Abstract
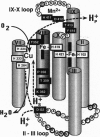
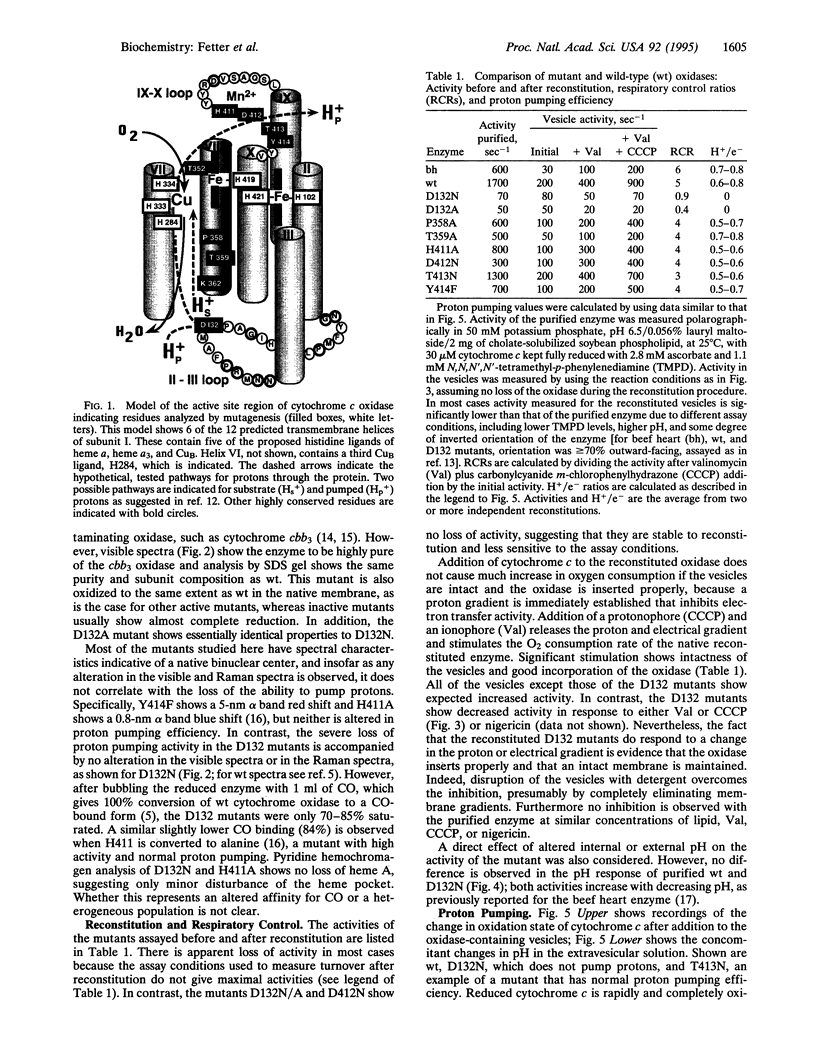
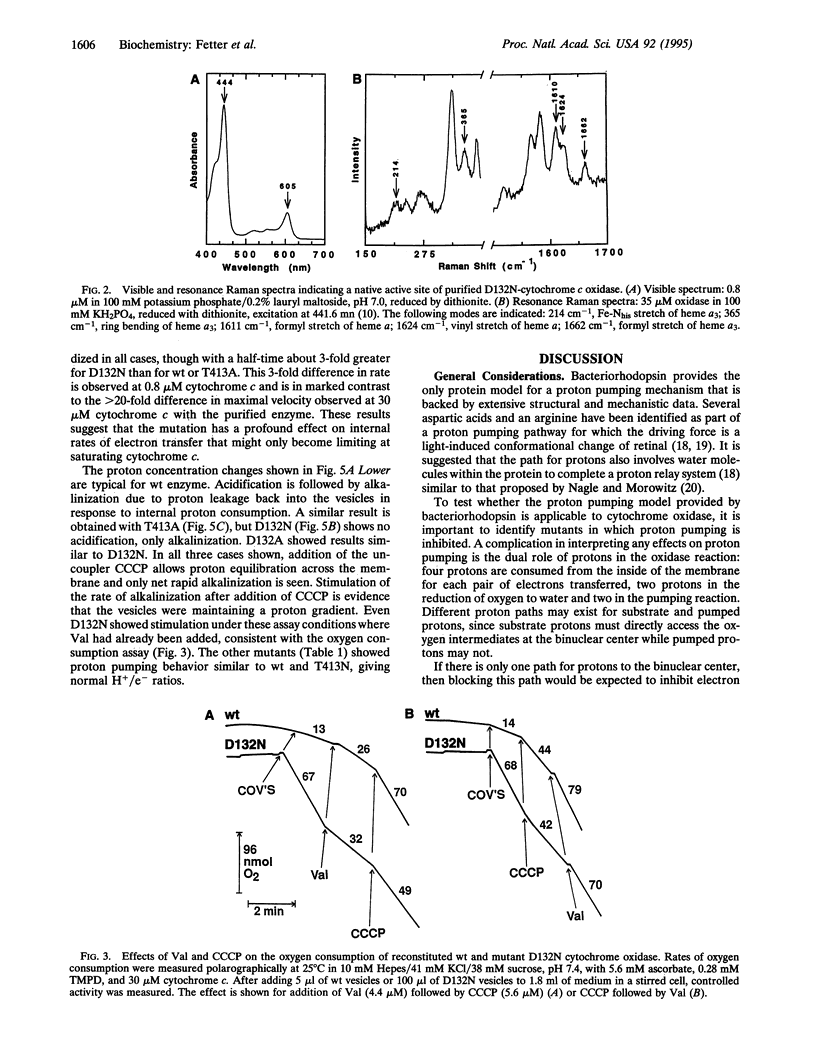
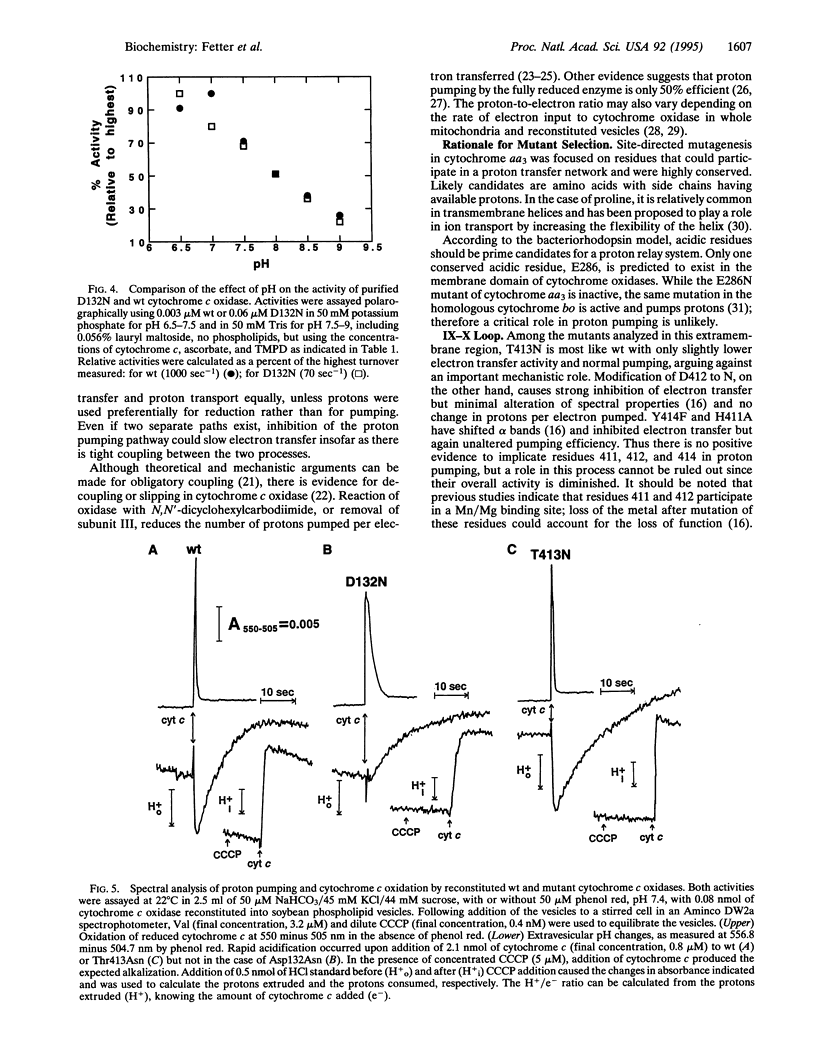
As the final electron acceptor in the respiratory chain of eukaryotic and many prokaryotic organisms, cytochrome c oxidase (EC 1.9.3.1) catalyzes the reduction of oxygen to water and generates a proton gradient. To test for proton pathways through the oxidase, site-directed mutagenesis was applied to subunit I of the Rhodobacter sphaeroides enzyme. Mutants were characterized in three highly conserved regions of the peptide, comprising possible proton loading, unloading, and transfer sites: an interior loop between helices II and III (Asp132Asn/Ala), an exterior loop between helices IX and X (His411Ala, Asp412Asn, Thr413Asn, Tyr414Phe), and the predicted transmembrane helix VIII (Thr352Ala, Pro358Ala, Thr359Ala, Lys362Met). Most of the mutants had lower activity than wild type, but only mutants at residue 132 lost proton pumping while retaining electron transfer activity. Although electron transfer was substantially inhibited, no major structural alteration appears to have occurred in D132 mutants, since resonance Raman and visible absorbance spectra were normal. However, lower CO binding (70-85% of wild type) suggests some minor change to the binuclear center. In addition, the activity of the reconstituted Asp132 mutants was inhibited rather than stimulated by ionophores or uncoupler. The inhibition was not observed with the purified enzyme and a direct pH effect was ruled out, suggesting an altered response to the electrical or pH gradient. The results support an important role for the conserved II-III loop in the proton pumping process and are consistent with the possibility of involvement of residues in helix VIII and the IX-X loop.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzi A., Casey R. P., Nałecz M. J. The effect of N,N'-dicyclohexylcarbodiimide on enzymes of bioenergetic relevance. Biochim Biophys Acta. 1984 Dec 17;768(3-4):209–226. doi: 10.1016/0304-4173(84)90017-x. [DOI] [PubMed] [Google Scholar]
