A model of a simple CO2-concentrating mechanism matches physiological data showing that, despite its simplicity, the system is functional and highly efficient.
Abstract
As an oligotrophic specialist, Prochlorococcus spp. has streamlined its genome and metabolism including the CO2-concentrating mechanism (CCM), which serves to elevate the CO2 concentration around Rubisco. The genomes of Prochlorococcus spp. indicate that they have a simple CCM composed of one or two HCO3− pumps and a carboxysome, but its functionality has not been examined. Here, we show that the CCM of Prochlorococcus spp. is effective and efficient, transporting only two molecules of HCO3− per molecule of CO2 fixed. A mechanistic, numerical model with a structure based on the CCM components present in the genome is able to match data on photosynthesis, CO2 efflux, and the intracellular inorganic carbon pool. The model requires the carboxysome shell to be a major barrier to CO2 efflux and shows that excess Rubisco capacity is critical to attaining a high-affinity CCM without CO2 recovery mechanisms or high-affinity HCO3− transporters. No differences in CCM physiology or gene expression were observed when Prochlorococcus spp. was fully acclimated to high-CO2 (1,000 µL L−1) or low-CO2 (150 µL L−1) conditions. Prochlorococcus spp. CCM components in the Global Ocean Survey metagenomes were very similar to those in the genomes of cultivated strains, indicating that the CCM in environmental populations is similar to that of cultured representatives.
The marine picocyanobacteria genus Prochlorococcus along with its sister group the marine genus Synechococcus dominate primary production in oligotrophic marine environments (Partensky et al., 1999). Prochlorococcus spp. is an oligotrophic specialist with several key adaptations allowing it to outcompete other phytoplankton in the stable, low-nutrient regions where it thrives. These adaptations include small cell size (less than 1 μm), allowing it to effectively capture nutrients and light, and genome streamlining, which minimizes nutrient requirements (Partensky and Garczarek, 2010). At approximately 1,900 genes, the genomes of high-light-adapted Prochlorococcus spp. are the smallest known among photoautotrophs, suggesting that this is about the minimum number of genes needed to make a cell from inorganic constituents and light (Rocap et al., 2003). Genome reduction has been accomplished by both the loss of entire pathways and complexes, such as the phycobilisomes and many regulatory capabilities, and the paring down of systems to their minimal components, as is the case for the circadian clock and the photosynthetic complexes (Rocap et al., 2003; Kettler et al., 2007; Partensky and Garczarek, 2010).
As part of this genome streamlining, the CO2-concentrating mechanism (CCM), which enhances the efficiency of photosynthesis by elevating the concentration of CO2 around Rubisco, has been reduced to what appears to be the minimal number of components necessary for a functional CCM (Badger and Price, 2003; Badger et al., 2006). In typical cyanobacteria, the CCM is composed of HCO3− transporters, CO2 uptake systems, and the carboxysome, a protein microcompartment in which Rubisco and carbonic anhydrase (CA) are enclosed. HCO3− is accumulated in the cytoplasm by direct import from the environment and by the active conversion of CO2 to HCO3− via an NADH-dependent process, which constitutes the CO2 uptake mechanism (Shibata et al., 2001). The accumulated HCO3− then diffuses into the carboxysome, where CA converts it to CO2, elevating the concentration of CO2 around Rubisco (Reinhold et al., 1987; Price and Badger, 1989).
Whereas some cyanobacteria have up to three different families of HCO3− transporters with differing affinities for use under different environmental conditions, Prochlorococcus spp. has only one or two families (Badger et al., 2006). Most cyanobacteria have low-affinity and high-affinity CO2 uptake systems, but no CO2 uptake systems are apparent in Prochlorococcus spp. genomes. The carboxysome of Prochlorococcus spp. and other α-cyanobacteria has apparently been laterally transferred from chemoautotrophs, but all of the required components of the carboxysome are present and it is functional (Badger et al., 2002; Roberts et al., 2012). Despite its simplicity, this CCM is likely functional. HCO3− can be accumulated in the cytoplasm by the HCO3− transporters and then diffuse into the carboxysome for conversion to CO2 and subsequent fixation by Rubisco. However, the functionality of the CCM in Prochlorococcus spp. has not yet been tested. Prochlorococcus spp. is a representative of the α-cyanobacteria, a group with distinct CCMs, which have been much less well studied than the CCMs of β-cyanobacteria (Rae et al., 2011, 2013; Whitehead et al., 2014).
We characterized inorganic carbon (Ci) acquisition and processing in Prochlorococcus spp. MED4, examined the effect of long-term acclimation to different CO2 concentrations on CCM physiology and gene expression, and searched metagenomes for Prochlorococcus spp. CCM genes to determine if CCMs in the natural populations are similar to cultured strains.
RESULTS
Photosynthesis and Ci Uptake versus Ci
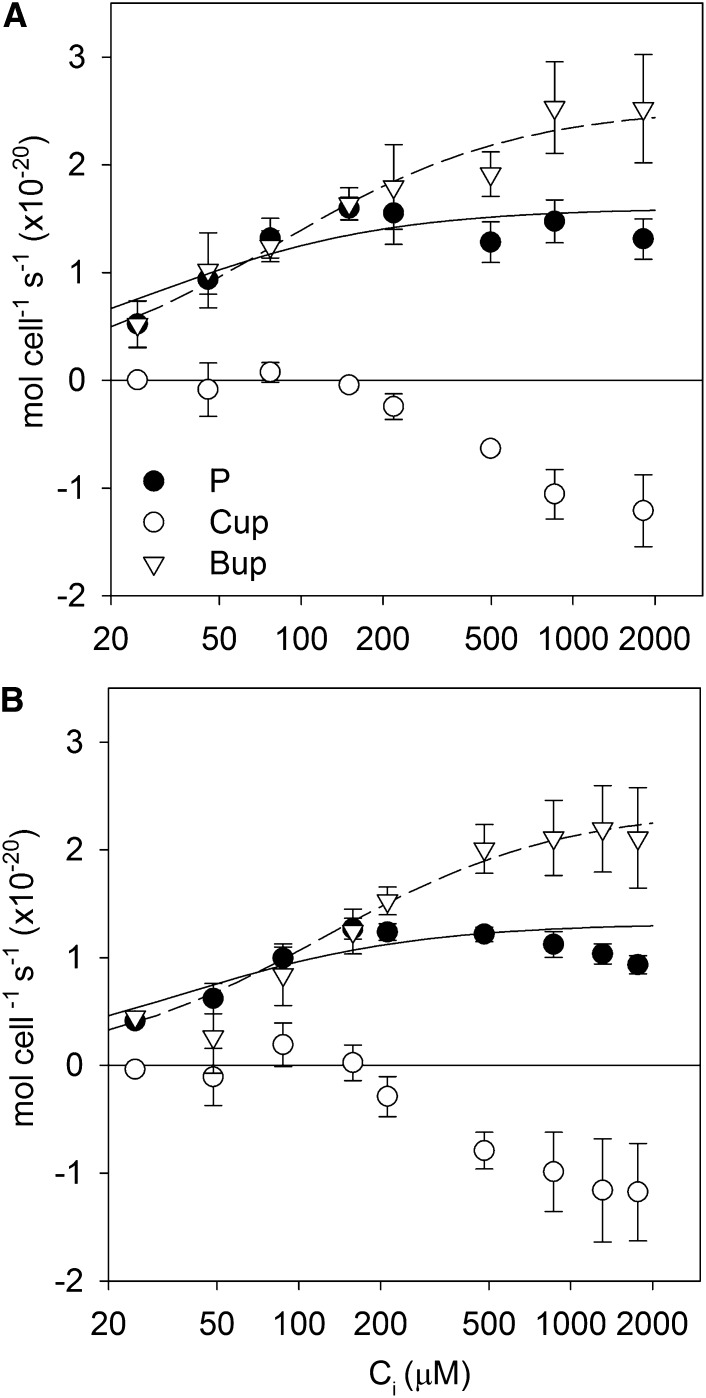
Rates of net photosynthesis, CO2 flux, and HCO3− flux were measured as Ci was gradually supplied to Prochlorococcus spp. MED4 acclimated to 150 and 1,000 µL L−1 CO2. The net photosynthesis and HCO3− flux data were fit with Michaelis-Menten functions to summarize the data (Fig. 1). Net photosynthetic rates began to decline slightly above approximately 1 mm Ci, most likely due to extended time in the assay chamber, so these data were excluded from the fits. The one-half-saturation constant of net photosynthesis for inorganic carbon (KP) was low (approximately 30 μm Ci) and unaffected by culture CO2 concentration (Fig. 1; Table I; 95% confidence intervals overlap based on se in the fit). Maximal photosynthetic rates (Pmax) were also not significantly different between the CO2 treatments. The one-half-saturation constant of HCO3− uptake for inorganic carbon (KB) was significantly higher than KP (95% confidence intervals do not overlap), but neither KB nor the maximal HCO3− uptake rate (Bmax) was significantly different between CO2 treatments. Net growth rates were not affected by CO2 (150 µL L−1 CO2, 0.37 ± 0.05 d−1; 1,000 µL L−1 CO2, 0.36 ± 0.01 d−1).
Figure 1.
Rates of photosynthesis (P), CO2 uptake or efflux (Cup), and HCO3− uptake (Bup) in Prochlorococcus spp. MED4 as a function of Ci concentration for cells grown at 150 µL L−1 CO2 (A) and 1,000 µL L−1 CO2 (B). Positive rates indicate uptake into the cell, and negative rates indicate efflux from the cell. Ci concentrations in the culture medium were approximately 1,850 μm at 150 µL L−1 CO2 and approximately 2,200 μm at 1,000 µL L−1 CO2.
Table I. Michaelis-Menten fits to photosynthesis and Ci fluxes as a function of Ci and se values in the fitted parameters.
For the net photosynthesis fits, the data at Ci greater than 1 mm were excluded from the fit.
