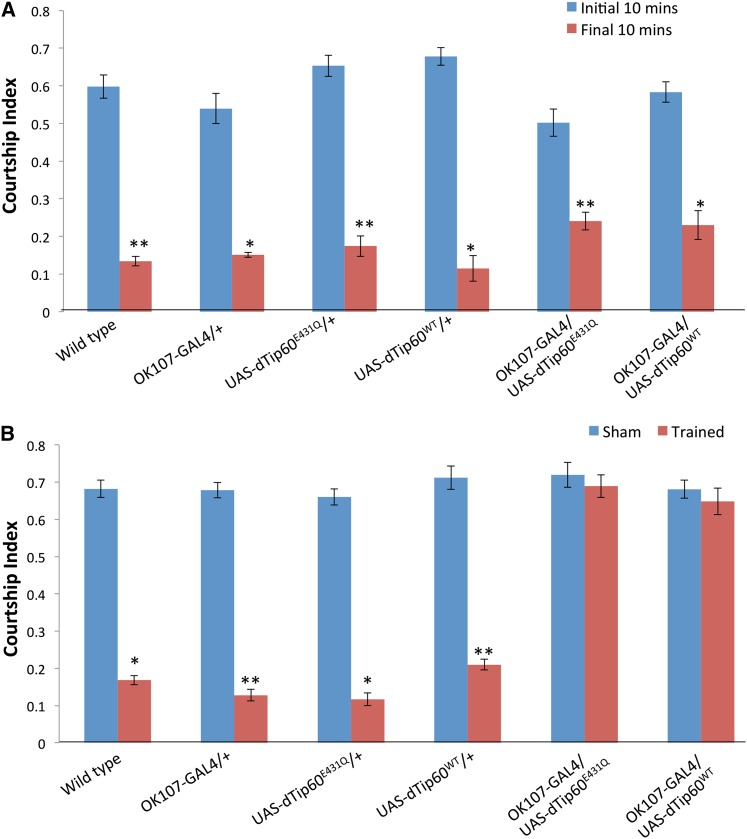
To examine the effect of Tip60 HAT function on learning and memory, we misregulated Drosophila Tip60 in the mushroom body by utilizing our previously reported transgenic lines that carry GAL4-responsive transgenes for either a dominant negative HAT defective version of dTip60 (dTip60E431Q) or wild-type dTip60 (dTip60WT) (Siegel and Hall 1979; Lorbeck et al. 2011). Expression of the respective transgenes was achieved continuously during development using the GAL4 driver OK107. This driver is expressed in discrete neuronal populations in the adult fly brain that includes high expression in the Kenyon cells, the intrinsic neurons of the MB, and the pars intercerebralis, suboesophageal ganglion, and optic lobes (Aso et al. 2009). To determine the effects on learning, male flies were placed in a courtship chamber with a previously mated (unreceptive) wild-type female for 60 min. The amount of time the male spent performing courtship behavior was assessed during the initial 10 min of this training and compared with the final 10 min of the training period. Male control flies (OK107-GAL4/+) show a significant drop in courtship behavior in the final 10 min of training when compared with the initial 10 min (Figure 3A), indicative of an appropriate learning response. A similar effect was observed in the UAS background control flies (UAS-dTip60E431Q/+ and UAS-dTip60WT/+) and in the wild-type Canton-S flies (Figure 3A). Male flies expressing either the Tip60 HAT mutant (dTip60E431Q) or additional copies of dTip60WT also showed a significant decrease in courtship behavior in the final 10 min of the training period compared with the initial 10 min (Figure 3A). This indicates that misregulation of Tip60 HAT activity in the MB does not interfere with the successful perception and interpretation of sensory stimuli required in this assay and that these flies are capable of altering their behavior appropriately (learn) in response to this training.
Figure 3.
Misregulation of Tip60 in Drosophila MB does not affect learning but leads to defects in immediate-recall memory. (A) Learning during the initial 10 min (blue columns) and final 10 min (red columns) of the training phase during the courtship suppression assay. Genotypes are indicated. Flies expressing either the mutant Tip60 defective in its HAT activity (dTip60E431Q) or additional copies of dTip60WT exhibit a marked decrease in courtship index during final 10 min compared to the initial 10 min, indicative of normal learning response. This is comparable to the response observed in wild type (Canton S) flies as well as in the corresponding GAL4 and UAS background controls. (B) Immediate-recall memory (0–2 min post-training) of trained males compared to untrained (sham) males of the same genotype. dTip60E431Q and dTip60WT flies show no significant difference between trained and sham males, indicative of no immediate recall of training. Error bars represent 95% confidence interval. In A, a single asterisk indicates P < 0.05 and a double asterisk indicates P < 0.001 compared with initial 10 min. In B, a single asterisk indicates P < 0.05 and a double asterisk indicates P < 0.001 compared with sham males. n = 20 for trained and untrained males in each genotype.
Different phases of memory have been defined in Drosophila and include immediate-recall memory (0–2 min post-training), short-term memory (up to 1 hr post-training), medium-term memory (up to 6 hr), anesthesia-resistant memory (up to 2 days) and long-term memory (up to 9 days) (Greenspan 1995; McBride et al. 1999, 2005). To test for the earliest phase of memory first, we assayed male flies expressing either the Tip60 HAT mutant (dTip60E431Q) or dTip60WT by transferring the respective trained males to clean mating chambers and pairing with a receptive virgin female within 2 min of training, following which their courtship behavior was monitored for 10 min. Trained male control flies (OK107-GAL4/+) showed a marked decrease in their courtship activity compared to untrained male flies (Sham) that were assayed in parallel (Figure 3B). A similar effect was observed in the UAS background control flies (UAS-dTip60E431Q/+ and UAS-dTip60WT/+) and in the wild-type Canton-S flies (Figure 3B). This indicates a change in behavior in these flies that is consistent with normal immediate-recall memory training. However, such a decrease in courtship behavior was not observed in flies expressing either the Tip60 HAT mutant (dTip60E431Q) or additional copies of the HAT competent dTip60WT (Figure 3B). Since these flies were capable of normal sensory perception and were also able to alter their behavior in response to their experience during the learning component of the assay, their inability to effectively suppress courtship behavior during the second component of the assay indicates that these flies are defective in immediate-recall memory of this form of learning.
Tip60 HAT activity is required for formation of normal MB structure in adult brains
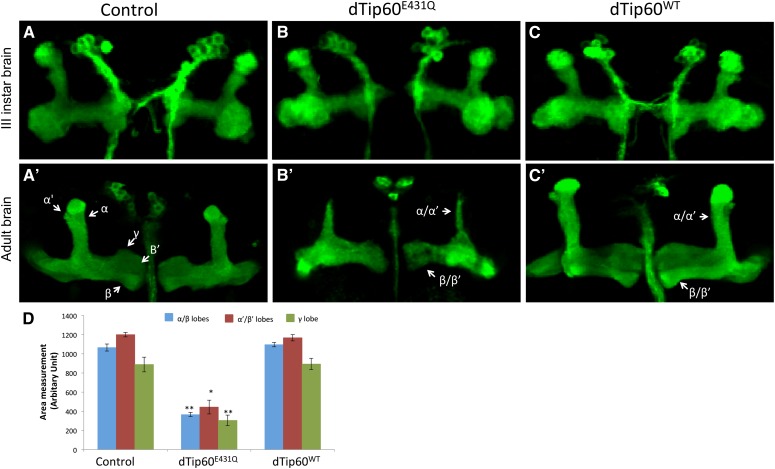
Development of precise axonal connectivity and plasticity in their connectivity are required for maintenance of functional neural circuits that facilitate learning and memory (Luo and O’Leary 2005). Accordingly, degeneration of neural circuits essential for learning and memory may lead to impaired behavioral plasticity. We have previously reported that Tip60 HAT function promotes axonal growth of the Drosophila sLNv, a well-characterized model system for axonal growth (Pirooznia et al. 2012a). We therefore wanted to examine if the observed memory deficits in the Tip60 mutant flies were accompanied by axonal growth defects in the MB. To examine the Tip60-mediated anatomical effects in the MB, we generated GAL4-responsive transgenic fly lines carrying a membrane-bound mCD8-GFP construct with either the dominant negative Tip60 HAT mutant (UAS-mCD8-GFP; UAS-dTip60E431Q) or wild-type Tip60 (UAS-mCD8-GFP; UAS-dTip60WT). Expression of the respective transgenes was directed by the MB-specific OK107-GAL4 driver. MB structural phenotypes under the different conditions were identified by immunostaining for GFP in whole brains dissected from adult animals.