- Babcock G. T., Wikström M. Oxygen activation and the conservation of energy in cell respiration. Nature. 1992 Mar 26;356(6367):301–309. doi: 10.1038/356301a0. [DOI] [PubMed] [Google Scholar]
- Brand M. D., Chien L. F., Diolez P. Experimental discrimination between proton leak and redox slip during mitochondrial electron transport. Biochem J. 1994 Jan 1;297(Pt 1):27–29. doi: 10.1042/bj2970027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl C. J., Deber C. M. Hypothesis about the function of membrane-buried proline residues in transport proteins. Proc Natl Acad Sci U S A. 1986 Feb;83(4):917–921. doi: 10.1073/pnas.83.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Hosler J., Shapleigh J., Revzin A., Ferguson-Miller S. Cytochrome aa3 of Rhodobacter sphaeroides as a model for mitochondrial cytochrome c oxidase. The coxII/coxIII operon codes for structural and assembly proteins homologous to those in yeast. J Biol Chem. 1992 Dec 5;267(34):24273–24278. [PubMed] [Google Scholar]
- Cao J., Shapleigh J., Gennis R., Revzin A., Ferguson-Miller S. The gene encoding cytochrome c oxidase subunit II from Rhodobacter sphaeroides; comparison of the deduced amino acid sequence with sequences of corresponding peptides from other species. Gene. 1991 May 15;101(1):133–137. doi: 10.1016/0378-1119(91)90235-4. [DOI] [PubMed] [Google Scholar]
- Capitanio N., Capitanio G., De Nitto E., Villani G., Papa S. H+/e- stoichiometry of mitochondrial cytochrome complexes reconstituted in liposomes. Rate-dependent changes of the stoichiometry in the cytochrome c oxidase vesicles. FEBS Lett. 1991 Aug 19;288(1-2):179–182. doi: 10.1016/0014-5793(91)81029-8. [DOI] [PubMed] [Google Scholar]
- García-Horsman J. A., Berry E., Shapleigh J. P., Alben J. O., Gennis R. B. A novel cytochrome c oxidase from Rhodobacter sphaeroides that lacks CuA. Biochemistry. 1994 Mar 15;33(10):3113–3119. doi: 10.1021/bi00176a046. [DOI] [PubMed] [Google Scholar]