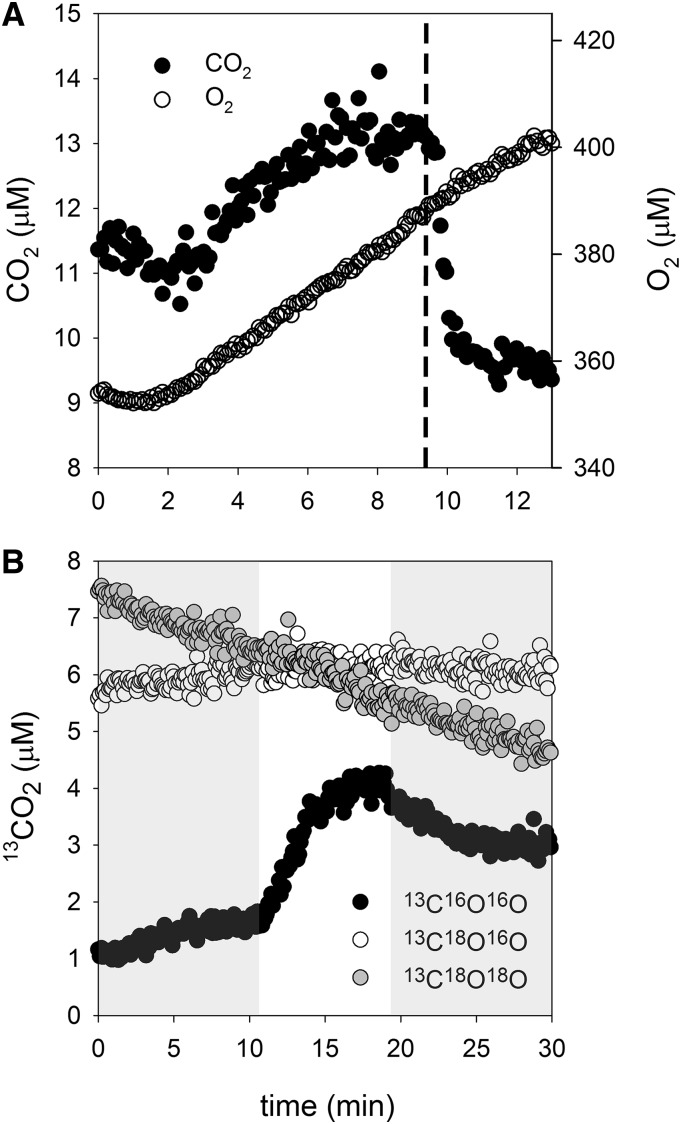
CO2 concentrations increased above equilibrium during photosynthesis, except at low Ci concentrations (200 μm or less), indicating CO2 efflux from Prochlorococcus spp. rather than CO2 uptake. We verified that CO2 was above equilibrium concentrations by adding bovine CA, which led to a rapid decline in the CO2 concentration to equilibrium levels (Fig. 2A). In a separate experiment, 13C18O-labeled Ci was added as the Ci source. During photosynthesis, an increase in 13C16O16O was observed, which indicates that the CO2 originates from Ci taken up for photosynthesis rather than respiratory 12CO2 and that the Ci has undergone many hydration/dehydration cycles leading to the removal of the 18O label. CO2 fluxes were near zero at low Ci (200 μm or less) but became negative (representing net CO2 efflux) beyond that, reaching their one-half-maximal level at approximately 400 to 500 μm Ci.
Figure 2.
A, Sample data showing increases in CO2 concentrations upon illumination in an assay chamber containing Prochlorococcus spp. MED4 and 2 mm Ci. That the CO2 concentration exceeds equilibrium with HCO3− is illustrated by the addition of bovine CA just after 9 min (dashed line), which establishes equilibrium between CO2 and HCO3−. Oxygen data show that photosynthesis was not affected by the addition of bovine CA. B, A light/dark cycle with 2 mm 13C18O-labeled Ci added to the assay chamber (darkness = gray background; illumination at 250 μmol photons m−2 s−1 = white background).
Intracellular Ci Pools
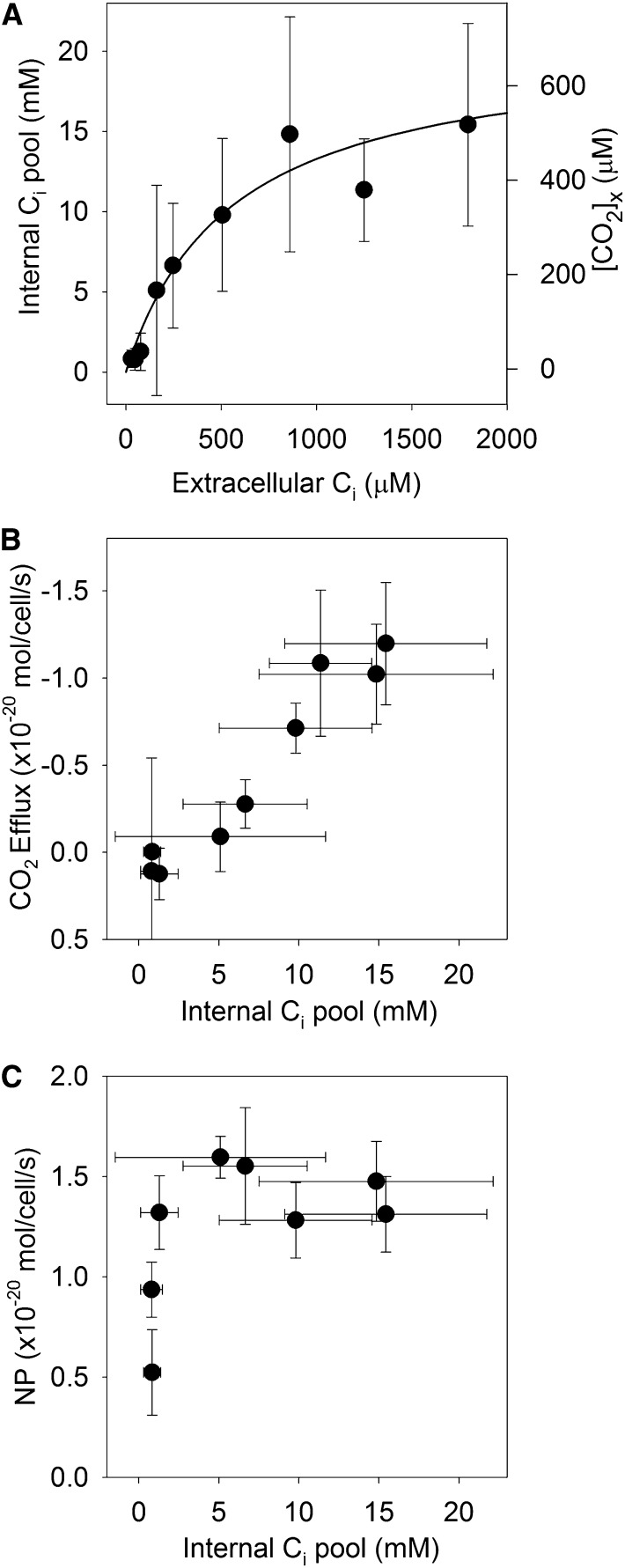
Intracellular Ci pools were estimated from the CO2 efflux that occurred at the beginning of each dark cycle. These data were highly variable, and there were no significant differences between the two CO2 treatments, so the data from the two treatments were pooled. The intracellular Ci pool increased as the extracellular Ci increased, reaching a maximal value of approximately 15 mm around 1,000 μm extracellular Ci (Fig. 3A). Cyanobacteria typically have somewhat larger intracellular Ci pools (Kaplan et al., 1980; Badger et al., 1985), but it should be noted that the calculated intracellular Ci pool is highly dependent on the cell diameter used to determine the volume of the spherical cells. We used a cell diameter of 0.7 μm, most commonly reported for the MED4 strain (Bertilsson et al., 2003; Ting et al., 2007), but values as low as 0.5 μm have been used (Morris et al., 2011), which would result in 3-fold lower volume and so 3-fold higher intracellular Ci. A Michaelis-Menten fit to the data had a one-half-saturation constant of 550 ± 210 μm Ci and a saturation value of 21 ± 3 mm. The intracellular Ci pool was linearly related to the rate of CO2 efflux (Fig. 3B; r2 = 0.94, P < 0.001) but was not directly related to the net photosynthetic rate (Fig. 3C). The CO2 concentration in the carboxysome was calculated from the intracellular Ci concentration by assuming that the cytoplasmic pH was 7.35 (Falkner et al., 1976; Kallas and Dahlquist, 1981; Belkin et al., 1987), that the carboxysome pH is the same as that of the cytoplasm (Menon et al., 2010), and that CO2 and HCO3− are in equilibrium in the carboxysome. At seawater Ci (2 mm), the internal Ci pool was approximately 15 mm and the estimated CO2 concentration in the carboxysome was approximately 500 μm.
Figure 3.
A, Intracellular Ci pool as a function of external Ci concentration as inferred from CO2 efflux following each light phase. The CO2 concentration in the carboxysome ([CO2]x) was calculated assuming that the intracellular pH is 7.35. A Michaelis-Menten fit to the data had a one-half-saturation constant of 550 ± 210 μm Ci and a saturation value of 21 ± 3 mm. B, Relationship between CO2 efflux and the intracellular Ci pool. C, Net photosynthesis (NP) of Prochlorococcus spp. MED4 acclimated to 150 µL L−1 CO2 as a function of the intracellular Ci pool.
Photosynthesis and Ci Uptake versus Irradiance
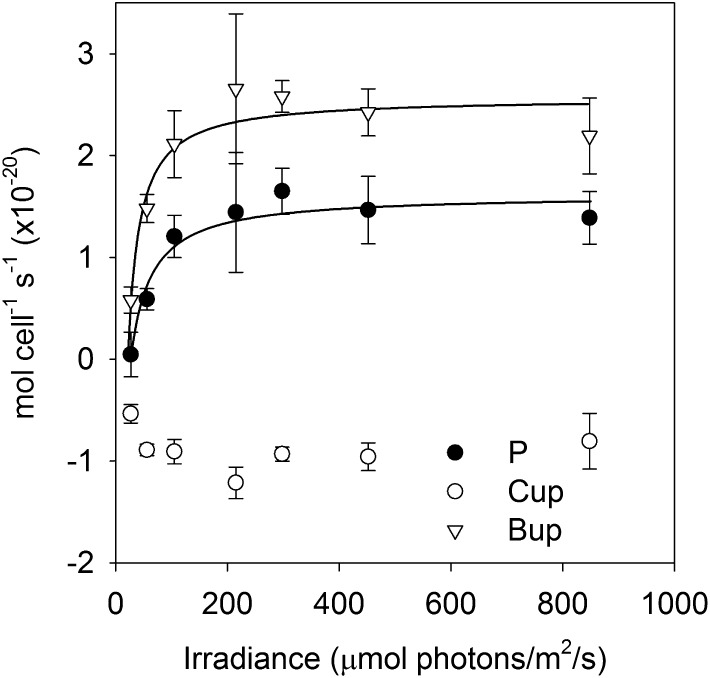
Rates of net photosynthesis, CO2 flux, and HCO3− flux were measured as irradiance was gradually increased on Prochlorococcus spp. MED4 cultures acclimated to 150 µL L−1 CO2 (Fig. 4; Table II). The net photosynthesis and HCO3− flux data were fit with Michaelis-Menten functions allowing an offset to account for negative net photosynthesis (respiration) in the dark (zero irradiance). The one-half-saturation of net photosynthesis for irradiance (Ik) was 63 μmol photons m−2 s−1 and Pmax was 1.62 × 10−20 mol cell−1 s−1. The one-half-saturation of HCO3− uptake for irradiance (IB) was 42 μmol photons m−2 s−1 and Bmax was 2.57 × 10−20 mol cell−1 s−1. The maximal rates of net photosynthesis and HCO3− uptake are indistinguishable from those measured in the photosynthesis versus Ci experiments, showing that rates were light saturated in these experiments, which were conducted at 200 μmol photons m−2 s−1. Additionally, Ik and IB are significantly below the incubator irradiance (100 μmol photons m−2 s−1), showing that these rates are light saturated, or nearly so, under growth conditions.