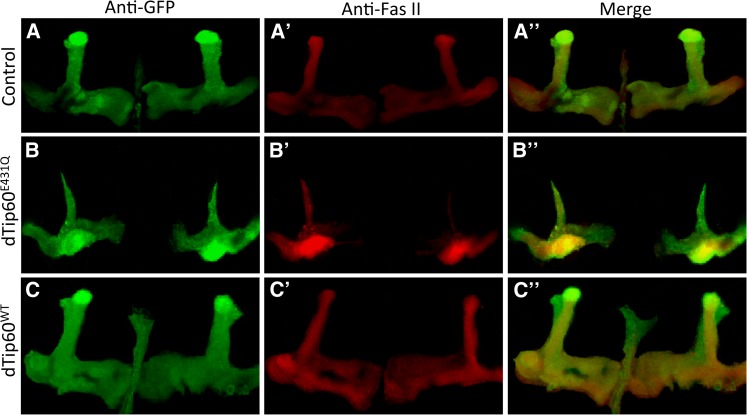
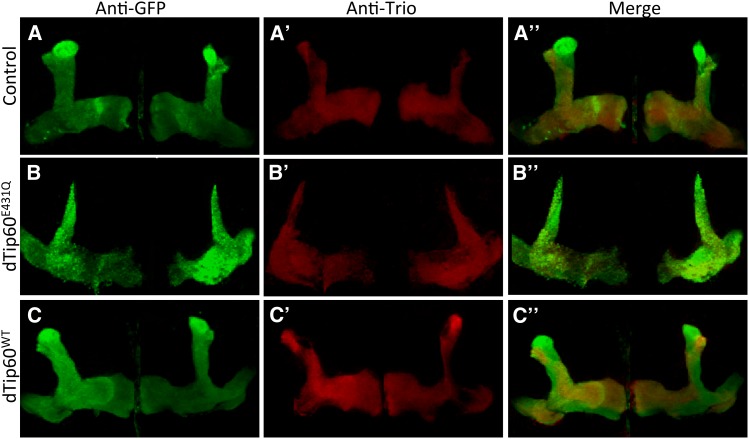
In the third instar larvae and adult control flies (OK107-GAL4/US-GFP), confocal microscopy revealed GFP immunolabeling of α/β neurons along the peduncles as well as distally as their axons bifurcate and project dorsally into the α/α′ lobes and medially into the β/β′ and γ lobes (Figure 4, A and A′). While the stereotyped morphology of the MB lobes was detected in third instar brain of flies expressing either the HAT mutant dTip60E431Q or dTip60WT (Figure 4, B and C), severe axonal defects were observed in the adult flies. GFP staining of adult brains from the dTip60E431Q mutants revealed dramatic reduction of the MB axonal fields, resulting in α/α′ lobes that were much thinner than those in the control flies (Figure 4, B′ and D). Additionally, severe reduction in the area of the β/β′ and γ lobes was also observed in these flies (Figure 4, B′ and D). Thinner α and β lobes were observed in both sides of the brain in the dTip60E431Q mutants, indicating that the axonal defects are common to both brain hemispheres. Developing axons of α/β neurons normally bifurcate at the base of the lobes, and the resulting sister branches subsequently extend in diverging directions: one dorsally to the α lobe and the other medially to the β lobe. Similarly, α′/β′ neurons also develop dorsal (α′) and medial (β′) lobes. While the defects that we observed were present in all of the dTip60E431Q MBs that we inspected, in some MBs the defects were more severe than in others. Therefore, to more accurately quantify the changes as well as to examine which particular lobe(s) were specifically affected in the dTip60E431Q flies, area measurements of the different MB lobes were carried out by costaining with anti-Fas II or anti-Trio antibodies that exhibit weak expression in the γ lobe while strongly labeling α/β and α′/β′ lobes, respectively. Fas II staining (Figure 5, B and B′) was used for quantification of α/β and γ lobes, and Trio staining (Figure 6, B and B′) was used for quantification of the area of α′/β′ lobes. Quantification using these lobe-specific markers revealed a marked decrease in the area of all the MB lobes in the dTip60E431Q flies compared to the control flies (OK107-GAL4; UAS-GFP) (Figure 4D). On the contrary, adult brains from the dTip60WT flies did not exhibit any significant effect on α/β, α′/β′, and γ lobes on either side of the brain as revealed by GFP (Figure 4, C′ and D), Fas II (Figure 5C′), and Trio labeling (Figure 6C′). Thus, production of Tip60 within the MB lobes and the axonal growth defects observed due to disruption of Tip60’s HAT function together suggest that Tip60 HAT activity may play essential roles in MB axonal outgrowth.
Figure 4.
Tip60 is required for normal structures of the adult MB. Representative confocal images of larval and adult MB visualized with mCD8-GFP driven by the pan-MB driver OK107-GAL4. Images were displayed as projections of 1-μM serial Z- sections and were adjusted for brightness and contrast using the ImageJ program to more clearly define the MBs. Unprocessed images are shown in Figure S1. Third instar larval brain in control flies (A). Flies expressing mutant Tip60 defective in its HAT activity (dTip60E431Q) (B) or additional copies of dTip60WT (C) show no effect on MB structure in the third instar larva. GFP labeling shows similar widths of and α/β lobes in (A′) adult control brains (OK107-GAL4; UAS-GFP), whereas adult flies expressing mutant dTip60E431Q display severe reduction in length and width of both α and β lobes (arrows) (B′) while overexpressing dTip60WT did not have any effect on the MB in the adult flies (C′). (D) Quantification of area in the different genotypes in adult flies. MBs stained with GFP to delineate MB structure were counterstained with either Fas II staining (Figure 5) for quantification of area of α/β and γ lobes or Trio staining (Figure 6) for quantification of area of α′/β′ lobes where n = 20. Error bars represent 95% confidence interval. A single asterisk indicates P < 0.05, and a double asterisk indicates P < 0.001 compared to the respective MB lobes in the control.
Figure 5.
Fasciclin II labeling in the MB. Representative confocal images of OK107-GAL4 driver carrying a GFP construct was used to drive expression of dTip60E431Q or dTip60WT, and the effect on (A, B, C) MB structure was visualized using GFP and Fas II staining (A', B', C') and co-staining of GFP and FasII (A“, B“, C“). Images were displayed as projections of 1-μM serial Z-sections and were adjusted for brightness and contrast using the ImageJ program to more clearly define MBs. Unprocessed images are shown in Figure S1. Compared to control brains (UAS-mCD8-GFP; OK107-GAL4), dTip60E431Q flies exhibit a marked decrease in α/β and γ lobes while dTip60WT flies did not exhibit any effects on α/β and γ lobes. Fas II labeling was used for quantifying area measurements in the different genotypes.