- Gennis R. B. Some recent advances relating to prokaryotic cytochrome c reductases and cytochrome c oxidases. Biochim Biophys Acta. 1991 May 23;1058(1):21–24. doi: 10.1016/s0005-2728(05)80260-9. [DOI] [PubMed] [Google Scholar]
- Gray K. A., Grooms M., Myllykallio H., Moomaw C., Slaughter C., Daldal F. Rhodobacter capsulatus contains a novel cb-type cytochrome c oxidase without a CuA center. Biochemistry. 1994 Mar 15;33(10):3120–3127. doi: 10.1021/bi00176a047. [DOI] [PubMed] [Google Scholar]
- Gregory L. C., Ferguson-Miller S. Effect of subunit III removal on control of cytochrome c oxidase activity by pH. Biochemistry. 1988 Aug 23;27(17):6307–6314. doi: 10.1021/bi00417a016. [DOI] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Hosler J. P., Ferguson-Miller S., Calhoun M. W., Thomas J. W., Hill J., Lemieux L., Ma J., Georgiou C., Fetter J., Shapleigh J. Insight into the active-site structure and function of cytochrome oxidase by analysis of site-directed mutants of bacterial cytochrome aa3 and cytochrome bo. J Bioenerg Biomembr. 1993 Apr;25(2):121–136. doi: 10.1007/BF00762854. [DOI] [PubMed] [Google Scholar]
- Hosler J. P., Fetter J., Tecklenburg M. M., Espe M., Lerma C., Ferguson-Miller S. Cytochrome aa3 of Rhodobacter sphaeroides as a model for mitochondrial cytochrome c oxidase. Purification, kinetics, proton pumping, and spectral analysis. J Biol Chem. 1992 Dec 5;267(34):24264–24272. [PubMed] [Google Scholar]
- Hosler J. P., Shapleigh J. P., Tecklenburg M. J., Thomas J. W., Kim Y., Espe M., Fetter J., Babcock G. T., Alben J. O., Gennis R. B. A loop between transmembrane helices IX and X of subunit I of cytochrome c oxidase caps the heme a-heme a3-CuB center. Biochemistry. 1994 Feb 8;33(5):1194–1201. doi: 10.1021/bi00171a019. [DOI] [PubMed] [Google Scholar]