Figure 4.
Rates of net photosynthesis (P), CO2 uptake or efflux (Cup), and HCO3− uptake (Bup) in Prochlorococcus spp. MED4 as a function of irradiance at saturating Ci concentration (1,000 μm).
Table II. Michaelis-Menten fits to photosynthesis and Ci fluxes as a function of irradiance and se values in the fitted parameters.
An offset, representing the irradiance at which rates are zero, was included in the fits. Ik, IB, and the offsets are in μmol photons m−2 s−1, and Pmax and Bmax are in ×10−20 mol cell−1 s−1.
Rubisco Content and Kinetics
Cellular Rubisco content as measured by quantitative western blots was 8.25 ± 0.7 × 10−22 mol Rubisco hexadecamer cell−1 (6.6 × 10−21 mol active site cell−1) at 150 µL L−1 CO2. The one-half-saturation constant of Rubisco for CO2 was 263 ± 5 μm (Supplemental Fig. S1), similar to a previously measured value of 295 μm (Roberts et al., 2012). Both of these Km values come from measurements made at pH 8, and the Roberts et al. (2012) measurements were made on purified carboxysomes, whereas ours were with a crude extract that likely contained a mix of intact carboxysomes and single Rubisco molecules. They are lower than the value reported for purified Prochlorococcus spp. Rubisco (750 μm) by Scott et al. (2007) made at pH 7.5. These differences may be accounted for by pH, since lower pH reduces the Km of cyanobacterial Rubisco (Badger 1980), or by differences between intact carboxysomes and purified Rubisco either due to actual changes in Km of the enzyme or environmental effects. The consequences of uncertainty in Km for the CCM model are discussed below.
Modeling
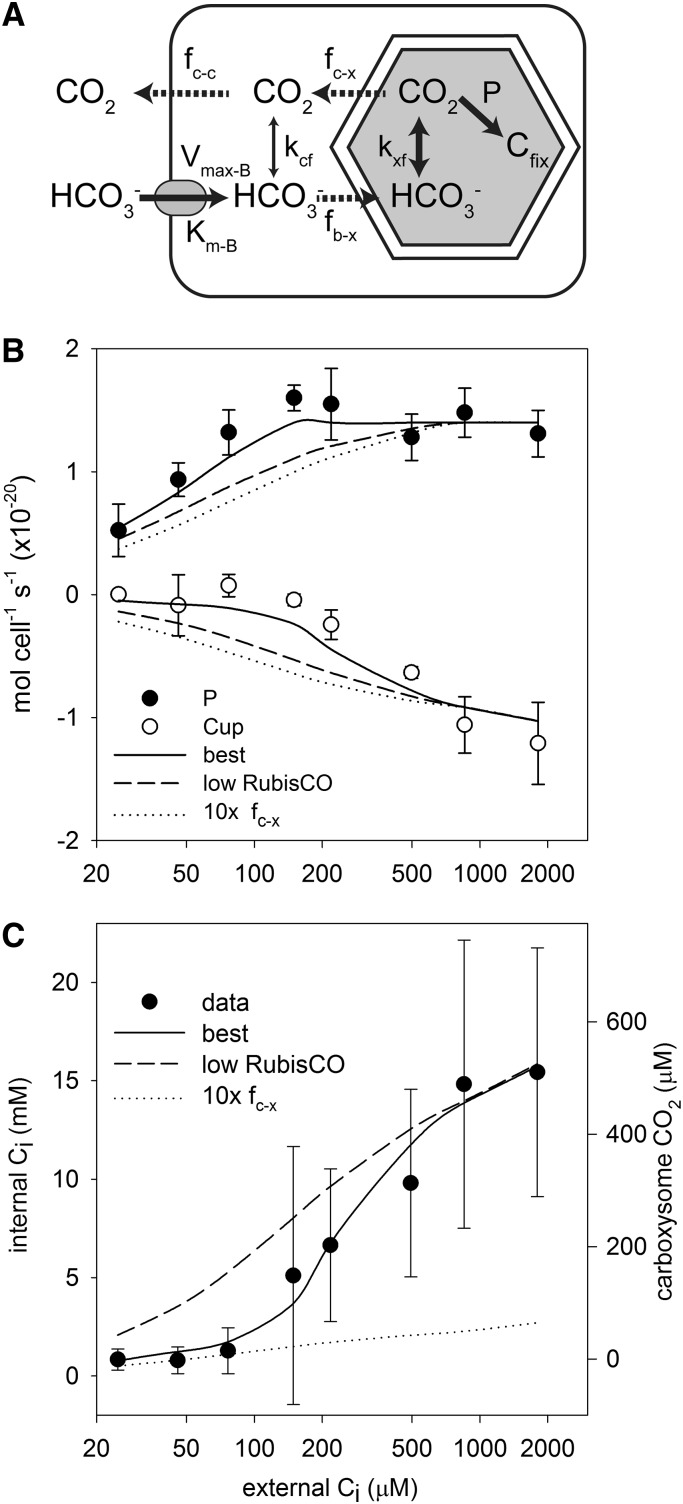
A mechanistic model of the Prochlorococcus spp. CCM (see “Materials and Methods”) was parameterized using data obtained in this study and values from the literature (Table III; Fig. 5A). The model was used to assess our understanding of CCM structure based on its ability to match rates of photosynthesis, CO2 efflux, and internal Ci concentrations observed under culture conditions. While the model structure should be applicable to a broader range of environmental conditions, the specific implementation used here should not be applied without modification to more general environmental conditions, since the model was parameterized based on cultures grown under a narrow range of conditions (constant temperature, light, etc.).
Table III. Model parameters.
| Symbola | Definition | Value | Unit | Source |
|---|---|---|---|---|
| cx | CO2 concentration | Varies | μm | |
| bx | HCO3− concentration | Varies | μm | |
| pHe | Extracellular pH | 8 | – | Measurement |
| pHc | Cytoplasmic and carboxysomal pH | 7.35 | – | “Results” |
| kcf | CO2 hydration rate constant in cytoplasm | 3 × 10−2 | s−1 | Uncatalyzed; Johnson (1982) |
| kxf | CO2 hydration rate constant in carboxysome | 1,000 | s−1 | Sufficient for equilibration |
| mR | Rubsico content | 6.6 × 10−21 | mol active site cell−1 | This study |
| Km-R | Rubisco one-half-saturation constant for CO2 | 263 | μm | This study |
| kcat-R | Rubisco maximal turnover rate | 10.6 | s−1 | Tcherkez et al. (2006) |
| Km-B | One-half-saturation constant for HCO3− uptake | 82 | μm | This study |
| Vmax-B | Maximal HCO3− uptake rate | 2.5 × 10−20 | mol cell−1 s−1 | This study |
| Nx | Number of carboxysomes per cell | 6 | Ting et al. (2007) | |
| fc-c | Cellular transfer coefficient for CO2 | 1 × 10−8 | cm3 s−1 | Assume diffusion limited (membrane is no barrier); Pasciak and Gavis (1974) |
| fc-x | Carboxysome transfer coefficient for CO2 | 2 × 10−15 | cm3 s−1 | Fitted to data |
| fb-x | Carboxysome transfer coefficient for HCO3− | 6 × 10−10 | cm3 s−1 | Assume diffusion limited (no barrier); Pasciak and Gavis (1974) |
| Vc | Cytoplasmic volume | 1.8 × 10−13 | cm3 | Ting et al. (2007) |
| Vx | Total carboxysome volume | 2.3 × 10−15 | cm3 | Ting et al. (2007) |
Subscripts are as follows: b, bicarbonate; c, cytoplasm; e, external environment; f, forward rate constant; and x, carboxysome.
Figure 5.
Model results. The model structure diagram (A) illustrates the active fluxes (solid arrows), passive fluxes (dashed arrows), and CO2/HCO3− interconversion (double-headed arrows) in the CCM, with the parameters controlling these fluxes indicated above each arrow (for notation, see Table III). Agreement of the various models with photosynthesis (P) and CO2 flux (Cup) data (B) and the internal Ci pool (C) is shown. Inferred carboxysome CO2 concentrations were calculated as described in “Results.”
The only critical parameter that has no literature constraints and was not measured in this study was the CO2 transfer coefficient of the carboxysome. This parameter was varied to optimize the fit of the model to observed photosynthetic rates, CO2 efflux rates, and internal Ci concentrations. The optimized model fit the data quite well and, in particular, captured the major regimes present in the data: a Ci-limited regime (less than 200 μm external Ci) where photosynthesis is increasing with Ci, CO2 efflux is low, and the internal Ci pool is small; and a Ci-replete regime (greater than 200 μm external Ci) where photosynthesis is saturated for Ci but the internal Ci pool accumulates and CO2 efflux becomes significant (Fig. 5). Two notable features of this best model are that the carboxysome CO2 transfer coefficient is low (2.4 ± 0.5 × 10−15 cm3 s−1) and there is excess Rubisco capacity (i.e. the CO2-saturated Rubisco fixation rate per cell exceeds the observed maximal photosynthetic rate). To show that the best model predicts several features of the data better than other models, we ran two alternative models: the first with no excess Rubisco capacity (low Rubisco in Fig. 5) and the second with a 10-fold higher carboxysome CO2 transfer coefficient (10× fc−x in Fig. 5). Both of these alternative models do not fit the data as well, lacking the clear distinction between the two regimes evident in the best model. Instead, in these models, photosynthesis, CO2 efflux, and the intracellular Ci pool increase gradually until saturation around 1,000 μm external Ci (Fig. 5, B and C).