Figure 6.
Trio labeling in the MB. Representative confocal images of OK107-GAL4 driver-carrying GFP construct was used to drive expression of dTip60E431Q or dTip60WT, and the effect on MB structure was visualized using GFP (A, B, C) and Trio staining (A', B', C') and co-staining of GFP and Trio (A“, B“, C“). Images were displayed as projections of 1-μM serial Z-sections and were adjusted for brightness and contrast using the ImageJ program to more clearly define MBs. Unprocessed images are shown in Figure S1. Compared to control brains (UAS-mCD8-GFP; OK107-GAL4), dTip60E431Q flies exhibit a marked decrease in α′/β′ while dTip60WT flies exhibit no significant effects on α′/β′ lobes. Trio labeling was used for quantifying area measurements in the different genotypes.
Tip60-associated target genes are enriched for functions in cognition-linked neuronal processes
Our prior findings demonstrated that gene control is a key mechanism by which Tip60 exerts its action in promoting function in a variety of neuronal processes. To determine whether Tip60 associates with target genes enriched for cognition-linked processes, we conducted genome-wide analysis of Tip60 occupancy using ChIP-Seq analysis. We also carried out ChIP-Seq analysis for the transcriptionally active elongation version of RNA polymerase II occupancy to assess which Tip60-associated gene targets were transcriptionally active. To identify early developmental targets, we used the Drosophila S2 embryonic cell line that robustly produces endogenous Tip60 (Zhu et al. 2007) and is composed of mixed cell types for an unbiased enrichment analysis.
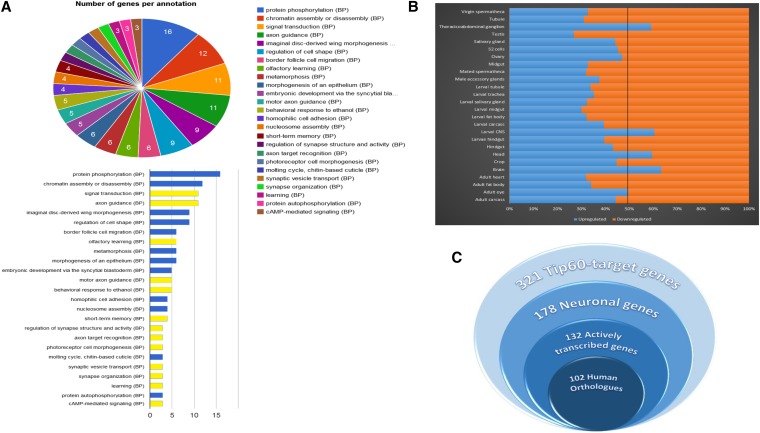
To identify Tip60-associated target genes, gene annotation of Tip60 and RNA Pol II ChIP-Seq peaks was performed using the D. melanogaster annotation database R5.5 and UCSC table browser, producing a list of 321 gene targets for Tip60 and 2210 for RNA Pol II. Functional annotation clustering analysis was performed on the list of 321 Tip60-associated genes using both DAVID and GeneCodis3 (Tabas-Madrid et al. 2012). Analysis of these functional clusters (Figure 7A) revealed that they were enriched for a variety of neuronal processes all linked to cognition. Next, tissue-specific expression data for the 321 Tip60-associated genes was obtained through FlyAtlas. Figure 7B shows the percentage of Tip60-associated genes that are upregulated and downregulated in the various fly tissue types. Importantly, these data show that in neuronal tissues (brain, head, larval CNS, and thoracicoabdominal ganglion), ∼60–65% of Tip60 target genes are robustly expressed, while in other tissue types, the majority of Tip60 target genes show low-level expression. To produce a comprehensive list of Tip60-associated “neuronal genes,” we combined the list of 46 neuronal genes identified through Gene Ontology functional annotation with additional Tip60-target genes that are upregulated in neuronal tissues relative to whole fly, as determined based on tissue-specific expression data from FlyAtlas. This produced a comprehensive list of 178 unique neuronal genes targeted by Tip60. Importantly, 132 of 178 neuronal genes targeted by Tip60 showed colocalization of RNA Pol II, suggesting that the majority of Tip60-target neuronal genes are actively transcribed (Figure 7C). Further analysis using the DAVID and Gene Codis3 classification system revealed that a substantial number of these genes were functionally linked to specific cognition-linked pathways (Table 1), many of which were activity-dependent signaling pathways. Full lists of total Tip60 gene targets and neuronal Tip60 gene targets are shown in Table S1 and Table S2, respectively. To identify the enrichment of known transcription factor DNA-binding motifs within Tip60-target-gene intervals, a motif enrichment analysis was performed using the MEME-ChIP suite as well as RSAT and Motif Enrichment Tool. DNA recognition sequences and statistical significance for top hit TFs involved in various neuronal functions are shown in Figure S2.
Figure 7.
Tip60 associates with genes enriched for neuronal functions. (A) Functional annotation clustering of Tip60-associated genes using GeneCodis. Only clusters containing more than two genes and having significant enrichment are shown. (B) Tissue-specific expression profiles for Tip60-associated genes. Expression profiles were obtained through FlyAtlas. Histogram analysis depicts that the majority of Tip60-associated genes are robustly expressed specifically in tissues with neuronal function. (C) Proportions of different gene categories in Tip60-target genes. A total of 132 neuronal genes targeted by Tip60 showed colocalization of an active elongation version of RNA Pol II, suggesting that these Tip60-target neuronal genes are actively transcribed.
Table 1. Activity-dependent, cognition-linked pathways enriched for Tip60-associated genes.
| Pathway name | P-value | Gene count |
|---|---|---|
| Signaling pathways | 0.033862 | 20 |
| Signaling by GPCR, G-protein coupled receptor | 0.000155 | 13 |
| GPCR downstream signaling | 0.00767 | 9 |
| Integration of energy metabolism | 0.018144 | 9 |
| Neuronal system | 0.025936 | 9 |
| Signaling by NGF, nerve growth factor | 0.028872 | 9 |
| Axon guidance | 0.022689 | 8 |
| NGF signaling via TRKA, tyrosine kinase, from the plasma membrane | 0.02838 | 8 |
| Gα signaling events | 0.024443 | 5 |
| Activation of NMDA receptor upon glutamate binding and postsynaptic events | 0.029943 | 4 |
| PKA activation | 0.039547 | 4 |
| Ca-dependent events | 0.039547 | 4 |
| Calmodulin-induced events | 0.039547 | 4 |
| CaM pathway | 0.039547 | 4 |
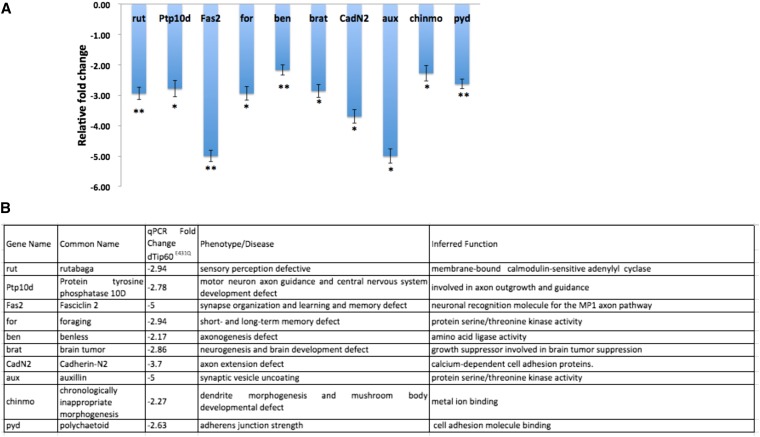
Based on our ChIP-Seq analysis, we hypothesized that the memory disruption and MB morphological defects that we observed upon Tip60 HAT misregulation was caused by inappropriate expression of genes required for these processes. To test this hypothesis, we performed quantitative real-time PCR analysis of 10 genes identified in our ChIP-Seq analysis that were well-characterized representatives of a particular cognition-linked pathway, this time using adult fly heads expressing dTip60E431Q under the control of the elav-GAL4 driver (Figure 8). Each of the 10 genes that we tested were significantly downregulated in response to Tip60 HAT. These genes included those required for axonal extension, guidance, and target recognition such as ben, brat, CadN2, and pyd, consistent with the MB axonal outgrowth defects that we observed in Tip60E431Q mutant fly brains (Figure 4). Moreover, genes required for memory such as rut, ptp10d, and fas2 as well as a core dendrite morphogenesis gene chinmo and the neurotransmitter release gene aux were also negatively affected, consistent with the memory defects that we observed in the Tip60E431Q flies (Figure 3). Taken together, our findings support a transcriptional regulatory role for Tip60 HAT activity in the activation of genes essential for cognitive processes.