- Krebs M. P., Khorana H. G. Mechanism of light-dependent proton translocation by bacteriorhodopsin. J Bacteriol. 1993 Mar;175(6):1555–1560. doi: 10.1128/jb.175.6.1555-1560.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux L. J., Calhoun M. W., Thomas J. W., Ingledew W. J., Gennis R. B. Determination of the ligands of the low spin heme of the cytochrome o ubiquinol oxidase complex using site-directed mutagenesis. J Biol Chem. 1992 Jan 25;267(3):2105–2113. [PubMed] [Google Scholar]
- Madden T. D., Redelmeier T. E. Transmembrane distribution of lipophilic cations in response to an electrochemical potential in reconstituted cytochrome c oxidase vesicles and in vesicles exhibiting a potassium ion diffusion potential. J Bioenerg Biomembr. 1994 Apr;26(2):221–230. doi: 10.1007/BF00763071. [DOI] [PubMed] [Google Scholar]
- Minagawa J., Mogi T., Gennis R. B., Anraku Y. Identification of heme and copper ligands in subunit I of the cytochrome bo complex in Escherichia coli. J Biol Chem. 1992 Jan 25;267(3):2096–2104. [PubMed] [Google Scholar]
- Murphy M. P. Slip and leak in mitochondrial oxidative phosphorylation. Biochim Biophys Acta. 1989 Nov 23;977(2):123–141. doi: 10.1016/s0005-2728(89)80063-5. [DOI] [PubMed] [Google Scholar]
- Nagle J. F., Morowitz H. J. Molecular mechanisms for proton transport in membranes. Proc Natl Acad Sci U S A. 1978 Jan;75(1):298–302. doi: 10.1073/pnas.75.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveberg M., Hallén S., Nilsson T. Uptake and release of protons during the reaction between cytochrome c oxidase and molecular oxygen: a flow-flash investigation. Biochemistry. 1991 Jan 15;30(2):436–440. doi: 10.1021/bi00216a019. [DOI] [PubMed] [Google Scholar]
- Papa S., Capitanio N., Capitanio G., De Nitto E., Minuto M. The cytochrome chain of mitochondria exhibits variable H+/e- stoichiometry. FEBS Lett. 1991 Aug 19;288(1-2):183–186. doi: 10.1016/0014-5793(91)81030-c. [DOI] [PubMed] [Google Scholar]