The absolute values of the modeled intracellular Ci are sensitive to internal pH and to the carboxysome CO2 transfer coefficient. Because the only loss processes for Ci inside the cell are photosynthesis and leakage of CO2, the internal pool will build up until these loss rates match the HCO3− uptake rate. Both these loss rates are dependent on the carboxysome CO2 concentration, which in turn is dependent on pH and the carboxysome CO2 transfer coefficient. The intracellular pH used in the model (7.35) was chosen from the range of reported values to obtain reasonable agreement between the modeled and observed absolute values of internal Ci. Nonetheless, agreement between the shape of the modeled intracellular Ci curve and the data is meaningful and distinguishes the best model from alternative models (Fig. 5C).
A sensitivity analysis of the model was conducted, the details of which are presented in “Supplemental Data.” Given the estimated parameter uncertainties for random error, the model is most sensitive to the internal pH and the turnover rate of Rubisco, although even the worst case scenarios for these parameters do not cause major issues with the model fit (Supplemental Text S1; Supplemental Tables S1–S3). There is also systematic uncertainty in the Km for Rubisco, which potentially ranges between 263 and 750 μm. First, we considered increasing Km for Rubisco from our measured value of 263 to 520 μm, the value estimated for pH 7.35, assuming that the pH sensitivity is similar to that of Rubisco from Anabaena variabilis (Badger, 1980). This change does reduce model performance, primarily through increasing the internal Ci pool at low external Ci, but the two regimes in the data are still resolved and the overall fit is reasonable (Supplemental Fig. S2). A further increase of Km for Rubisco to 750 μm, the value obtained by Scott et al. (2007), degrades the model fit further, with the most notable discrepancy being a higher modeled internal Ci pool at low external Ci (Supplemental Fig. S2). There is still some evidence for a distinction between the Ci-limited and Ci-replete regimes, but as Km for Rubisco increases, the model begins to look more similar to the low-Rubisco model, which lacks excess Rubisco capacity. The sensitivity of the model to Km for Rubisco is primarily a concern with respect to the effects of pH on Km for Rubisco. If confinement to the carboxysome imparts a lower apparent Km for Rubisco through environmental or allosteric effects, then the lower Km for Rubisco is more appropriate for our model, since such effects are not treated explicitly in the model.
CCM Gene Expression
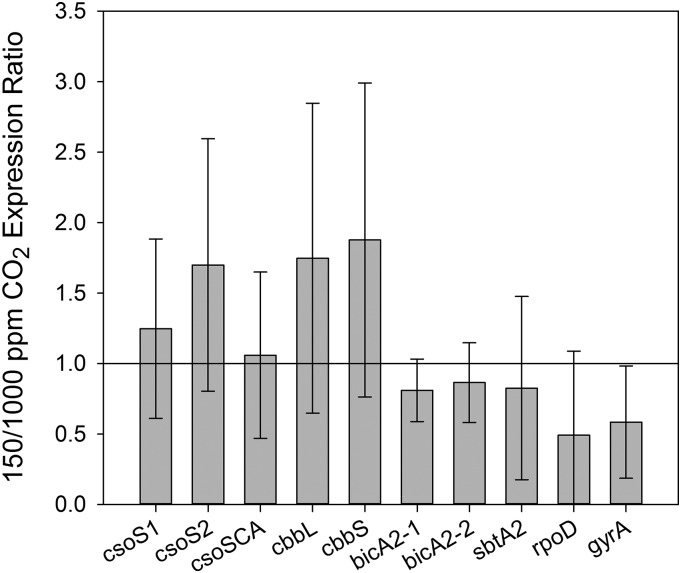
The expression of putative CCM genes and two housekeeping genes was measured in cultures acclimated to 150 and 1,000 µL L−1 CO2 (Fig. 6). None of the genes examined was significantly up- or down-regulated by the CO2 treatments. The CCM genes assessed include components of the carboxysome shell (csoS1, csoS2, and csoSCA), the large and small subunits of Rubisco (cbbL and cbbS), a strong homolog to the low-affinity Na+-dependent HCO3− transporter A (bicA2-1), a weaker homolog to this transporter (bicA2-2), and a weak homolog to the high-affinity Na+-dependent HCO3− transporter A (sbtA2). The two housekeeping genes assessed, whose regulation was not expected to be affected by CO2, were RNA polymerase σ factor 70 (rpoD) and DNA gyrase subunit A (gyrA).
Figure 6.
Effects of long-term acclimation to different CO2 concentrations on the expression of genes involved in the CCM of Prochlorococcus spp. MED4 and selected housekeeping genes, as assessed using reverse transcription-qPCR. Although there is some indication that CCM genes are slightly up-regulated at low CO2, none of the differences are statistically significant (Student’s t test, n = 3). CCM genes include carboxysome shell proteins (csoS1, csoS2, and csoSCA), Rubisco (cbbL and cbbS), and potential HCO3− transporters (bicA2-1, bicA2-2, and sbtA2). Housekeeping genes are σ factor 70 (rpoD) and DNA gyrase (gyrA).
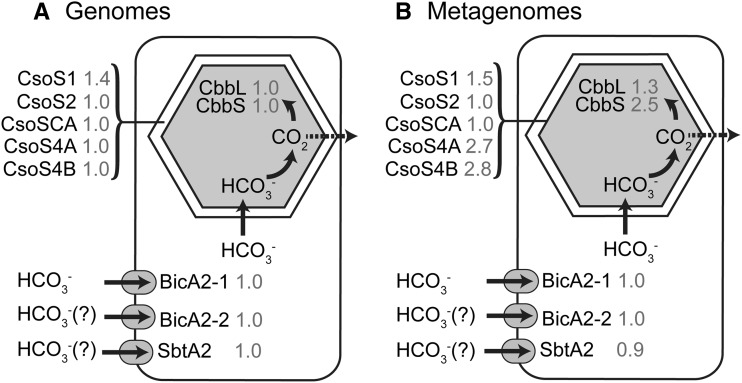
Prochlorococcus spp. CCM Components in Marine Metagenomes
The Global Ocean Survey (GOS) metagenomes were searched for Prochlorococcus spp. CCM components using a reciprocal best BLAST hit approach. Carboxysome components were all present at reasonable abundances, although some components differed from the frequencies expected from the sequenced Prochlorococcus spp. genomes (Fig. 7). In particular, the abundances of CsoS4A, CsoS4B, and CbbS appear to be more than two times more common in the field than in the sequenced culture genomes. While we cannot rule out the possibility that their higher abundances are real, these genes are the smallest genes examined (CsoS4A, 261 nucleotides; CsoS4B, 246; and CbbS, 333), which leads us to suspect that the normalization procedures artificially increased abundance. A manual inspection of selected BLAST results for these genes confirmed that there was no difficulty in distinguishing Prochlorococcus spp. sequences from those of its closest relative, genus Synechococcus spp. We also searched for components of CO2 uptake systems, focusing on CO2 hydration protein X (ChpX) and CO2 hydration protein Y (ChpY) genes, since these genes are unique to cyanobacterial CO2 uptake systems (Badger and Price, 2003; Ogawa and Mi, 2007). ChpX and ChpY sequences from marine and freshwater Synechococcus spp. were used to query the metagenomes in this case, since these genes are not present in sequenced Prochlorococcus spp. genomes. Only ChpX genes were found in the GOS metagenomes using a reciprocal best BLAST hit analysis. The mate pairs of these sequences were examined to see if any hit to Prochlorococcus spp. Out of 134 ChpX sequences found, 127 mate pairs had best hits to genes in Synechococcus spp. genomes and three hit to components of a heme uptake system in Prochlorococcus spp. MIT9202, with the remaining mate pairs (four) hitting heterotrophic bacteria.
Figure 7.
Frequency of Prochlorococcus spp. CCM components in genomes (A) and GOS metagenomes (B). Prochlorococcus spp. CCM components in the genomes were identified by BLAST analysis. In the metagenomes, Prochlorococcus spp. CCM components were identified by reciprocal BLAST analysis, and the counts were length normalized relative to RecA and then divided by the mean counts of four single-copy genes to estimate CCM genes per Prochlorococcus spp. genome (gray numbers next to protein names).
DISCUSSION
The CCM of Prochlorococcus spp. Is Functional and Efficient
The KP was approximately 30 μm, much lower than the approximately 2 mm Ci available in seawater, showing that photosynthesis is fully saturated with Ci in the ocean. This low KP is similar to that of cyanobacteria with a greater repertoire of HCO3− and CO2 uptake systems (Badger and Andrews, 1982; Price et al., 2004; Rae et al., 2011). As discussed in more detail below, neither KP nor any other parameter was affected by growth at either 150 or 1,000 µL L−1 CO2, so the two treatments will not be distinguished here. HCO3− uptake as a function of Ci availability followed Michaelis-Menten kinetics, consistent with a single transporter dominating HCO3− uptake. The KB was between 80 and 130 μm, similar to KB values for BicA transporters from several different cyanobacteria (38–171 μm) and, most notably, close to the KB for BicA from the marine Synechococcus sp. WH8102 (approximately 75 μm; Price et al., 2004), suggesting that the BicA homolog in Prochlorococcus spp. is the primary HCO3− transporter. The homolog of the high-affinity SbtA may be active in Prochlorococcus spp., but it would likely only be responsible for a small fraction of overall HCO3− uptake under the conditions examined, since the KB of SbtA is much lower (2–16 μm; Shibata et al., 2002; Price et al., 2004). HCO3− uptake continues to increase after photosynthesis is saturated for Ci, and this additional HCO3− uptake simply leaks back out of the cell as CO2. However, it does increase the internal Ci pool, and thus the CO2 concentration in the carboxysome, which could serve to reduce photorespiration by increasing the CO2-to-oxygen ratio.
Net CO2 efflux was observed from the cells at Ci concentrations of 200 μm and above (Fig. 1). As shown by the addition of 13C18O-labeled Ci, the CO2 originates from freshly transported Ci rather than from respiration, which would produce predominantly 12CO2. The effluxed CO2 is entirely depleted of 18O label, indicating that the Ci has undergone many hydration/dehydration cycles prior to exiting the cell (Tchernov et al., 1997). Since the only known CA in Prochlorococcus spp. is in the carboxysome (So et al., 2004), this shows that the leaked CO2 originates in the carboxysome, where CA removes the 18O label and increases the CO2 concentration, driving efflux. Unlike many cyanobacteria that have active CO2-to-HCO3− conversion mechanisms to take up and recover leaked CO2, Prochlorococcus spp. has no known CO2 recovery mechanisms. The observation of CO2 efflux from the cell is consistent with this, as is the pattern of 18O exchange in the light. Upon illumination, cyanobacteria that possess CO2 recovery mechanisms draw down 13C18O18O and 13C18O16O while 13C16O16O increases, whereas cyanobacteria with inactivated CO2 recovery systems only show increases of 13C16O16O, as was observed here for Prochlorococcus spp. (Maeda et al., 2002; Whitehead et al., 2014).