Figure 8.
Tip60 HAT loss in the CNS causes misregulation of neuronal genes. (A) Real-time PCR was performed on cDNA isolated from adult fly heads expressing dTip60 E431Q under the elav C155 pan-neuronal GAL4 driver. Histogram represents relative fold change in expression level of neuronal target genes. Real-time PCRs were performed in triplicate, and the fold change was calculated using the ΔΔCT method. Statistical significance was calculated using an unpaired Student’s t-test: *P < 0.05 and **P < 0.01. (B) List of selected learning- and memory-related genes and their functions that are identified in ChIP-seq analysis and validated in adult head tissue using quantitative RT-PCR.
Increasing Tip60 levels in the MB of the fly CNS rescues learning and memory defects under APP-induced neurodegenerative conditions
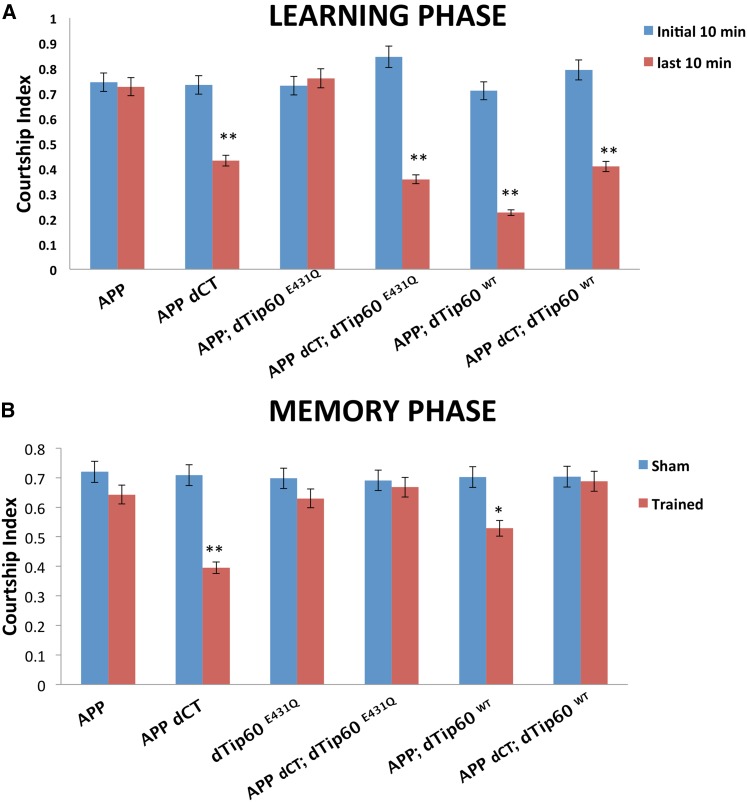
We previously reported that Tip60 plays a neuroprotective role in AD neurodegenerative progression by demonstrating that increased levels of Tip60 HAT activity suppress AD deficits that include apoptotic-induced neurodegeneration, axonal outgrowth and transport defects, and associated sleep and locomotion behavioral phenotypes in a Drosophila model overexpressing APP (Pirooznia et al. 2012a,b; Johnson et al. 2013; Pirooznia and Elefant 2013b). Given the importance of histone acetylation in cognitive ability (Pirooznia and Elefant 2012; Peixoto 2013; Pirooznia and Elefant 2013a; Fischer 2014) and our finding that appropriate levels of Tip60 HAT activity are required for short-term memory (Figure 3, A and B), we hypothesized that excess Tip60 might also rescue AD-linked cognitive deficits. To test this hypothesis, we again used the conditioned courtship suppression assay to assess learning and memory, this time using a unique AD fly model generated in our laboratory that co-expresses equivalent levels of dTip60WT or dTip60E431Q with either APP or APP dCT (APP lacking the C terminus that forms the transcriptional regulatory Tip60/AICD complex) (Siegel and Hall 1979). We found that flies exhibited both learning and short-term memory defects when APP expression was targeted specifically to the fly MB using GAL4 driver OK-107 GAL4 and that these cognitive deficits were dependent upon the presence of the Tip60-interacting C terminus of APP. Remarkably, our studies revealed that excess levels of Tip60 in the fly MB rescued such APP-induced learning and memory defects (Figure 9, A and B) and that this rescue was dependent upon both a functioning Tip60 HAT domain and the Tip60-interacting C terminus of APP (Figure 9, A and B). (Gunawardena and Goldstein 2001; Merdes et al. 2004). Taken together, our results support a neuroprotective role for Tip60 HAT activity in APP-induced cognitive deficits associated with AD.
Figure 9.
Increased level of Tip60 HAT activity rescues learning and memory deficits under APP-induced neurodegenerative conditions. (A) Learning during the initial 10 min (blue columns) and final 10 min (red columns) of the training phase during the courtship suppression assay. Genotypes are indicated. Flies expressing either hAPP or co-expressing equivalent levels of hAPP with HAT mutant dTip60E431Q exhibit no marked decrease in courtship during final 10 min compared to intitial 10 min, indicative of learning defects. Flies co-expressing hAPP with dTip60WT exhibit marked decrease in courtship index during final 10 min compared to the initial 10 min, indicative of normal learning response. Flies expressing either hAPP dCT or co-expressing and hAPPdCT; dTip60E431Q exhibits marked decrease in courtship index during final 10 min compared to initial 10 min, indicating that learning effects are dependent upon the C-terminal of hAPP. (B) Immediate-recall memory (0–2 min post-training) of trained males compared to untrained (sham) males of the same genotype. Both hAPP and hAPP;dTip60E431Q flies show no significant difference between trained and sham males, indicative of no immediate-recall memory, while hAPP; dTip60WT show significant difference, indicative of memory rescue. hAPP dCT with dTip60E431Q and dTip60WT flies shows no significant difference between trained and sham males, indicative of no immediate recall of training. Error bars represent 95% confidence interval. A single asterisk indicates P < 0.05 and a double asterisk indicates P < 0.001 compared with sham males. n = 20 for trained and untrained males in each genotype.
