Reduced root cortical cell file number substantially reduces root respiration, permitting greater root growth and exploration of deep soil domains, thereby improving water acquisition, plant growth, and yield under drought.
Abstract
We tested the hypothesis that reduced root cortical cell file number (CCFN) would improve drought tolerance in maize (Zea mays) by reducing the metabolic costs of soil exploration. Maize genotypes with contrasting CCFN were grown under well-watered and water-stressed conditions in greenhouse mesocosms and in the field in the United States and Malawi. CCFN ranged from six to 19 among maize genotypes. In mesocosms, reduced CCFN was correlated with 57% reduction of root respiration per unit of root length. Under water stress in the mesocosms, genotypes with reduced CCFN had between 15% and 60% deeper rooting, 78% greater stomatal conductance, 36% greater leaf CO2 assimilation, and between 52% to 139% greater shoot biomass than genotypes with many cell files. Under water stress in the field, genotypes with reduced CCFN had between 33% and 40% deeper rooting, 28% lighter stem water oxygen isotope enrichment (δ18O) signature signifying deeper water capture, between 10% and 35% greater leaf relative water content, between 35% and 70% greater shoot biomass at flowering, and between 33% and 114% greater yield than genotypes with many cell files. These results support the hypothesis that reduced CCFN improves drought tolerance by reducing the metabolic costs of soil exploration, enabling deeper soil exploration, greater water acquisition, and improved growth and yield under water stress. The large genetic variation for CCFN in maize germplasm suggests that CCFN merits attention as a breeding target to improve the drought tolerance of maize and possibly other cereal crops.
Drought is a primary constraint to global crop production (Schmidhuber and Tubiello, 2007), and global climate change is likely to increase the risk of drought, especially in rain-fed agriculture (Battisti and Naylor, 2009; Burke et al., 2009; Mishra and Cherkauer, 2010; Lobell et al., 2011). Therefore, the development of crops with greater drought tolerance is an important global objective. Yield under drought is often not an efficient selection criterion in drought breeding programs, since yield is affected by many elements of the phenotype and the environment, interacting in complex and often unknown ways. Trait-based selection or ideotype breeding is generally a more efficient selection strategy, permitting the identification of useful sources of variation among lines that have poor agronomic adaptation, elucidation of genotype-by-environment interactions, and informed trait stacking (Araus et al., 2002, 2008; Manschadi et al., 2006; Lynch, 2007b, 2011; York et al., 2013).
In most agroecosystems, the topsoil dries before the subsoil as drought progresses. In such environments, plants with deeper roots are able to acquire water available in deeper soil domains that may not be available to plants with shallower roots (Ludlow and Muchow, 1990; Ho et al., 2005; Hammer et al., 2009). An ideotype has been proposed to guide the breeding of crops with deeper roots and, therefore, greater water acquisition from drying soil, called Steep, Cheap, and Deep, integrating architectural, anatomical, and physiological phenes (Lynch, 2013). The term Cheap denotes phenes that reduce the metabolic cost of soil exploration, which is an important limitation to the acquisition of scarce soil resources, including water in dry soil (Fan et al., 2003; Lynch, 2007b; Zhu et al., 2010; Postma and Lynch, 2011a, 2011b; Jaramillo et al., 2013). Plant resource allocation to root growth typically increases under drought to enhance water acquisition; therefore, the metabolic cost of root growth becomes a significant component of plant fitness and adaptation under drought (Lynch, 2007b, 2013). Therefore, a plant that is able to access water in deep soil domains at reduced metabolic cost will have superior productivity, because it will have more metabolic resources available for further resource acquisition, growth, and reproduction. Evidence in support of this hypothesis comes from empirical and modeling studies for maize (Zea mays) under water and edaphic stress (Lynch, 2007a; Zhu et al., 2010; Postma and Lynch, 2011a, 2011b; Jaramillo et al., 2013).