If there are no CO2 recovery mechanisms, HCO3− uptake into the cytoplasm is the only active transport step, and the efficiency of the CCM can be estimated as the rate of HCO3− uptake divided by the photosynthetic rate (Tchernov et al., 1997; Hopkinson et al., 2011). Under growth conditions, which are similar to typical conditions in the ocean, the CCM is remarkably efficient, with only two molecules of HCO3− transported per CO2 molecule fixed. For comparison, the diatom Phaeodactylum tricornutum transports 3.5 molecules of HCO3− per CO2 fixed (Hopkinson et al., 2011) and Synechococcus sp. WH7803 transports six molecules of HCO3− per CO2 fixed (Tchernov et al., 1997), making the Prochlorococcus spp. CCM the most efficient of the few that have been examined.
The Minimal CCM Model Is Consistent with Physiological Data
The simple CCM indicated by an analysis of Prochlorococcus spp. genomes implies that there should be straightforward relationships between the intracellular Ci pool, photosynthesis, and CO2 efflux. Without a CO2 recovery mechanism, CO2 efflux should be linearly related to the CO2 gradient between the carboxysome and the extracellular solution. Because the CO2 concentration in the carboxysome was always much greater than the CO2 in the external solution (maximum of 15 μm), to first order CO2 efflux should be directly related to the CO2 concentration in the carboxysome, which is proportional to the intracellular Ci pool. A linear relationship was observed between the CO2 efflux rate and the intracellular Ci pool, consistent with the simple CCM model (Fig. 3B). However, a closer examination of the data shows that there are two distinct regimes to the data. In the first regime (less than 200 μm external Ci), photosynthesis increases with external Ci while little to no CO2 efflux is observed and the intracellular Ci pool remains small (Figs. 1 and 3). In the second regime, at higher external Ci (greater than 200 μm), photosynthesis is saturated for Ci but the internal Ci pool continues to increase and CO2 efflux becomes significant.
The CCM model is able to explain these two regimes and shows that the key characteristics producing these two regimes are excess Rubisco capacity and a low permeability of the carboxysome to CO2, as illustrated by the failure of alternative models lacking these characteristics to match the data (Fig. 5). In many algae, there is little excess Rubisco capacity, so photosynthesis should follow a Michaelis-Menten relationship with the CO2 concentration around Rubisco (Badger et al., 1980; Losh et al., 2013; Hopkinson, 2014). In Prochlorococcus spp., CO2 efflux should be linearly related to the CO2 concentration in the carboxysome and so would also show a Michaelis-Menten relationship if there was no excess Rubisco capacity. This is clearly not the case here: photosynthesis became saturated at an intracellular Ci concentration of approximately 5 mm when CO2 in the carboxysome was approximately 200 μm, and only minimal CO2 efflux was observed (Fig. 3C). Further increase in the carboxysome CO2 concentration (to 500 μm at 2 mm external Ci) did not lead to higher rates of photosynthesis, meaning that a factor other than CO2, such as electron transport or ribulose 1,5-bisphosphate (RuBP) regeneration, was limiting carbon fixation in this regime.
Consistent with this, an estimate of the maximal CO2 fixation rate by Rubisco at saturating CO2 based on quantitative measurements of Rubisco abundance combined with estimated turnover rates (10.6 s−1 active site−1 and eight active sites per molecule) shows that there was approximately 4-fold excess Rubisco capacity above the measured rates of CO2 fixation. This excess Rubisco capacity contributes to the low KP of Prochlorococcus spp. and may explain how the low KP is achieved in the absence of CO2 recovery mechanisms or high-affinity HCO3− transporters. Other cyanobacteria with a greater repertoire of Ci transporters have little excess Rubisco capacity (Whitehead et al., 2014). Once photosynthesis is saturated for CO2, further HCO3− import leads to the buildup of the intracellular Ci pool and carboxysome CO2 concentration, such that the rate of CO2 leakage matches the rate of HCO3− import in excess of photosynthesis.
Given the reduced features of the Prochlorococcus spp. CCM, the carboxysome must be a significant barrier to CO2 efflux. The best model fit was obtained with a carboxysome CO2 transfer coefficient of 2.4 × 10−15 cm3 s−1 or, approximating the carboxysomes as spheres of 45-nm radius (Ting et al., 2007), a permeability of 1 × 10−5 cm s−1. The mass transfer coefficient is approximately 4 orders of magnitude lower than the diffusion-controlled transfer coefficient (9 × 10−10 cm3 s−1), obtained assuming that the carboxysome structure does not hinder CO2 diffusion (Pasciak and Gavis, 1974). A maximal estimate of the carboxysome CO2 transfer coefficient can be made using our measurements of Rubisco content and one-half-saturation constant for CO2 combined with literature values for the maximum turnover rate of cyanobacterial Rubisco (Table III) to calculate the minimal CO2 concentration in the carboxysome (84 μm) required to match the maximal rate of photosynthesis. Since membranes are highly permeable to CO2, the resistance of the carboxysome should be the major control on CO2 efflux, in which case the efflux rate can be described by the CO2 transfer coefficient for the carboxysome (fc-x) and the CO2 concentration difference between the carboxysome and the extracellular solution: efflux = fc-xNx([CO2]x − [CO2]e), where Nx = the number of carboxysomes per cell. Using the minimal carboxysome CO2 concentration and the observed efflux rates, the maximal carboxysome CO2 transfer coefficient was estimated as 2.4 × 10−14 cm3 s−1, or permeability of 1 × 10−4 cm s−1, still several orders of magnitude lower than the diffusion-controlled estimate. These best and maximal permeability values are very similar to values that have been found necessary to obtain efficient CCMs in previous models (1 × 10−5 to 2.5 × 10−4 cm s−1; Reinhold et al., 1987, 1991). If the carboxysome did not act as a CO2 barrier, the efficiency of the CCM would be massively reduced, limiting its usefulness. The carboxysome has long been postulated to be a barrier to CO2 efflux (Reinhold et al., 1987), and analysis of isolated carboxysomes lacking CA has provided support for this hypothesis (Dou et al., 2008), but the simple structure of the Prochlorococcus spp. CCM has allowed us to quantitatively assess the extent to which the carboxysome prevents CO2 efflux.
Regulation of the CCM by CO2
In most microalgae, CO2 availability is the primary factor regulating CCM expression, with decreased CO2 availability inducing up-regulation of the CCM (Woodger et al., 2005; Matsuda et al., 2011). One common manifestation of CCM up-regulation is a decline in KP and KB (Kaplan et al., 1980; Rost et al., 2003). Despite some reduction in KP and KB at 150 µL L−1 CO2, these parameters were not significantly different from their values at 1,000 µL L−1 CO2, nor were any other physiological characteristics (Pmax, Bmax, and CO2 efflux; Table I). At the genetic level, no CCM genes were differentially expressed between the two long-term CO2 acclimation treatments (Fig. 6). Consistent with this lack of a long-term response to CO2 treatment is the absence of canonical CCM transcription factors (CcmR and CmpR) in the Prochlorococcus spp. genome (Omata et al., 2001; Woodger et al., 2007; Nishimura et al., 2008). However, Prochlorococcus spp. does have a PII protein, which contributes to CCM regulation in some cyanobacteria (Palinska et al., 2002), and we did not explore the potential for short-term responses to CO2 deficiency, which could induce transient changes in gene and protein expression as observed in response to nitrogen starvation in Prochlorococcus spp. (Tolonen et al., 2006). Another α-cyanobacterium, Synechococcus sp. WH5701, also showed no physiological changes when acclimated to different CO2 concentrations, but some changes in gene expression were observed (Rae et al., 2011). This is not the case for all α-cyanobacteria, as some have strong physiological responses to changes in long-term CO2 availability (Hassidim et al., 1997; Whitehead et al., 2014).
Some algae are able to take advantage of rising ocean CO2 concentrations by down-regulating their CCMs and redirecting energy toward increased growth (Rost et al., 2008; Hopkinson et al., 2011). The lack of response to CO2 suggests that Prochlorococcus spp. cannot take advantage of rising ocean CO2 concentrations in this way. Consistent with this, the growth of Prochlorococcus spp. does not increase with increasing CO2 (Fu et al., 2007). Although, in principle, the increased extracellular CO2 concentration should decrease CO2 leakage, conserving some energy, the CO2 concentration in the carboxysome is so high (approximately 500 μm) compared with external CO2 (5 μm at 150 µL L−1 CO2 and 32 μm at 1,000 µL L−1 CO2) that the change in the absolute gradient, which controls the leakage rate, would be small.
Prochlorococcus spp. CCM Genes in the Environment
The set of CCM genes found in Prochlorococcus spp. genomes obtained from cultured isolates is nearly constant, with the exception of some small variations in the carboxysome proteins (Badger et al., 2006; Roberts et al., 2012). The genetic complement and physiological characteristics of isolated Prochlorococcus spp. strains are generally similar to those in the environment, but some differences have been identified (Martiny et al., 2009; Rusch et al., 2010). To determine if the complement of CCM genes found in natural Prochlorococcus spp. populations is similar to that of cultured isolates, we searched the GOS metagenomes for core CCM genes using a reciprocal BLAST approach to ensure that only Prochlorococcus spp. sequences were matched. The natural populations show a similar gene frequency to cultured isolates, suggesting that the CCM in the environment is similar to that in cultured strains (Fig. 7). The inferred high abundance of short carboxysome genes (CsoS4A, CsoS4B, and CbbS) is thought to be an artifact due to the short length of these genes. We also searched the metagenomes for genes diagnostic for CO2 uptake systems in cyanobacteria (ChpX and ChpY; Shibata et al., 2001; Maeda et al., 2002). Using mate-pair analysis, three sequences were identified that may have been in Prochlorococcus spp., but in all three cases, the mate pairs hit components of a heme uptake system, which itself is thought to be horizontally acquired and rare in Prochlorococcus spp. (Hopkinson and Barbeau, 2012). These results suggest that environmental Prochlorococcus spp., like their cultured representatives, generally cannot take up CO2, although on extremely rare occasions they may have this ability.