Discussion
Our previous work using a Drosophila model system demonstrated that Tip60 HAT function is critical for a variety of cognition-linked processes that include synaptic plasticity, axonal outgrowth and transport, and neuronal cell apoptotic control in the CNS (Sarthi and Elefant 2011; Pirooznia et al. 2012a,b; Johnson et al. 2013; Pirooznia and Elefant 2013a). Our present study extends these findings to demonstrate that Tip60 HAT activity plays an integral functional role in memory formation in Drosophila. Here, we show that loss of dTip60 HAT activity or increased levels of dTip60 HAT activity in all the lobes of the MB, the learning and memory center of the Drosophila CNS, disrupt immediate-recall memory while there is no effect on learning. In the dTip60E431Q HAT mutant flies, memory defects are also accompanied by axonal growth defects that are evident in dorsal α/α′ and medial β/β′ and γ lobes of the adult MB in these flies with no marked effect on the larval MB structure. The α, β, and γ neurons composing the MB undergo considerable structural reorganization during embryonic, larval, and pupal development. The γ neurons are the earliest born and develop during first instar larval stages while development of α′/β′ axons and α/β axons takes place during the third instar larval and pupal stages, respectively (Scott et al. 2001). Although the α/β lobes appear much later in development than the α′/β′ lobes, the dramatic effects that we observe on both these lobes in the dTip60E431Q adult flies indicate that Tip60 HAT activity may be crucial for development of α/β lobes as well as for maintaining branch stability in the larval-born α′/β′ lobes as development proceeds. During metamorphosis, the γ neurons undergo a stereotypical process of axon elimination wherein the dorsal and medial segments of its axon are pruned back (Watts et al. 2003, 2004). The γ axons subsequently re-extend medially during pupal remodeling. Tip60 HAT activity likely mediates regeneration of γ axons as well during pupal development as evidenced by the severe reduction of these axons in the adult flies that express the HAT-defective dTip60E431Q mutant. Consistent with these findings, we recently reported a similar effect in the axons of Drosophila sLNv, a well-characterized model system for studying axonal growth, wherein Tip60 HAT loss inhibits sLNv axon outgrowth in the adult flies while no axonal defects were observed in the third instar larva (Pirooznia et al. 2012a). Together, these results support a role for Tip60 HAT activity in mediating MB axonal outgrowth required for proper adult MB axonal formation.
Transcription of genes involved in synaptic plasticity is a highly regulated process, and it is becoming increasingly clear that HATs and HDACs are key regulators in this process (Graff and Mansuy 2008; Sweatt 2009). As such, epigenetic PTMs of histone proteins, most notably histone acetylation, that control nuclear chromatin packaging and gene expression profiles are emerging as a fundamental mechanism by which neurons adapt their transcriptional response to environmental cues (Feng et al. 2007; Sweatt 2009; Bousiges et al. 2010; Meaney and Ferguson-Smith 2010; Riccio 2010; Nelson and Monteggia 2011; Pirooznia and Elefant 2012). The implicit hypothesis is that environmental signals drive changes in histone acetylation modifications that are inherently dynamic in nature. Such changes allow for activity-dependent transcriptional “plasticity” that mediates sustained variation in neural function (Sweatt 2009; Riccio 2010; Graff et al. 2012b; Bousiges et al. 2013). One of the most important of such experience-driven behavioral changes is learning and memory formation, as it directly impacts cognitive ability (Levenson and Sweatt 2005; Carulli et al. 2011; Nelson and Monteggia 2011; West and Greenberg 2011). Importantly, the MB is a highly plastic brain region in the Drosophila CNS that is well known for its role in multimodal sensory integration and associative learning. Thus, it serves as a powerful model to study not only developmental synaptic reorganization but also experience-dependent, cognition-linked plasticity (Technau 1984; Heisenberg et al. 1995; Barth and Heisenberg 1997). Here we show that robust production of Tip60 is localized in the MB Kenyon cell nuclei. Moreover, our ChIP-Seq analysis reveals that Tip60 direct target genes are enriched for functions in cognitive processes and that key genes representing these pathways are misregulated in the adult Tip60 HAT mutant fly brain. We acknowledge that, because theTip60 target genes that we show here were identified in S2 cells, they cannot be assumed to be the same in vivo. However, our findings are consistent with our previous microarray study performed on Tip60 HAT mutant second instar larvae in vivo that showed misregulation of genes enriched for neuronal function (Lorbeck et al. 2011). Intriguingly, we found that many of the pathways enriched for Tip60-associated genes are activity dependent (Table 1). Taken together, our studies uncover an epigenetic transcriptional regulatory role for Tip60 HAT action in cognitive function and suggest that Tip60 function in gene control may rely on experience-driven modes of action.
Several other brain regions in addition to the MB have been identified to be important for courtship learning. Basic courtship involves communication between the projection neurons from the antennal glomeruli with higher centers in the lateral protocerebrum and MBs (Mehren et al. 2004). Recent studies using cobalt labeling and ectopic expression of the ATP receptor P2X2 in the MB Kenyon cells also suggest the existence of functional feedback from MBs to the antennal lobes, a process crucial for sensory processing (Rybak and Menzel 1993; Hu et al. 2010). Furthermore, such functional feedbacks from the Kenyon cells are thought to be mediated by the β and γ lobes (Hu et al. 2010), which are also severely affected in the dTip60E431Q flies. Changes in neuronal connectivity in the CNS are also thought to contribute to behavioral defects in several Drosophila learning mutants that alter cAMP signaling (Guan et al. 2011). Thus, the axonal growth defects that we observe in the dTip60E431Q flies may result in disruption of synaptic connectivity between the MB and neural circuits in the protocerebrum essential for sensory processing, subsequently leading to the observed memory impairment. Intriguingly, although overexpression of dTip60WT did not have an observable effect on the MB structure per se, the dTip60WT-expressing flies exhibit defects in immediate-recall memory similar to the dTip60E431Q flies. Importantly, under normal conditions, maintaining the balance between HAT and HDAC levels and activity is critical for establishing proper histone modification patterns that serve to regulate both stable and rapidly changing gene expression profiles crucial for both neuronal homeostasis and appropriate neurophysiological response outputs such as long-term potentiation, learning, and memory, respectively (Morris 2003). Thus, we speculate that increasing Tip60-mediated acetylation in the MB can lead to complex changes in the chromatin landscape, causing misregulation of genes that are induced following patterned synaptic stimulation, such as behavioral experiences. Such changes play a critical role in translating the activity in neural circuits into accessible memories in the brain (Pirooznia and Elefant 2012, 2013a).
Outgrowth and stabilization of axons during development of the nervous system and reorganization of axonal connections in the adult in response to environmental cues critical for cognition are based on the dynamic rearrangement of the cytoskeleton (Baas and Luo 2001; Dent and Gertler 2003). Axon growth and elongation depends, among other factors, on microtubule polymerization (Conde and Caceres 2009), and acetylation of α-tubulin has been reported to stabilize microtubules and promote polymerization (Creppe et al. 2009). Accordingly, reducing levels of HDAC6 has been reported to restore learning and memory as well as α-tubulin acetylation in a mouse model for AD (Govindarajan et al. 2013). Consistent with these findings, we previously reported that Tip60 partially acetylates microtubules in the larval neuromuscular junction (NMJ), an effect that was dependent on its HAT function (Sarthi and Elefant 2011), although we did not observe this effect in motor neuron axons of the Drosophila CNS (Johnson et al. 2013). Here, our analysis reveals that Tip60 is localized not only within the nucleus of the Kenyon cells in the MB but also within all the MB axonal lobes, indicative of both nuclear and cytoplasmic localization for Tip60. Such cytoplasmic and nuclear Tip60 localization is recapitulated in the Drosophila NMJ, where we previously reported Tip60 to be localized both pre- and postsynaptically (Sarthi and Elefant 2011). Therefore, we cannot rule out the possibility that Tip60 may also mediate axonal growth by modulating cytoskeletal dynamics in the MB through direct binding and acetylation of cytoskeletal proteins that function to promote and stabilize axon growth. Notably, neural activity has been shown to modulate chromatin acetylation in hippocampal neurons in part by controlling the shuttling of certain HDACs in and out of the nucleus that, in turn, influences their activity in gene control (Chawla et al. 2003; Riccio 2010). Thus, Tip60 may also utilize a similar cytoplasmic/nuclear shuttling mechanism, an area that we are currently exploring.