Root cortical aerenchyma (RCA) is the enlarged air space in the root cortex that forms either through cell death or cell separation (Evans, 2004). RCA is associated with a disproportionate reduction of root respiration in maize by converting living cortical tissue to air volume (Fan et al., 2003; Zhu et al., 2010). Reduction of root metabolic costs permits more internal resources to be allocated to greater root growth and, consequently, greater soil resource acquisition. RCA formation is also associated with a reduction of phosphorus content in root tissue on a volume basis, since air spaces do not contain phosphorus (Fan et al., 2003), and with improved growth in low-phosphorus soil (Lynch, 2011). RCA also reduces the nitrogen content of root tissue and is beneficial for nitrogen capture and maize growth on low-nitrogen soils (Saengwilai, 2014a). Modeling studies suggest that RCA improves crop adaptation to suboptimal nutrient availability by reducing the metabolic costs of soil exploration (Postma and Lynch, 2011a, 2011b). Under drought, Zhu et al. (2010) found that maize genotypes with more RCA had five times greater biomass and eight times greater yield than genotypes with less RCA. Living cortical area (LCA) is total transverse root cortical area minus RCA area. Jaramillo et al. (2013) found that root respiration is positively correlated with LCA, and a 3.5-fold reduction in LCA is associated with a 2.5-fold improvement in plant growth under drought. These results indicate that the metabolic demand of living cortical tissue is a primary determinant of root growth, soil exploration, and resource acquisition in soil environments with suboptimal resource availability.
This study builds on earlier studies indicating that substantial reduction of root metabolic cost is associated with variation in LCA. The cortex of the maize root is composed of several concentric layers of parenchyma cells, the number of which we refer to as the cortical cell file number (CCFN). Recently, Burton et al. (2013) reported that there is 3-fold variation for CCFN in Zea spp. In that study, the variation was wider in maize landraces (six to 16 cell files) than in wild Zea spp. (seven to 13 cell files). It has been proposed that reduced CCFN would decrease the metabolic costs of root growth and maintenance, in terms of both the carbon cost of root respiration and the nutrient content of living tissue, by reducing the proportion of root volume occupied by living cortical tissue, which has greater metabolic demands than the stele (Lynch, 2013). However, the physiological utility of CCFN has not been explored.
The objective of this study was to test the hypothesis that reduced CCFN would reduce root respiration, permitting greater rooting depth, thereby enhancing water acquisition and improving both plant growth and yield under water stress.
RESULTS
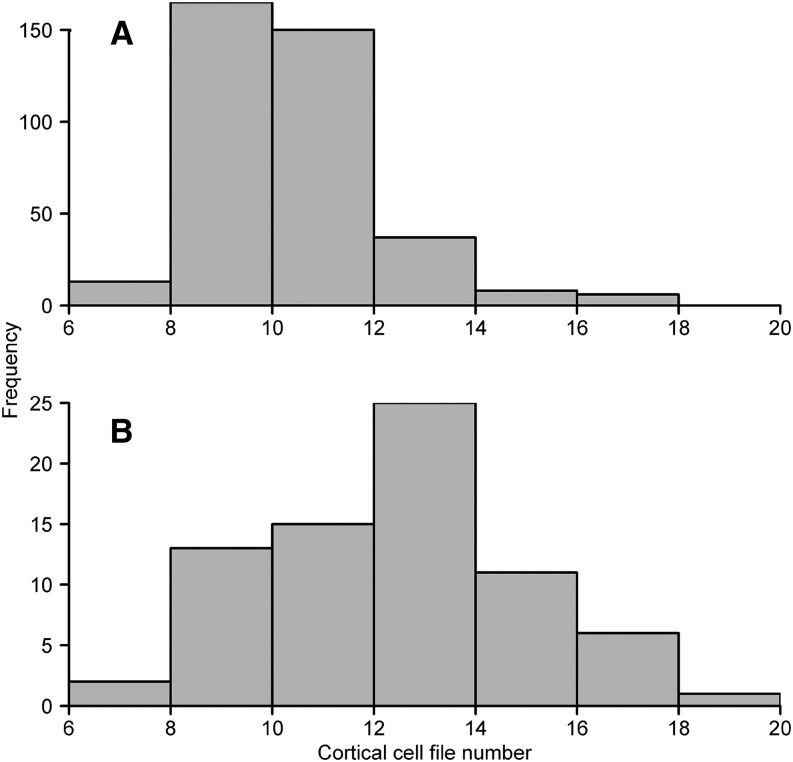
We observed substantial phenotypic variation for CCFN within maize recombinant inbred lines (RILs; Fig. 1). In mesocosms (GH1), CCFN ranged from eight to 17 in the intermated B73 × Mo17 (IBM) population (Fig. 2A). In the field in Malawi (MW2011), CCFN ranged from six to 19 among lines from the maize breeding program at the Lilongwe University of Agriculture and Natural Resources (Fig. 2B). The stability of CCFN across environments was estimated as the correlation coefficient between CCFN measured on the same genotypes in mesocosms (30-d-old plants [GH2]) and in the field (70-d-old plants [PA2011]) and across environments in the field, Bunda (BU2012) and Chitala (CH2012). Strong positive correlations were found between CCFN in mesocosms (GH2) and in the field (PA2011; r = 0.85, P < 0.05) and between CCFN measured in two environments in Malawi (BU2012 and CH2012; r = 0.68, P < 0.05).