CONCLUSION
The agreement between the CCM model and physiological data suggests we have a good understanding of the mechanics of the Prochlorococcus spp. CCM and, to the extent that it is similar to the CCMs in other cyanobacteria, a good understanding of those CCMs as well. However, some questions about the function and ecological role of the Prochlorococcus spp. CCM remain. The CCM is quite efficient, pumping two molecules of HCO3− per CO2 fixed, compared with a few other marine algae that have been studied. This may help Prochlorococcus spp. conserve energy and outcompete other algae, especially deep in the ocean where light intensity is low, but the efficiency of the CCM has only been assessed in a few selected algae. Although this efficiency is impressive, Prochlorococcus spp. shows the potential to be even more efficient. Photosynthesis becomes saturated at low Ci concentrations (approximately 200 μm), when essentially no CO2 efflux is observed, and the CCM is perfectly efficient in that every HCO3− molecule imported is fixed. However, as Ci increases further, the cell imports more HCO3−, which is not fixed but instead leaks back out of the cell. The increased rates of HCO3− uptake do increase the intracellular Ci pool and carboxysomal CO2 concentration, which would help to reduce photorespiration by further increasing the CO2-to-oxygen ratio. Nonetheless, the Prochlorococcus spp. CCM is surprisingly effective and efficient despite its simplicity, another example of the remarkable adaptations that allow this simple organism to dominate large parts of the ocean. More generally, this study confirms that the CCMs of α-cyanobacteria, even with the minimal components present in Prochlorococcus spp., function similarly to the well-studied CCMs of β-cyanobacteria (Whitehead et al., 2014).
MATERIALS AND METHODS
Culturing
Prochlorococcus spp. MED4 (CCMP 1986) was obtained from the National Center for Marine Algae and Microbiota and maintained in PRO99 medium using natural Gulf Stream seawater as a base (Moore et al., 2007). The seawater base was sterilized by autoclaving, and the nutrient stocks were 0.2 μm filtered. All culture vessels were acid washed (10% [v/v] HCl) and thoroughly rinsed with deionized water and then ultrapure (greater than 18 MΩ cm−1) water. Cultures were maintained at 18°C and a light intensity of 100 μmol photons m−2 s−1 on a light/dark cycle of 16/8 h. Growth was tracked by measurement of in vivo chlorophyll fluorescence. Culture CO2 conditions were controlled by bubbling with premixed CO2-air mixtures (150 or 1,000 µL L−1 CO2). To ensure that the bubbling rate was sufficient to achieve equilibrium, medium pH was routinely measured using Thymol Blue (Zhang and Byrne, 1996), and dissolved Ci was measured on occasion using membrane inlet mass spectrometry (MIMS). Cultures were allowed to acclimate for at least 2 weeks to the CO2 conditions. Cultures were always harvested in midmorning, approximately 4 h after the light period began, to ensure that cells were in a consistent state with respect to diel variability.
Cell Counts
Samples from experimental suspensions were preserved with glutaraldehyde (0.125% [v/v] final concentration), frozen, and stored in liquid nitrogen. Just prior to analysis, samples were defrosted at 35°C and diluted 400-fold in 0.2 μm filtered artificial seawater. Cells were counted flow cytometrically as described previously (Worden and Binder, 2003).
Ci Acquisition
A membrane inlet mass spectrometer (Pfeiffer QMS220) was used to measure net CO2 and HCO3− fluxes and photosynthetic oxygen production. Cells were harvested by centrifugation at 11,000g for 15 min, resuspended in 1 to 2 mL of Ci-free artificial seawater, centrifuged again at 7,000g for 3 min, and finally resuspended in 1 mL of Ci-free artificial seawater with 20 mm Tris buffer at pH 8 for experimentation. This cell suspension was then placed in the cuvette of the MIMS system. Fifty micromolar acetazolamide, a CA inhibitor that does not pass through cell membranes, was added to ensure that CO2 hydration and HCO3− dehydration rates were at background, uncatalyzed rates. To assess Ci acquisition as a function of Ci availability, any residual Ci in the assay solution was first consumed through photosynthesis by turning on a light-emitting diode light (200 μmol photons m−2 s−1). 13C-labeled Ci was then gradually added back to the sample using alternating dark and light phases to determine net CO2 and HCO3− fluxes into or out of the cells and net photosynthesis rates following the methods of Badger et al. (1994). Net photosynthetic rates were determined from the rate of oxygen production. Net CO2 flux was calculated from the extent to which the CO2 concentration was drawn down below (uptake) or raised above (efflux) equilibrium. Net HCO3− uptake was then calculated as the difference between photosynthesis and net CO2 flux (with positive CO2 flux indicating uptake and negative indicating efflux). Rates were normalized by cell number as counted using flow cytometry. Michaelis-Menten curves were fit to the net photosynthesis and HCO3− uptake data.
After each dark cycle, CO2 concentrations rose above equilibrium levels, which we interpret to be the result of leakage of accumulated intracellular Ci out of the cell. The rate of leakage can be determined by tracking the CO2 concentration and accounting for net loss due to hydration (Supplemental Fig. S3):
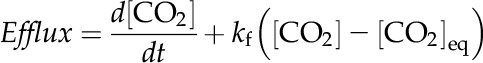 |
(1) |
where kf is the spontaneous CO2 hydration rate, [CO2]eq is the CO2 concentration at equilibrium, d indicates differentiation, and t is time. The leakage rate can then be integrated over time until the CO2 concentration returns to equilibrium, giving the total amount of Ci that was lost from the cell, which is used here as a measure of the intracellular Ci pool (Supplemental Fig S3). This assumes that the main loss process for accumulated Ci is by leakage of CO2 out of the cell. The approach is similar to that taken by Badger et al. (1985), except that in their experiments, CA was added, so that the CO2 measurements were a direct measurement of the total external Ci concentration. Recent comparisons of the MIMS method with the more traditional silicone oil centrifugation method found that the approaches were generally similar, but the MIMS method underestimated Ci pools at low external Ci in some cases (Whitehead et al., 2014). The cytoplasmic volume, which is required to calculate the internal Ci concentration, was estimated from the three-dimensional reconstructions of Prochlorococcus spp. MED4 cells of Ting et al. (2007). The total cell volume was calculated assuming that the cell is a sphere of diameter 0.7 μm, and then the thylakoid volume, estimated to take up approximately 30% of the cell volume by analysis of the images in Ting et al. (2007), was subtracted from the total volume to calculate the cytoplasmic volume.
In another set of experiments, the effect of light intensity on Ci uptake was assessed similarly, except that 1 mm Ci was added and light intensity was gradually increased from 25 to 850 μmol photons m−2 s−1.
Rubisco Quantification and Kinetics
Quantification of Rubisco protein was performed as described by Losh et al. (2013). Briefly, cells were pelleted via centrifugation, and total protein was extracted by vortexing and boiling the pellet for 5 min in SDS buffer (50 mm Tris-HCl, 2% [w/v] SDS, 10% [v/v] glycerol, and 12.5 mm EDTA). Total protein was quantified using the bicinchoninic acid assay against a bovine serum albumin protein standard according to the manufacturer’s instructions (Pierce, Thermo Scientific). Picomoles of CbbL (the large subunit of Rubisco) were determined through quantitative western blotting with global antibodies and standards according to the manufacturer’s instructions (Agrisera). Since there are equimolar concentrations of CbbL and CbbS (the small subunit), total Rubisco protein was calculated from the pmol of CbbL and masses of 52.57 and 12.94 kD for CbbL and CbbS, respectively (Rocap et al., 2003).
The one-half-saturation constant for Rubisco CO2 fixation was determined at 20°C in crude protein extracts using a 14C assay. In 2 mL of N2-sparged gas-tight vials, 10 μg of Rubisco (in 20 μL of crude extract) was added to 500 μL of assay buffer (50 mm Bicine, 20 mm, MgCl2, 1 mm EDTA, 5 mm dithiothreitol, 0.1 mg mL−1 CA, and 0.4 mm RuBP, pH 8, bubbled with N2 to remove all CO2 and oxygen). The reaction was started by the addition of varying amounts of NaH14CO3 from 0 to 1 m, and vials were incubated for 4 min. Reaction was stopped by the addition of 0.5 mL of 6 n HCl. Vials were left to degas of inorganic 14C overnight. Organic 14C was counted with a scintillation counter (PerkinElmer). To confirm that there was no nonspecific activity, we tested 14C assays with activated crude extract without RuBP and used these values as blanks
Gene Expression
The relative expression of putative CCM genes at 150 and 1,000 µL L−1 CO2 was assessed using reverse transcription-quantitative polymerase chain reaction (qPCR). Prochlorococcus spp. cultures were allowed to acclimate to 150 and 1,000 µL L−1 CO2 as described above, at which point cells were harvested by centrifugation and RNA was isolated using TRIzol (Invitrogen) following the manufacturer’s instructions. The isolated total RNA was treated with 2 units of amplification-grade DNase to remove any potential contaminating DNA. One microgram of total RNA was reverse transcribed using random hexamer primers to complementary DNA (cDNA) with SuperScript III reverse transcriptase (Invitrogen). Parallel reactions were run without reverse transcriptase for use as no-template controls in the qPCR analysis.
Relative abundances of putative CCM genes were quantified by qPCR using a Bio-Rad iCycler iQ. Primers for the CCM genes and controls were designed using the Prochlorococcus spp. MED4 genome sequence and are listed in Supplemental Table S4. qPCR mixtures (25 μL) consisted of 12.5 μL of Bio-Rad iQ SYBR Green 2× Supermix, 0.75 μL of forward and reverse primers (200 nm final concentration), 9 μL of ultrapure water, and 2 μL of cDNA or no-template control. Temperature profiles for the PCR consisted of an initial 10 min at 50°C and then 5 min at 95°C, followed by 45 cycles of 95°C for 10 s (melting) and 30 s at 58°C (annealing and extension), and finally 1 min at 95°C and 1 min at 55°C. Five dilutions of genomic DNA were analyzed for each gene to produce a standard curve for the quantification of relative gene expression based on the cycle at which fluorescence crossed a threshold level. Following each qPCR, a melting-curve analysis was performed, and selected products were run on a 1% (w/v) agarose gel to verify that a single product of the expected length was amplified. No-template controls amplified much later in the reaction than cDNA samples (worst case three cycles later).