Tip60 has been implicated in AD via its interaction with the AICD, a fragment generated by the processing of APP by γ-secretase that is subsequently released into the cytoplasm (Muller et al. 2008). AICD forms a transcriptional competent protein complex with the HAT Tip60 (Cao and Sudhof 2001) and is recruited to the promoters of specific neuronal target genes where it acts to acetylate select histone proteins to epigenetically regulate gene transcription (Cao and Sudhof 2001; Von Rotz et al. 2004; Ryan and Pimplikar 2005). Importantly, misexpression of certain Tip60/AICD target genes such as neprilysin, EGFR, LRP, and KAI-1 have been associated with AD pathophysiology (Baek et al. 2002; Muller et al. 2007; Slomnicki and Lesniak 2008). Based on these findings, it has been proposed that the inappropriate AICD/Tip60 complex formation and/or its recruitment may contribute or lead to AD pathology via epigenetic transcriptional misregulation of target genes required for neuronal functions (Pirooznia and Elefant 2012, 2013a; Johnson et al. 2013). Here, we report that, while misregulation of Tip60 HAT activity alone causes short-term memory deficits in the fly, excess production of Tip60 in conjunction with APP rescue both APP-induced learning and immediate-recall memory deficits in a fly model overexpressing APP. Importantly, reversal of these cognitive deficits is dependent upon the C terminus of APP that forms the transcriptional regulatory Tip60/AICD complex as well as the functional HAT activity of Tip60. Accordingly, we show by ChIP-Seq that Tip60-associated genes are enriched for cognition function and that key Tip60 genes representing learning- and memory-linked pathways are misregulated in the Tip60 HAT mutant fly brain in vivo. Consistent with these findings, we previously reported that increasing in vivo Tip60 HAT levels in the nervous system of flies under APP-induced neurodegenerative conditions rescues Tip60-mediated, cognition-linked processes impaired in AD that include apoptotic neurodegeneration (Pirooznia et al. 2012b), axonal outgrowth (Pirooznia et al. 2012a; Pirooznia and Elefant 2013b) and transport (Johnson et al. 2013) and restores associated disrupted complex functional abilities that include sleep cycles (Pirooznia et al. 2012a; Pirooznia and Elefant 2013b) and locomotor function (Johnson et al. 2013). Gene expression analysis revealed that these flies exhibit repression of cassettes of pro-apoptotic genes and induction of pro-survival genes (Pirooznia and Elefant 2012, 2013a; Pirooznia et al. 2012b). Together, our results point to an all-encompassing neuroprotective role for Tip60 in early AD progression and support a model by which Tip60 epigenetic reprograms select gene sets that redirect neuronal cell fate from cell death toward cell survival and function (Pirooznia and Elefant 2013a). Tip60 might exert such gene reprogramming either by itself or by complexing with other peptides such as AICD for its recruitment to select genes via promoter-bound TFs such as those that we identified in our ChIP-Seq analysis. Our findings that Tip60 exerts neuroprotective effects under neurodegenerative conditions via epigenetic gene reprogramming are not unprecedented. Indeed, gene transfer of CBP was shown to ameliorate learning and memory deficits in a mouse AD model by increasing brain-derived neurotrophic factor (Caccamo et al. 2010), and p300, but not HDAC inhibitors, were found to enhance axonal regeneration by inducing axonal outgrowth genes (Gaub et al. 2011). In light of these findings, it is important consider that modulation of specific HAT levels and/or activity may alter the expression of many genes or “cassettes” of specific genes that act together to produce a neuroprotective effect, as indicated by our ChIP-Seq analysis for Tip60 (Pirooznia and Elefant 2012, 2013a). Therefore, it will be critical to determine the identity of specific cognition-linked gene target sets regulated by Tip60 to further dissect their neuroprotective nature for more effective design of HAT-based therapeutic strategies.
Supplementary Material
Acknowledgments
We thank Dr. Amita Sehgal for generously contributing a variety of mushroom body GAL4 drivers. This work was supported by National Institutes of Health grant R01HD057939 (to F.E.).
Footnotes
Supporting information is available online at http://www.genetics.org/lookup/suppl/doi:10.1534/genetics.114.171660/-/DC1.
Communicating editor: H. J. Bellen
Literature Cited
- Akalai D. B., Wilson C. F., Zong L., Tanaka N. K., Ito K., et al. , 2006. Roles for Drosophila mushroom body neurons in olfactory learning and memory. Learn. Mem. 13: 659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Saigh R., Elefanti A., Velegraki A., Zerva L., Meletiadis J., 2012. In vitro pharmacokinetic/pharmacodynamic modeling of voriconazole activity against Aspergillus species in a new in vitro dynamic model. Antimicrob. Agents Chemother. 56: 5321–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y., Grubel K., Busch S., Friedrich A. B., Siwanowicz I., et al. , 2009. The mushroom body of adult Drosophila characterized by GAL4 drivers. J. Neurogenet. 23: 156–172. [DOI] [PubMed] [Google Scholar]
- Awasaki T., Saito M., Sone M., Suzuki E., Sakai R., et al. , 2000. The Drosophila trio plays an essential role in patterning of axons by regulating their directional extension. Neuron 26: 119–131. [DOI] [PubMed] [Google Scholar]
- Baas P. W., Luo L., 2001. Signaling at the growth cone: the scientific progeny of Cajal meet in Madrid. Neuron 32: 981–984. [DOI] [PubMed] [Google Scholar]
- Baek S. H., Ohgi K. A., Rose D. W., Koo E. H., Glass C. K., et al. , 2002. Exchange of N-CoR corepressor and Tip60 coactivator complexes links gene expression by NF-kappaB and beta-amyloid precursor protein. Cell 110: 55–67. [DOI] [PubMed] [Google Scholar]
- Barth M., Heisenberg M., 1997. Vision affects mushroom bodies and central complex in Drosophila melanogaster. Learn. Mem. 4: 219–229. [DOI] [PubMed] [Google Scholar]
- Bates K. E., Sung C. S., Robinow S., 2010. The unfulfilled gene is required for the development of mushroom body neuropil in Drosophila. Neural Dev. 5: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli E., Nestler E. J., Allis C. D., Sassone-Corsi P., 2008. Decoding the epigenetic language of neuronal plasticity. Neuron 60: 961–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousiges O., Vasconcelos A. P., Neidi R., Cosquer B., Herbeux K., et al. , 2010. Spatial memory consolidation is associated with induction of several lysine-acetyltransferase (histone acetylatransferase) expression levels and H2B/H4 acetylation-dependent transcriptional events in the rat hippocampus. Neuropsychopharmacology 35: 2521–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousiges O., Neidl R., Majchrzak M., Muller M. A., Bergelivien A., et al. , 2013. Detection of histone acetylation levels in the dorsal hippocampus reveals early tagging on specific residues of H2B and H4 histones in response to learning. PLoS ONE 8: e57816. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Broughton S. J., Tully T., Greenspan R. J., 2003. Conditioning deficits of CaM-kinase transgenic Drosophila melanogaster in a new excitatory courtship assay. J. Neurogenet. 17: 91–102. [PubMed] [Google Scholar]