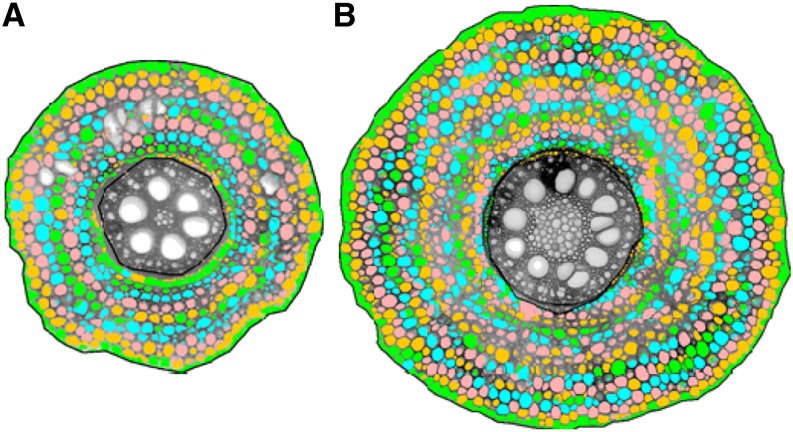
Figure 1.
Cross-section images showing genotypic differences in root CCFN in maize: eight cell files (A) and 14 cell files (B). Cross sections are from standard reference tissue collected 10 to 20 cm from the base of the second nodal crown root at 70 d after planting from field-grown plants. Images were obtained from laser ablation tomography.
Figure 2.
Genetic variation for root CCFN in maize selected IBM lines (GH1; A) and RILs from the Malawi maize breeding program (MW2011; B). The data shown are from standard reference tissue collected 10 to 20 cm from the base of the second nodal crown root. In the greenhouse, roots were sampled 30 d after planting, and in the field, roots were sampled 70 d after planting.