Metagenomic Analyses
Putative CCM genes from the 18 publicly available Prochlorococcus spp. genomes were used to search for homologs in the GOS metagenomic data set using a reciprocal BLAST analysis. These genes include components of the carboxysome shell (CsoS1, CsoS2, CsoSCA, CsoS4A, and CsoS4B; National Center for Biotechnology Information accession numbers WP011819929, WP011132186, WP011132187, WP011132188, and WP011132189), Rubisco large and small subunits (CbbL and CbbS; WP011130576 and WP011132185), and putative bicarbonate transporters (BicA2-1, BicA2-2, and SbtA2; WP011131853, WP011132278, and WP011131852). Sets of protein sequences from the Prochlorococcus spp. genomes were compiled and used as query sequences in a tBLASTn search against the GOS metagenomes, keeping hits with E-values less than 1E-5. These metagenome sequences were then used as query sequences in a BLASTx search against a database of 206 marine bacterial genomes (Hopkinson and Barbeau, 2012). If the top BLASTx hit in this search was a Prochlorococcus spp. sequence used in the initial tBLASTn search (i.e. a reciprocal best BLAST hit), then the metagenome sequence was retained as a member of the protein family of interest. The raw sequence counts were scaled by the average length of genes in the family of interest relative to the length of recombinase A (RecA; i.e. length-corrected counts = raw counts × [RecA length/gene of interest length]) to correct for more frequent sampling of longer genes. CCM gene frequency, in genes per Prochlorococcus spp. genome, was calculated by dividing the length-corrected counts by the average number of single-copy Prochlorococcus spp. genes found (DNA gyrase B, HSP70, RecA, and RNA polymerase B). Because the number of counts at most individual GOS sites was low, counts were summed over the entire GOS data set prior to normalization by single-copy gene number.
Modeling
A numerical model of the Prochlorococcus spp. CCM, similar in structure to that of Reinhold et al. (1987), was used to assess the consistency of the data with the streamlined CCM indicated by genomic analysis (Fig. 5A). CO2 and HCO3− concentrations are modeled in the cytoplasm and carboxysome, with CO32− treated implicitly as part of the HCO3− pool, since equilibrium between these species is established very rapidly (Zeebe and Wolf-Gladrow, 2001). The only active transport is the import of HCO3− into the cytoplasm, which is described by Michaelis-Menten kinetics and parameterized from the HCO3− uptake data. HCO3− accumulates in the cytoplasm, which lacks CA, and then diffuses into the carboxysome, which is taken to be highly permeable to HCO3− as a result of positively charged pores in the carboxysome shell (Klein et al., 2009; Kinney et al., 2011). In the carboxysome, CA catalyzes the conversion of HCO3− to CO2, elevating the CO2 concentration around Rubisco. Rubisco fixes CO2 in the carboxysome at a rate dependent on the CO2 concentration and capped at the observed maximal CO2 fixation rate to simulate limitation by another factor such as electron transport rate or RuBP regeneration. CO2 can diffuse passively out of the carboxysome and cell, as parameterized by transfer coefficients. The transfer coefficient between the cytoplasm and bulk solution was calculated assuming that the cytoplasmic membrane is not a significant barrier to CO2 flux, as has been found for many other algae and red blood cells (Silverman et al., 1981; Hopkinson et al., 2011). The CO2 transfer coefficient for carboxysomes has not been determined and instead was optimized to fit the observed rates of photosynthesis, CO2 efflux, and the internal Ci concentration. The model is described by a system of differential equations:
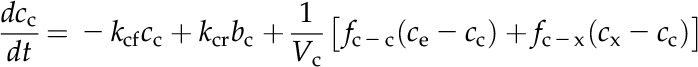 |
(2) |
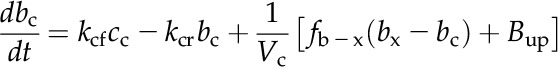 |
(3) |
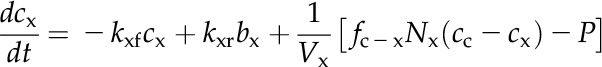 |
(4) |
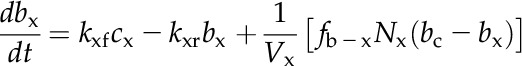 |
(5) |
where the HCO3− uptake (Bup) and photosynthetic (P) rates are described as follows:
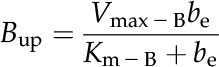 |
(6) |
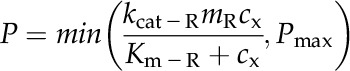 |
(7) |
The notation is detailed in Table III.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers WP011819929, WP011132186, WP011132187, WP011132188, WP011132189, WP011130576, WP011132185, WP011131853, WP011132278, and WP011131852.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Rubisco fixation rate as a function of CO2.
Supplemental Figure S2. Effect of Rubisco Km on model-data agreement.
Supplemental Figure S3. Determination of the internal Ci pool from CO2 efflux.
Supplemental Table S1. Model parameters and variability.
Supplemental Table S2. Results of sensitivity analysis.
Supplemental Table S3. Interactions between model parameters.
Supplemental Table S4. qPCR primers.
Supplemental Text S1. Model sensitivity analysis.
Supplementary Material
Acknowledgments
We thank François Morel (Princeton University) for helpful advice and discussions, and two anonymous reviewers for careful analysis of the work and helpful comments that improved the article.
Glossary
- CCM
CO2-concentrating mechanism
- CA
carbonic anhydrase
- Ci
inorganic carbon
- KP
one-half-saturation constant of net photosynthesis for inorganic carbon
- Pmax
maximal photosynthetic rate
- KB
one-half-saturation constant of HCO3− uptake for inorganic carbon
- Bmax
maximal HCO3− uptake rate
- Ik
one-half-saturation of net photosynthesis for irradiance
- IB
one-half-saturation of HCO3− uptake for irradiance
- GOS
Global Ocean Survey
- RuBP
ribulose 1,5-bisphosphate
- MIMS
membrane inlet mass spectrometry
- qPCR
quantitative polymerase chain reaction
- cDNA
complementary DNA
Footnotes
This work was supported by the National Science Foundation (grant nos. EF 1041023 and MCB 1129326).
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Badger MR. (1980) Kinetic properties of ribulose 1,5-bisphosphate carboxylase/oxygenase from Anabaena variabilis. Arch Biochem Biophys 201: 247–254 [DOI] [PubMed] [Google Scholar]
- Badger MR, Andrews TJ (1982) Photosynthesis and inorganic carbon usage by the marine cyanobacterium, Synechococcus sp. Plant Physiol 70: 517–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger MR, Bassett M, Comins HN (1985) A model for HCO3− accumulation and photosynthesis in the cyanobacterium Synechococcus sp.: theoretical predictions and experimental observations. Plant Physiol 77: 465–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger MR, Hanson D, Price GD (2002) Evolution and diversity of CO2 concentrating mechanisms in cyanobacteria. Funct Plant Biol 29: 161–173 [DOI] [PubMed] [Google Scholar]
- Badger MR, Kaplan A, Berry JA (1980) Internal inorganic carbon pool of Chlamydomonas reinhardtii: evidence for a carbon dioxide concentrating mechanism. Plant Physiol 66: 407–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger MR, Palmqvist K, Yu J (1994) Measurement of CO2 and HCO3− fluxes in cyanobacteria and microalgae during steady-state photosynthesis. Physiol Plant 90: 529–536 [Google Scholar]
- Badger MR, Price GD (2003) CO2 concentrating mechanisms in cyanobacteria: molecular components, their diversity and evolution. J Exp Bot 54: 609–622 [DOI] [PubMed] [Google Scholar]
- Badger MR, Price GD, Long BM, Woodger FJ (2006) The environmental plasticity and ecological genomics of the cyanobacterial CO2 concentrating mechanism. J Exp Bot 57: 249–265 [DOI] [PubMed] [Google Scholar]
- Belkin S, Mehlhorn RJ, Packer L (1987) Proton gradients in intact cyanobacteria. Plant Physiol 84: 25–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertilsson S, Berglund O, Karl DM, Chisholm SW (2003) Elemental composition of marine Prochlorococcus and Synechococcus: implications for the ecological stoichiometry of the sea. Limnol Oceanogr 48: 1721–1731 [Google Scholar]
- Dou Z, Heinhorst S, Williams EB, Murin CD, Shively JM, Cannon GC (2008) CO2 fixation kinetics of Halothiobacillus neapolitanus mutant carboxysomes lacking carbonic anhydrase suggest the shell acts as a diffusional barrier for CO2. J Biol Chem 283: 10377–10384 [DOI] [PubMed] [Google Scholar]
- Falkner G, Horner F, Werdan K, Heldt HW (1976) pH changes in cytoplasm of the blue-green alga Anacystis nidulans caused by light-dependent proton flux into the thylakoid space. Plant Physiol 58: 717–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu FX, Warner ME, Zhang YH, Feng YY, Hutchins DA (2007) Effects of increased temperature and CO2 on photosynthesis, growth, and elemental ratios in marine Synechococcus and Prochlorococcus (cyanobacteria). J Phycol 43: 485–496 [Google Scholar]
- Hassidim M, Keren N, Ohad I, Reinhold L, Kaplan A (1997) Acclimation of Synechococcus strain WH7803 to ambient CO2 concentration and to elevated light intensity. J Phycol 33: 811–817 [Google Scholar]
- Hopkinson BM. (2014) A chloroplast pump model for the CO2 concentrating mechanism in the diatom Phaeodactylum tricornutum. Photosynth Res 121: 223–233 [DOI] [PubMed] [Google Scholar]
- Hopkinson BM, Barbeau KA (2012) Iron transporters in marine prokaryotic genomes and metagenomes. Environ Microbiol 14: 114–128 [DOI] [PubMed] [Google Scholar]
- Hopkinson BM, Dupont CL, Allen AE, Morel FMM (2011) Efficiency of the CO2-concentrating mechanism of diatoms. Proc Natl Acad Sci USA 108: 3830–3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KS. (1982) Carbon dioxide hydration and dehydration kinetics in seawater. Limnol Oceanogr 27: 849–855 [Google Scholar]
- Kallas T, Dahlquist FW (1981) Phosphorus-31 nuclear magnetic resonance analysis of internal pH during photosynthesis in the cyanobacterium Synechococcus. Biochemistry 20: 5900–5907 [DOI] [PubMed] [Google Scholar]
- Kaplan A, Badger MR, Berry JA (1980) Photosynthesis and the intracellular inorganic carbon pool in the bluegreen alga Anabaena variabilis: response to external CO2 concentration. Planta 149: 219–226 [DOI] [PubMed] [Google Scholar]