- Busto G. U., Cervantes-sandoval I., Davis R. L., 2010. Olfactory learning in Drosophila. Physiology (Bethesda) 26: 338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A., Maldonado M. A., Bokov A. F., Majumder S., Oddo S., 2010. CBP gene transfer increases BDNF levels and ameliorates learning and memory deficits in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 107: 22687–22692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., Sudhof T. C., 2001. A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science 293: 115–120. [DOI] [PubMed] [Google Scholar]
- Cao X., Sudhof T. C., 2004. Dissection of amyloid-beta precursor protein-dependent transcriptional transactivation. J. Biol. Chem. 279: 24601–24611. [DOI] [PubMed] [Google Scholar]
- Carulli D., Foscarin S., Rossi F., 2011. Activity-dependent plasticity and gene expression modifications in the adult CNS. Front. Mol. Neurosci. 4: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla S., Vanhoutte P., Arnold F. J., Huang C. L., Bading H., 2003. Neuronal activity-dependant nucleocytoplasmic shuttling of HDAC4 and HDAC5. J. Neurochem. 85: 151–159. [DOI] [PubMed] [Google Scholar]
- Conde C., Caceres A., 2009. Microtubule assembly, organization and dynamics in axons and dendrites. Nat. Rev. Neurosci. 10: 319–332. [DOI] [PubMed] [Google Scholar]
- Creppe C., Malinouskaya L., Volvert M. L., Gillard M., Close P., et al. , 2009. Elongator controls the migration and differentiation of cortical neurons through acetylation of alpha-tubulin. Cell 136: 551–564. [DOI] [PubMed] [Google Scholar]
- Crittenden J. R., Skoulakis E. M., Han K. A., Kalderon D., Davis R. L., 1998. Tripartite mushroom body architecture revealed by antigenic markers. Learn. Mem. 5: 38–51. [PMC free article] [PubMed] [Google Scholar]
- Dent E. W., Gertler F. B., 2003. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron 40: 209–227. [DOI] [PubMed] [Google Scholar]
- Dubnau J., and T. Tully, 2001. Functional anatomy: from molecule to memory. Curr. Biol. 11: R240–R243. [DOI] [PubMed] [Google Scholar]
- Ebert D. H., Greenberg M. E., 2013. Activity-dependent neuronal signalling and autism spectrum disorder. Nature 493: 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris S. M., 2013. Evolution of complex higher brain centers and behaviors: behavioral correlates of mushroom body elaboration in insects. Brain Behav. Evol. 82: 9–18. [DOI] [PubMed] [Google Scholar]
- Feng J., Fouse S., Fan G., 2007. Epigenetic regulation of neural gene expression and neuronal function. Pediatr. Res. 61: 58R–63R. [DOI] [PubMed] [Google Scholar]
- Fiala A., 2007. Olfaction and olfactory learning in Drosophila: recent progress. Curr. Opin. Neurobiol. 17: 720–726. [DOI] [PubMed] [Google Scholar]
- Fischer, A., 2014. Targeting histone-modifications in Alzheimer’s disease: What is the evidence that this is a promising therapeutic avenue? Neuropharmacology 80: 95–102. [DOI] [PubMed] [Google Scholar]
- Fushima K., Tsujimura H., 2007. Precise control of fasciclin II expression is required for adult mushroom body development in Drosophila. Dev. Growth Differ. 49: 215–227. [DOI] [PubMed] [Google Scholar]
- Gaub P., Joshi Y., Wuttke A., Naumann U., Schnichels S., et al. , 2011. The histone acetyltransferase p300 promotes intrinsic axonal regeneration. Brain 134: 2134–2148. [DOI] [PubMed] [Google Scholar]
- Govindarajan M., Rao P., Burkhardt S., Sananbenesi F., Schluter O., et al. , 2013. Reducing HDAC6 ameliorates cognitive deficits in a mouse model for Alzheimer’s disease. EMBO Mol. Med. 5: 52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J., Mansuy I. M., 2008. Epigenetic codes in cognition and behaviour. Behav. Brain Res. 192: 70–87. [DOI] [PubMed] [Google Scholar]
- Graff J., Rei D., Guan J. S., Wang W. Y., Seo J., et al. , 2012a An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature 483: 222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J., Woldemichael B. T., Berchtold D., Dewarrat G., Mansuy I. M., 2012b Dynamic histone marks in the hippocampus and cortex facilitate memory consolidation. Nat. Commun. 3: 991. [DOI] [PubMed] [Google Scholar]
- Greenspan R. J., 1995. Flies, genes, learning, and memory. Neuron 15: 747–750. [DOI] [PubMed] [Google Scholar]
- Guan Z., Buhl L. K., Quinn W. G., Littleton J. T., 2011. Altered gene regulation and synaptic morphology in Drosophila learning and memory mutants. Learn. Mem. 18: 191–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardena S., Goldstein L. S., 2001. Disruption of axonal transport and neuronal viability by amyloid precursor protein mutations in Drosophila. Neuron 32: 389–401. [DOI] [PubMed] [Google Scholar]
- Guven-Ozkan T., and R. L. Davis, 2014. Functional neuroanatomy of Drosophila olfactory memory formation. Learn. Mem. 21: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg M., 2003. Mushroom body memoir: from maps to models. Nat. Rev. Neurosci. 4: 266–275. [DOI] [PubMed] [Google Scholar]
- Heisenberg M., Heusipp M., Wanke C., 1995. Structural plasticity in the Drosophila brain. J. Neurosci. 15: 1951–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu A., Zhang W., Wang Z., 2010. Functional feedback from mushroom bodies to antennal lobes in the Drosophila olfactory pathway. Proc. Natl. Acad. Sci. USA 107: 10262–10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. A., Sarthi J., Pirooznia S. K., Reube W., Elefant F., 2013. Increasing Tip60 HAT levels rescues axonal transport defects and associated behavioral phenotypes in a Drosophila Alzheimer’s disease model. J. Neurosci. 33: 7535–7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahsai L., Zars T., 2011. Learning and memory in Drosophila: behaviour, genetics and neural systems. Int. Rev. Neurobiol. 99: 139–167. [DOI] [PubMed] [Google Scholar]
- Kazantsev A. G., Thompson L. M., 2008. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat. Rev. Drug Discov. 7: 854–868. [DOI] [PubMed] [Google Scholar]
- Keene A. C., Waddell S., 2007. Drosophila olfactory memory: single genes to complex neural circuits. Nat. Rev. Neurosci. 8: 341–354. [DOI] [PubMed] [Google Scholar]
- Korzus E., Rosenfeld M. G., Mayford M., 2004. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron 42: 961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T., Lee A., Luo L., 1999. Development of the Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast. Development 126: 4065–4076. [DOI] [PubMed] [Google Scholar]
- Legube G., Trouche D., 2003. Regulating histone acetyltransferases and deacetylases. EMBO Rep. 4: 944–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson J. M., Sweatt J. D., 2005. Epigenetic mechanisms in memory formation. Nat. Rev. Neurosci. 6: 108–118. [DOI] [PubMed] [Google Scholar]
- Levenson J. M., O’Riordan K. J., Brown K. D., Trinh M. A., Molfese D. L., et al. , 2004. Regulation of histone acetylation during memory formation in the hippocampus. J. Biol. Chem. 279: 40545–40559. [DOI] [PubMed] [Google Scholar]
- Lorbeck M., Pirooznia K., Sarthi J., Zhu X., Elefant F., 2011. Microarray analysis uncovers a role for Tip60 in nervous system function and general metabolism. PLoS ONE 6: e18412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L., O’Leary D. D., 2005. Axon retraction and degeneration in development and disease. Annu. Rev. Neurosci. 28: 127–156. [DOI] [PubMed] [Google Scholar]