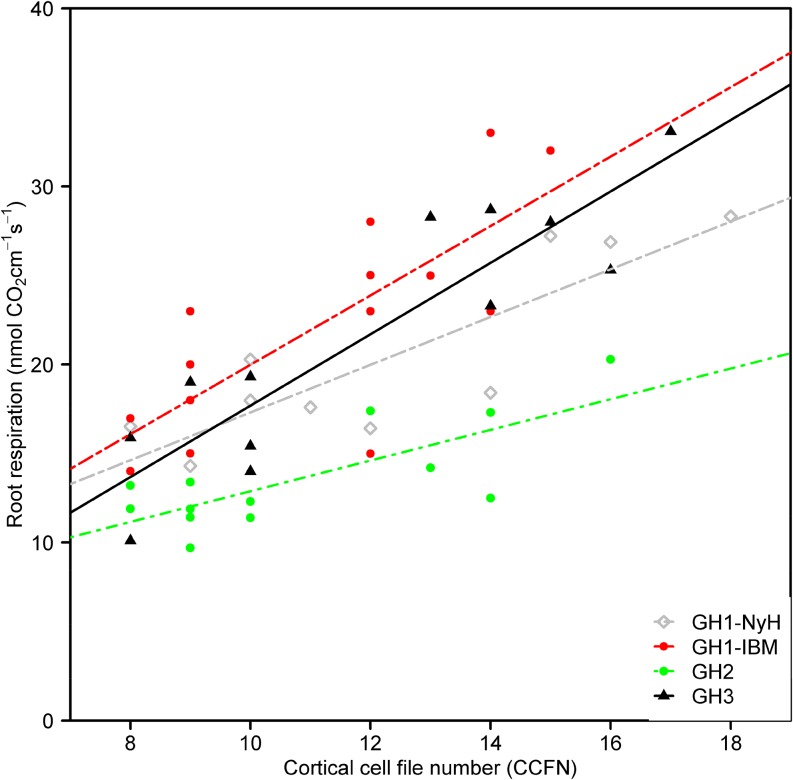
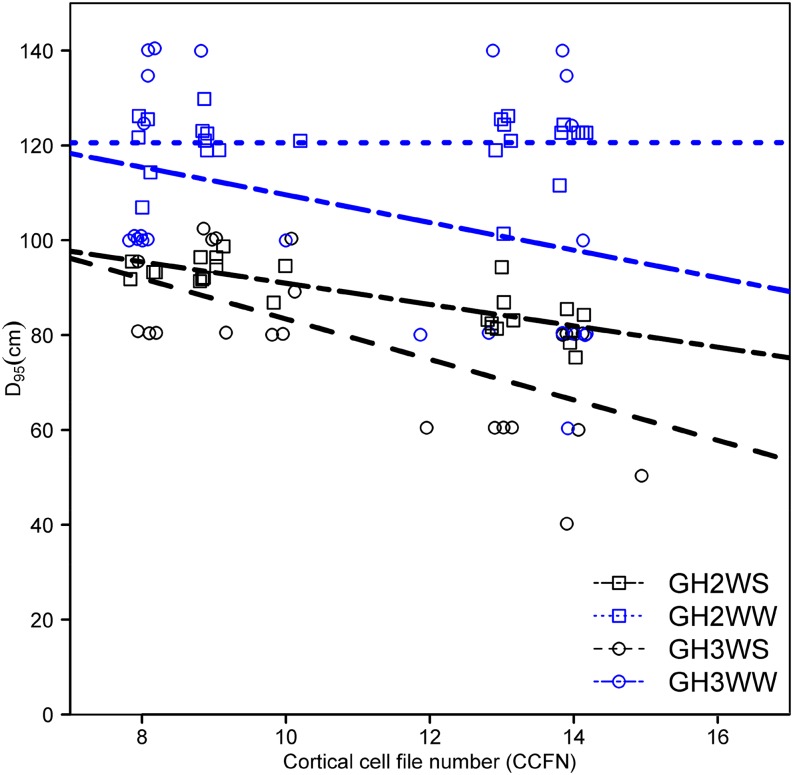
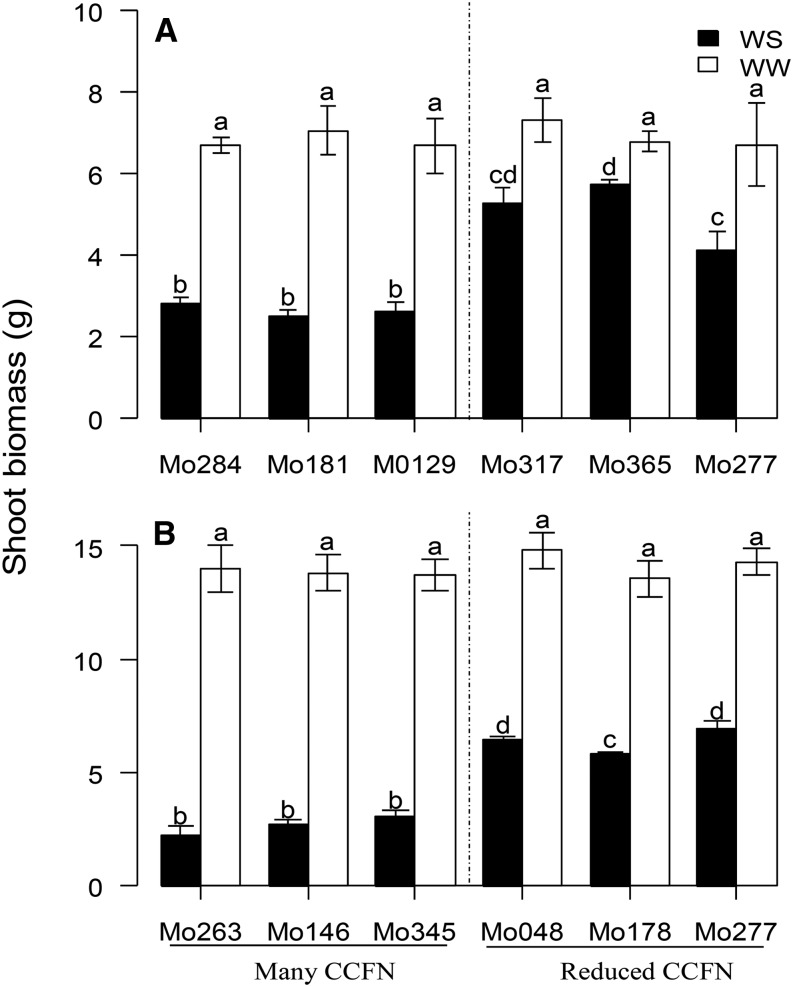
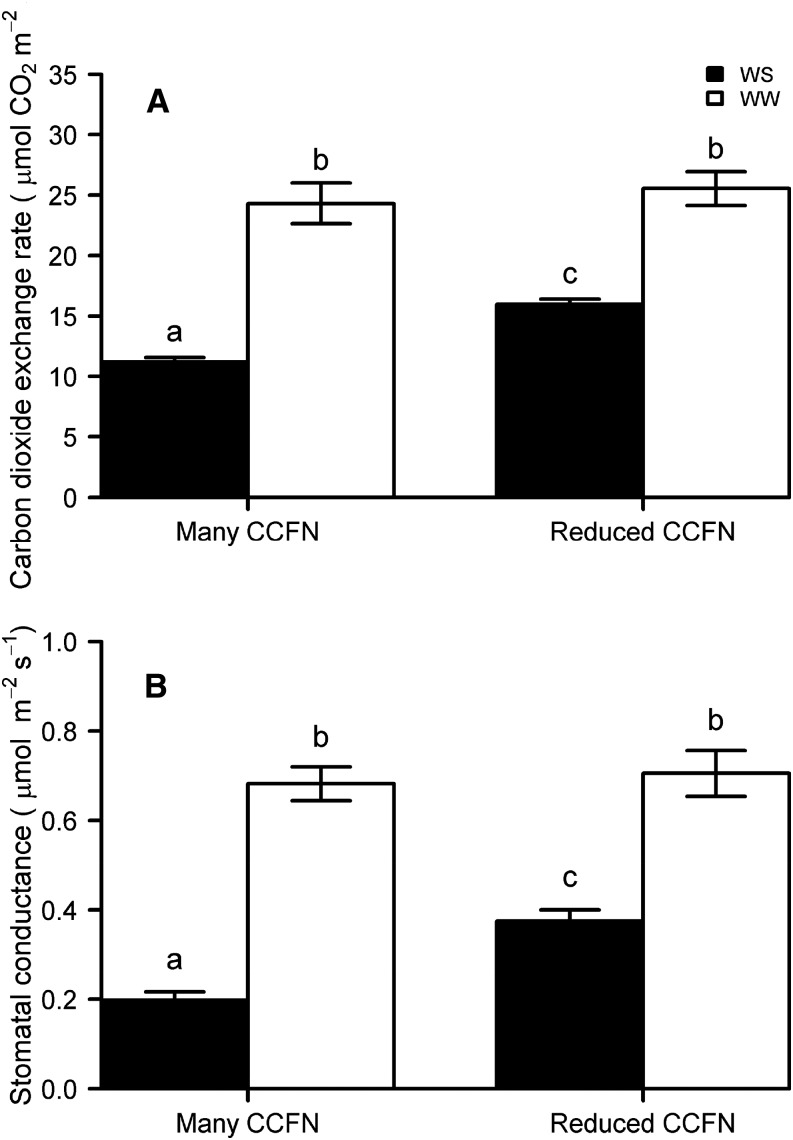
To understand the effects of CCFN on root respiratory costs, CO2 production from excised root segments was measured in diverse sets of maize lines in mesocosms (GH1–GH3). Reduced CCFN was correlated with reductions of specific root respiration by 57% (GH1-IBM), 46% (GH1-NY821 × H99 [NyH]), 52% (GH2), and 69% (GH3; Fig. 3). However, there was no significant difference in respiration rates between well-watered and water-stressed roots in GH2 and GH3 (Table I). In GH1, CCFN was correlated with specific root length (SRL) in both IBM lines (r = −0.55, P < 0.05) and NyH lines (r = −0.48, P < 0.05). CCFN was a better predictor of root respiration than SRL (Table II). In well-watered mesocosms, CCFN had no relationship with rooting depth, stomatal conductance, photosynthesis rate, or plant biomass. Under water stress, genotypes with reduced CCFN had 15% (GH1) and 60% (GH2) deeper rooting, 78% greater stomatal conductance (GH3), 36% greater leaf photosynthetic rate (GH3), and 52% (GH2) and 139% (GH3) greater biomass than genotypes with many cell files (Table I; Figs. 4–6). Reduced CCFN genotypes proliferated more roots in soil domains below 60 cm compared with many CCFN genotypes under water-stressed conditions (Supplemental Fig. S1A).