- Kettler GC, Martiny AC, Huang K, Zucker J, Coleman ML, Rodrigue S, Chen F, Lapidus A, Ferriera S, Johnson J, et al. (2007) Patterns and implications of gene gain and loss in the evolution of Prochlorococcus. PLoS Genet 3: e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney JN, Axen SD, Kerfeld CA (2011) Comparative analysis of carboxysome shell proteins. Photosynth Res 109: 21–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein MG, Zwart P, Bagby SC, Cai F, Chisholm SW, Heinhorst S, Cannon GC, Kerfeld CA (2009) Identification and structural analysis of a novel carboxysome shell protein with implications for metabolite transport. J Mol Biol 392: 319–333 [DOI] [PubMed] [Google Scholar]
- Losh JL, Young JN, Morel FM (2013) Rubisco is a small fraction of total protein in marine phytoplankton. New Phytol 198: 52–58 [DOI] [PubMed] [Google Scholar]
- Maeda S, Badger MR, Price GD (2002) Novel gene products associated with NdhD3/D4-containing NDH-1 complexes are involved in photosynthetic CO2 hydration in the cyanobacterium, Synechococcus sp. PCC7942. Mol Microbiol 43: 425–435 [DOI] [PubMed] [Google Scholar]
- Martiny AC, Kathuria S, Berube PM (2009) Widespread metabolic potential for nitrite and nitrate assimilation among Prochlorococcus ecotypes. Proc Natl Acad Sci USA 106: 10787–10792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y, Nakajima K, Tachibana M (2011) Recent progresses on the genetic basis of the regulation of CO2 acquisition systems in response to CO2 concentration. Photosynth Res 109: 191–203 [DOI] [PubMed] [Google Scholar]
- Menon BB, Heinhorst S, Shively JM, Cannon GC (2010) The carboxysome shell is permeable to protons. J Bacteriol 192: 5881–5886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore L, Coe A, Zinser E, Saito M, Sullivan M, Lindell D, Frois-Moniz K, Waterbury J, Chisholm S (2007) Culturing the marine cyanobacterium Prochlorococcus. Limnol Oceanogr Methods 5: 353–362 [Google Scholar]
- Morris JJ, Johnson ZI, Szul MJ, Keller M, Zinser ER (2011) Dependence of the cyanobacterium Prochlorococcus on hydrogen peroxide scavenging microbes for growth at the ocean’s surface. PLoS ONE 6: e16805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Takahashi Y, Yamaguchi O, Suzuki H, Maeda S, Omata T (2008) Mechanism of low CO2-induced activation of the cmp bicarbonate transporter operon by a LysR family protein in the cyanobacterium Synechococcus elongatus strain PCC 7942. Mol Microbiol 68: 98–109 [DOI] [PubMed] [Google Scholar]
- Ogawa T, Mi H (2007) Cyanobacterial NADPH dehydrogenase complexes. Photosynth Res 93: 69–77 [DOI] [PubMed] [Google Scholar]
- Omata T, Gohta S, Takahashi Y, Harano Y, Maeda S (2001) Involvement of a CbbR homolog in low CO2-induced activation of the bicarbonate transporter operon in cyanobacteria. J Bacteriol 183: 1891–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinska KA, Laloui W, Bédu S, Loiseaux-de Goër S, Castets AM, Rippka R, Tandeau de Marsac N (2002) The signal transducer PII and bicarbonate acquisition in Prochlorococcus marinus PCC 9511, a marine cyanobacterium naturally deficient in nitrate and nitrite assimilation. Microbiology 148: 2405–2412 [DOI] [PubMed] [Google Scholar]
- Partensky F, Garczarek L (2010) Prochlorococcus: advantages and limits of minimalism. Annu Rev Mar Sci 2: 305–331 [DOI] [PubMed] [Google Scholar]
- Partensky F, Hess WR, Vaulot D (1999) Prochlorococcus, a marine photosynthetic prokaryote of global significance. Microbiol Mol Biol Rev 63: 106–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasciak WJ, Gavis J (1974) Transport limitation of nutrient uptake in phytoplankton. Limnol Oceanogr 19: 881–898 [Google Scholar]
- Price GD, Badger MR (1989) Expression of human carbonic anhydrase in the cyanobacterium Synechococcus PCC7942 creates a high CO2-requiring phenotype: evidence for a central role for carboxysomes in the CO2 concentrating mechanism. Plant Physiol 91: 505–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GD, Woodger FJ, Badger MR, Howitt SM, Tucker L (2004) Identification of a SulP-type bicarbonate transporter in marine cyanobacteria. Proc Natl Acad Sci USA 101: 18228–18233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae BD, Förster B, Badger MR, Price GD (2011) The CO2-concentrating mechanism of Synechococcus WH5701 is composed of native and horizontally-acquired components. Photosynth Res 109: 59–72 [DOI] [PubMed] [Google Scholar]
- Rae BD, Long BM, Badger MR, Price GD (2013) Functions, compositions, and evolution of the two types of carboxysomes: polyhedral microcompartments that facilitate CO2 fixation in cyanobacteria and some proteobacteria. Microbiol Mol Biol Rev 77: 357–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold L, Kosloff R, Kaplan A (1991) A model for inorganic carbon fluxes and photosynthesis in cyanobacterial carboxysomes. Can J Bot 69: 984–988 [Google Scholar]
- Reinhold L, Zviman M, Kaplan A (1987) Inorganic carbon fluxes and photosynthesis in cyanobacteria: a quantitative model InBiggins J, ed, Progress in Photosynthesis. Martinus Nijhoff, Dordrecht, The Netherlands, pp 289–296 [Google Scholar]
- Roberts EW, Cai F, Kerfeld CA, Cannon GC, Heinhorst S (2012) Isolation and characterization of the Prochlorococcus carboxysome reveal the presence of the novel shell protein CsoS1D. J Bacteriol 194: 787–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocap G, Larimer FW, Lamerdin J, Malfatti S, Chain P, Ahlgren NA, Arellano A, Coleman M, Hauser L, Hess WR, et al. (2003) Genome divergence in two Prochlorococcus ecotypes reflects oceanic niche differentiation. Nature 424: 1042–1047 [DOI] [PubMed] [Google Scholar]
- Rost B, Riebesell U, Burkhardt S, Sültemeyer D (2003) Carbon acquisition of bloom-forming marine phytoplankton. Limnol Oceanogr 48: 55–67 [Google Scholar]
- Rost B, Zondervan I, Wolf-Gladrow D (2008) Sensitivity of phytoplankton to future changes in ocean carbonate chemistry: current knowledge, contradictions and research directions. Mar Ecol Prog Ser 373: 227–237 [Google Scholar]
- Rusch DB, Martiny AC, Dupont CL, Halpern AL, Venter JC (2010) Characterization of Prochlorococcus clades from iron-depleted oceanic regions. Proc Natl Acad Sci USA 107: 16184–16189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott KM, Henn-Sax M, Harmer TL, Longo DL, Framer CH, Cavanaugh CM (2007) Kinetic isotope effect and biochemical characterization of form IA RubisCO from the marine cyanobacterium Prochlorococcus marinus MIT9313. Limnol Oceanogr 52: 2199–2204 [Google Scholar]
- Shibata M, Katoh H, Sonoda M, Ohkawa H, Shimoyama M, Fukuzawa H, Kaplan A, Ogawa T (2002) Genes essential to sodium-dependent bicarbonate transport in cyanobacteria: function and phylogenetic analysis. J Biol Chem 277: 18658–18664 [DOI] [PubMed] [Google Scholar]
- Shibata M, Ohkawa H, Kaneko T, Fukuzawa H, Tabata S, Kaplan A, Ogawa T (2001) Distinct constitutive and low-CO2-induced CO2 uptake systems in cyanobacteria: genes involved and their phylogenetic relationship with homologous genes in other organisms. Proc Natl Acad Sci USA 98: 11789–11794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman DN, Tu CK, Roessler N (1981) Diffusion-limited exchange of 18O between CO2 and water in red cell suspensions. Respir Physiol 44: 285–298 [DOI] [PubMed] [Google Scholar]
- So AKC, Espie GS, Williams EB, Shively JM, Heinhorst S, Cannon GC (2004) A novel evolutionary lineage of carbonic anhydrase (epsilon class) is a component of the carboxysome shell. J Bacteriol 186: 623–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tcherkez GGB, Farquhar GD, Andrews TJ (2006) Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proc Natl Acad Sci USA 103: 7246–7251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernov D, Hassidim M, Luz B, Sukenik A, Reinhold L, Kaplan A (1997) Sustained net CO2 evolution during photosynthesis by marine microorganisms. Curr Biol 7: 723–728 [DOI] [PubMed] [Google Scholar]
- Ting CS, Hsieh C, Sundararaman S, Mannella C, Marko M (2007) Cryo-electron tomography reveals the comparative three-dimensional architecture of Prochlorococcus, a globally important marine cyanobacterium. J Bacteriol 189: 4485–4493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolonen AC, Aach J, Lindell D, Johnson ZI, Rector T, Steen R, Church GM, Chisholm SW (2006) Global gene expression of Prochlorococcus ecotypes in response to changes in nitrogen availability. Mol Syst Biol 2: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead L, Long BM, Price GD, Badger MR (2014) Comparing the in vivo function of α-carboxysomes and β-carboxysomes in two model cyanobacteria. Plant Physiol 165: 398–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodger FJ, Badger MR, Price GD (2005) Sensing of inorganic carbon limitation in Synechococcus PCC7942 is correlated with the size of the internal inorganic carbon pool and involves oxygen. Plant Physiol 139: 1959–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodger FJ, Bryant DA, Price GD (2007) Transcriptional regulation of the CO2-concentrating mechanism in a euryhaline, coastal marine cyanobacterium, Synechococcus sp. strain PCC 7002: role of NdhR/CcmR. J Bacteriol 189: 3335–3347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worden AZ, Binder BJ (2003) Application of dilution experiments for measuring growth and mortality rates among Prochlorococcus and Synechococcus populations in oligotrophic environments. Aquat Microb Ecol 30: 159–174 [Google Scholar]
- Zeebe RE, Wolf-Gladrow D (2001) CO2 in Seawater: Equilibrium, Kinetics, Isotopes. Elsevier, Amsterdam [Google Scholar]
- Zhang HN, Byrne RH (1996) Spectrophotometric pH measurements of surface seawater at in-situ conditions: absorbance and protonation behavior of thymol blue. Mar Chem 52: 17–25 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.