- Margulies C., T. Tully, and J. Dubnau, 2005. Deconstructing memory in Drosophila. Curr. Biol. 15: R700–R713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride S. M., Giuliani G., Choi C., Krause P., Correale D., et al. , 1999. Mushroom body ablation impairs short-term memory and long-term memory of courtship conditioning in Drosophila melanogaster. Neuron 24: 967–977. [DOI] [PubMed] [Google Scholar]
- McBride S. M., Choi C. H., Wang Y., Liebelt D., Braunstein E., et al. , 2005. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron 45: 753–764. [DOI] [PubMed] [Google Scholar]
- Meaney M. J., Ferguson-Smith A. C., 2010. Epigenetic regulation of the neural transcriptome: the meaning of the marks. Nat. Neurosci. 13: 1313–1318. [DOI] [PubMed] [Google Scholar]
- Mehren J. E., Ejima A., Griffith L. C., 2004. Unconventional sex: fresh approaches to courtship learning. Curr. Opin. Neurobiol. 14: 745–750. [DOI] [PubMed] [Google Scholar]
- Melicharek D. J., Ramirez L. C., Singh S., Thompson R., Marenda D. R., 2010. Kismet/CHD7 regulates axon morphology, memory and locomotion in a Drosophila model of CHARGE syndrome. Hum. Mol. Genet. 19: 4253–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes G., Soba P., Loewer A., Bilic M. V., Beyreuther K., et al. , 2004. Interference of human and Drosophila APP and APP-like proteins with PNS development in Drosophila. EMBO J. 23: 4082–4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. G., 2003. Long-term potentiation and memory. Philos. Trans. R. Soc. Lond. B Biol. Sci. 358: 643–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T., Concannon C. G., Ward M. W., Walsh C. M., Tirniceriu A. L., et al. , 2007. Modulation of gene expression and cytoskeletal dynamics by the amyloid precursor protein intracellular domain (AICD). Mol. Biol. Cell 18: 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T., Meyer H. E., Egensperger R., Marcus K., 2008. The amyloid precursor protein intracellular domain (AICD) as modulator of gene expression, apoptosis, and cytoskeletal dynamics-relevance for Alzheimer’s disease. Prog. Neurobiol. 85: 393–406. [DOI] [PubMed] [Google Scholar]
- Nelson E. D., Monteggia L. M., 2011. Epigenetics in the mature mammalian brain: effects on behavior and synaptic transmission. Neurobiol. Learn. Mem. 96: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto L., Abel T., 2013. The role of histone acetylation in memory formation and cognitive impairments. Neuropsychopharmacology 38: 62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirooznia K., Elefant F., 2012. Modulating epigenetic HAT activity: A promising therapuetic option for neurological disease? J. Mol. Cloning Genet. Recomb. 1: 1–3. [Google Scholar]
- Pirooznia S. K., Chiu K., Chan M. T., Zimmerman J. E., Elefant F., 2012a Epigenetic regulation of axonal growth of Drosophila pacemaker cells by histone acetyltransferase tip60 controls sleep. Genetics 192: 1327–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirooznia S. K., Sarthi J., Johnson A. A., Toth M. S., Chiu K., et al. , 2012b Tip60 HAT activity mediates APP induced lethality and apoptotic cell death in the CNS of a Drosophila Alzheimer’s disease model. PLoS ONE 7: e41776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirooznia K., Elefant F., 2013a Targeting specific HATs for neurodegenerative disease treatment: translating basic biology to therapeutic possibilities. Front. Mol. Neurosci. 7: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirooznia S. K., Elefant F., 2013b A HAT for sleep?: epigenetic regulation of sleep by Tip60 in Drosophila. Fly (Austin) 7: 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio A., 2010. Dynamic epigenetic regulation in neurons: enzymes, stimuli and signaling pathways. Nat. Neurosci. 13: 1330–1337. [DOI] [PubMed] [Google Scholar]
- Ryan K. A., Pimplikar S. W., 2005. Activation of GSK-3 and phosphorylation of CRMP2 in transgenic mice expressing APP intracellular domain. J. Cell Biol. 171: 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak J., Menzel R., 1993. Anatomy of the mushroom bodies in the honey bee brain: the neuronal connections of the alpha-lobe. J. Comp. Neurol. 334: 444–465. [DOI] [PubMed] [Google Scholar]
- Sarthi J., Elefant F., 2011. dTip60 HAT activity controls synaptic bouton expansion at the Drosophila neuromuscular junction. PLoS ONE 6: e26202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott E. K., Lee T., Luo L., 2001. enok encodes a Drosophila putative histone acetyltransferase required for mushroom body neuroblast proliferation. Curr. Biol. 11: 99–104. [DOI] [PubMed] [Google Scholar]
- Siegel R. W., Hall J. C., 1979. Conditioned responses in courtship behavior of normal and mutant Drosophila. Proc. Natl. Acad. Sci. USA 76: 3430–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwicki K. K., Ladewski L., 2003. Associative learning and memory in Drosophila: beyond olfactory conditioning. Behav. Processes 64: 225–238. [DOI] [PubMed] [Google Scholar]
- Siwicki K. K., Riccio P., Ladewski L., Marcillac F., Dartevelle L., et al. , 2005. The role of cuticular pheromones in courtship conditioning of Drosophila males. Learn. Mem. 12: 636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomnicki L. P., Lesniak W., 2008. A putative role of the Amyloid Precursor Protein Intracellular Domain (AICD) in transcription. Acta Neurobiol. Exp. (Warsz.) 68: 219–228. [DOI] [PubMed] [Google Scholar]
- Sweatt J. D., 2009. Experience-dependent epigenetic modifications in the central nervous system. Biol. Psychiatry 65: 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas-Madrid, D., R. Nogales-Cadenas, and A. Pascual-Montano, 2012 GeneCodis3: a non-redundant and modular enrichment analysis tool for functional genomics. Nucleic Acids Res. 40(Web server issue): W478–W483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Technau G. M., 1984. Fiber number in the mushroom bodies of adult Drosophila melanogaster depends on age, sex and experience. J. Neurogenet. 1: 113–126. [DOI] [PubMed] [Google Scholar]
- von Rotz R. C., Kohli B. M., Bosset J., Meier M., Suzuki T., et al. , 2004. The APP intracellular domain forms nuclear multiprotein complexes and regulates the transcription of its own precursor. J. Cell Sci. 117: 4435–4448. [DOI] [PubMed] [Google Scholar]
- Watts R. J., Hoopfer E. D., Luo L., 2003. Axon pruning during Drosophila metamorphosis: evidence for local degeneration and requirement of the ubiquitin-proteasome system. Neuron 38: 871–885. [DOI] [PubMed] [Google Scholar]
- Watts R. J., Schuldiner O., Perrino J., Larsen C., Luo L., 2004. Glia engulf degenerating axons during developmental axon pruning. Curr. Biol. 14: 678–684. [DOI] [PubMed] [Google Scholar]
- West A. E., Greenberg M. E., 2011. Neuronal activity-regulated gene transcription in synapse development and cognitive function. Cold Spring Harb. Perspect. Biol. 1: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Singh N., Donnelly C., Boimel P., Elefant F., 2007. The cloning and characterization of the histone acetyltransferase human homolog Dmel/TIP60 in Drosophila melanogaster: Dmel/TIP60 is essential for multicellular development. Genetics 175: 1229–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.