- Prochaska L. J., Fink P. S. On the role of subunit III in proton translocation in cytochrome c oxidase. J Bioenerg Biomembr. 1987 Apr;19(2):143–166. doi: 10.1007/BF00762722. [DOI] [PubMed] [Google Scholar]
- Saraste M. Structural features of cytochrome oxidase. Q Rev Biophys. 1990 Nov;23(4):331–366. doi: 10.1017/s0033583500005588. [DOI] [PubMed] [Google Scholar]
- Shapleigh J. P., Gennis R. B. Cloning, sequencing and deletion from the chromosome of the gene encoding subunit I of the aa3-type cytochrome c oxidase of Rhodobacter sphaeroides. Mol Microbiol. 1992 Mar;6(5):635–642. doi: 10.1111/j.1365-2958.1992.tb01511.x. [DOI] [PubMed] [Google Scholar]
- Shapleigh J. P., Hosler J. P., Tecklenburg M. M., Kim Y., Babcock G. T., Gennis R. B., Ferguson-Miller S. Definition of the catalytic site of cytochrome c oxidase: specific ligands of heme a and the heme a3-CuB center. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4786–4790. doi: 10.1073/pnas.89.11.4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. W., Puustinen A., Alben J. O., Gennis R. B., Wikström M. Substitution of asparagine for aspartate-135 in subunit I of the cytochrome bo ubiquinol oxidase of Escherichia coli eliminates proton-pumping activity. Biochemistry. 1993 Oct 12;32(40):10923–10928. doi: 10.1021/bi00091a048. [DOI] [PubMed] [Google Scholar]
- Wikström M., Bogachev A., Finel M., Morgan J. E., Puustinen A., Raitio M., Verkhovskaya M., Verkhovsky M. I. Mechanism of proton translocation by the respiratory oxidases. The histidine cycle. Biochim Biophys Acta. 1994 Aug 30;1187(2):106–111. doi: 10.1016/0005-2728(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Wikström M. Pumping of protons from the mitochondrial matrix by cytochrome oxidase. Nature. 1984 Apr 5;308(5959):558–560. doi: 10.1038/308558a0. [DOI] [PubMed] [Google Scholar]
- Wrigglesworth J. M., Nicholls P. Turnover and vectorial properties of cytochrome c oxidase in reconstituted vesicles. Biochim Biophys Acta. 1979 Jul 10;547(1):36–46. doi: 10.1016/0005-2728(79)90093-8. [DOI] [PubMed] [Google Scholar]