Figure 3.
Correlation of root respiration per unit of length and CCFN for GH1-NyH (y = 1.7x − 0.31, r2 = 0.46, P = 0.009), GH1-IBM (y = 1.9x − 0.49, r2 = 0.46, P = 0.009), GH2 (y = 0.8x − 4.32, r2 = 0.59, P = 0.001), and GH3 (y = 2.11x − 3.09, r2 = 0.52, P = 0.018) in the mesocosms 30 d after planting. Each point is the mean of at least three measurements of respiration from the second nodal crown root per genotype. [See online article for color version of this figure.]
Table I. Summary of ANOVA for respiration, root depth (D95), stomatal conductance, carbon dioxide exchange rate (CER), and shoot biomass as influenced by soil moisture regime (treatment) and genotype in the greenhouse mesocosms experiments (GH2 and GH3).
The associated F values and probabilities (ns, not significant; *P < 0.05; **P < 0.01; and ***P < 0.001) are shown.
Table II. Summary of linear models (y = a + bx) of root respiration as predicted by root CCFN and SLR in 15 IBM RILs (GH1-IBM) and 11 NyH RILs (GH1-NyH) under moderate drought in the greenhouse (GH1).
Figure 4.
Correlation of root depth (D95) and CCFN for GH2WS (y = 113.4 – 2.4x, r2 = 0.57, P < 0.001), GH2WW (y = 120.6 + 0.003x, r2 = 0.003, P not significant), GH3WS (y = 124.9 – 4.2x, r2 = 0.41, P < 0.01), and GHWW (y = 138.6 – 2.9x, r2 = 0.10, P not significant) in greenhouse mesocosms 30 d after planting. Data include water-stressed (WS) and well-watered (WW) conditions. [See online article for color version of this figure.]
Figure 6.
Shoot dry weight of genotypes contrasting in CCFN at 30 d after planting in well-watered (WW) and water-stressed (WS) conditions in mesocosms (A) and GH2 and GH3 (B). Bars show means ± se of four replicates per treatment. Bars with the same letters are not significantly different within the same frame (P < 0.05).
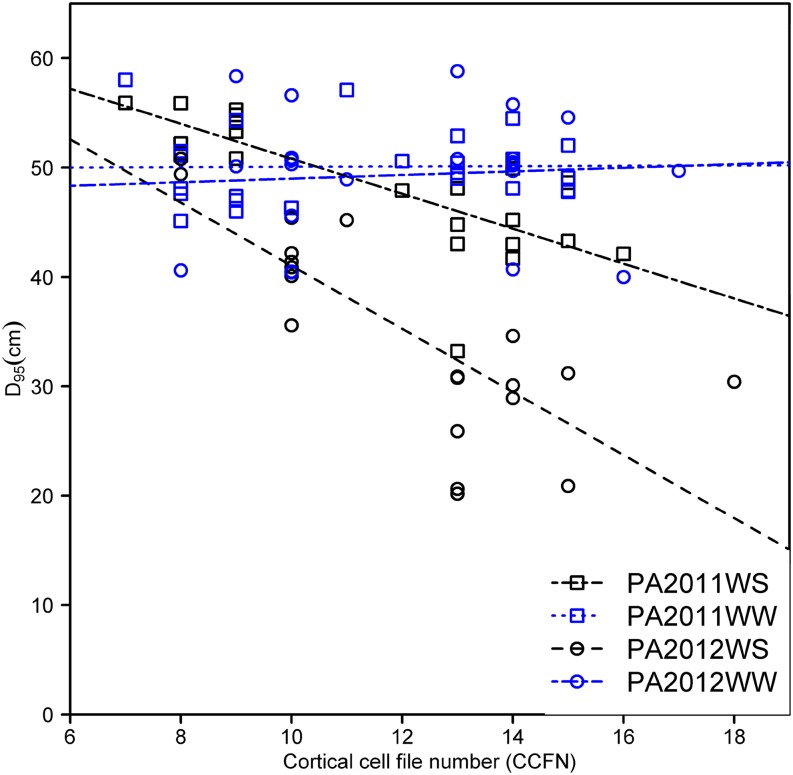
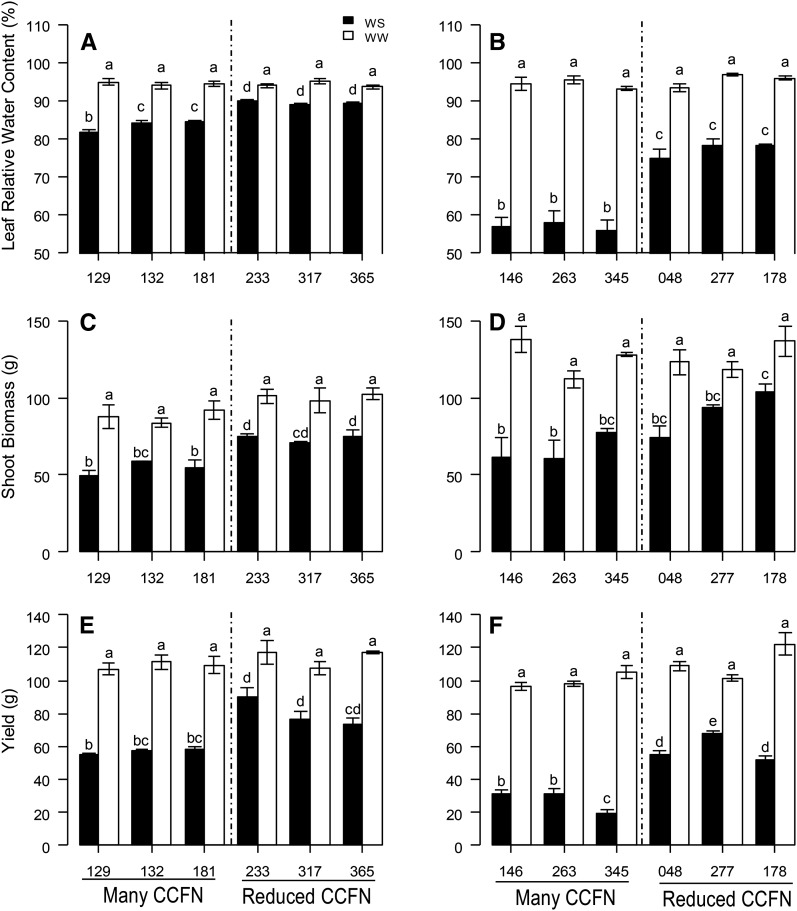
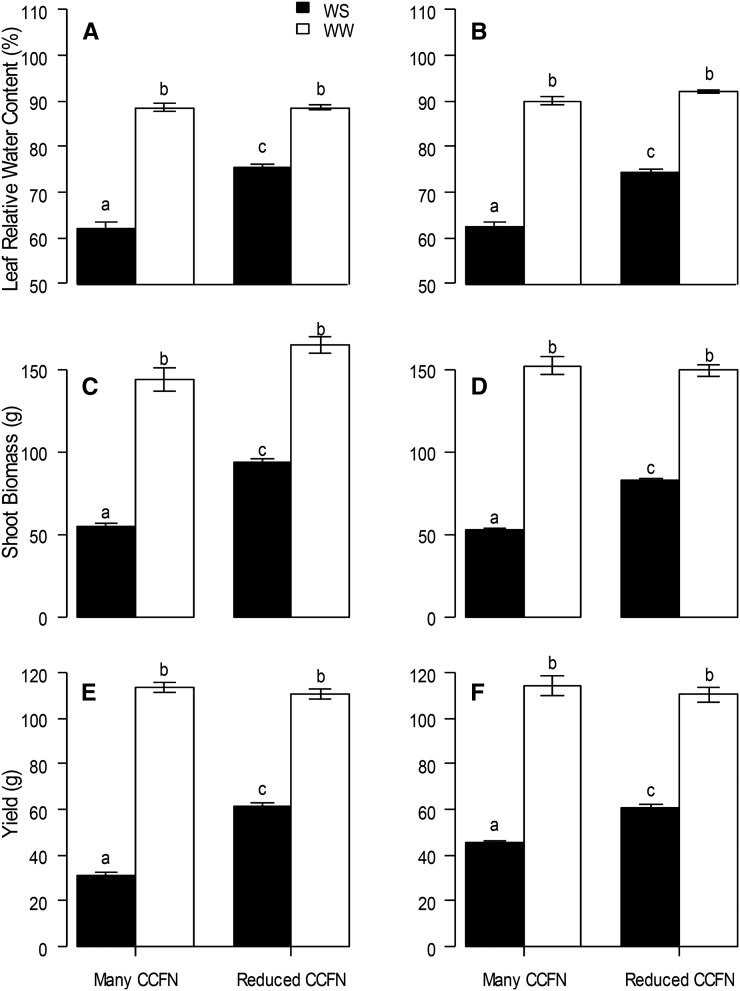
In the field at Rock Springs, Pennsylvania, under water stress, genotypes with reduced CCFN had 33% (PA2011) and 40% (PA2012) deeper rooting depth (D95, the depth above which 95% of total root length is located in the soil profile) and 10% (PA2011) and 35% (PA2012) greater leaf relative water content (RWC) than genotypes with many cell files (Table III; Figs. 7 and 8, A and B). In addition, genotypes with deeper D95 had greater leaf water status than genotypes with shallow D95, while there was no relationship in well-watered conditions (PA2011; r = 0. 51, P < 0.000). In addition, reduced CCFN genotypes proliferated more roots in soil domains below 30 cm compared with many CCFN genotypes under water-stressed conditions (Supplemental Fig. S1B).
Table III. Summary of ANOVA for leaf RWC (%), shoot biomass, and yield as influenced by soil moisture regime (treatment) and genotype in the rainout shelters at Rock Springs, Pennsylvania (PA2011 and 2012).
The associated F values and probabilities (ns, not significant; **P < 0.01; and ***P < 0.001) are shown.
| Source of Variation | PA2011 |
PA2012 |
||||||
|---|---|---|---|---|---|---|---|---|
| D95 | RWC | Biomass | Yield | D95 | RWC | Biomass | Yield | |
| Treatment (T) | 1.80ns | 522.10*** | 119.80*** | 310.2*** | 1.88ns | 722.4*** | 108.06*** | 1341.8** |
| Genotype (G) | 6.90*** | 14.8*** | 7.01*** | 8.00*** | 6.92*** | 21.81*** | 4.21** | 33.58*** |
| G × T | 6.71*** | 18.12*** | 0.75ns | 3.64** | 6.77*** | 16.78*** | 2.49ns | 18.36** |
Figure 7.
Correlation of root depth (D95) and root CCFN in rainout shelters at Rock Springs, Pennsylvania, for PA2011WS (y = 66.7 – 1.59x, r2 = 0.59, P < 0.01), PA2012WW (y = 49.89 + 0.02x, r2 = 0.002, P not significant), PA2012WS (r2 = 0.42, P < 0.05), and PA2012WW (y = 47.4 + 0.02x, r2 = 0.05, P not significant) 80 d after planting. Data include water-stressed (WS) and well-watered (WW) conditions. [See online article for color version of this figure.]
Figure 8.
Performance of maize lines contrasting in CCFN in water-stressed (WS) and well-watered (WW) conditions in rainout shelters at Rock Springs, Pennsylvania. Leaf RWC is shown at 60 d after planting for PA2011 (A) and PA2012 (B); shoot biomass per plant is shown at 70 d after planting for PA2011 (C) and PA2012 (D); and yield per plant is shown for PA2011 (E) and PA2012 (F). Bars show means ± se of four replicates per treatment. Bars with the same letters are not significantly different within the same frame (P < 0.05).
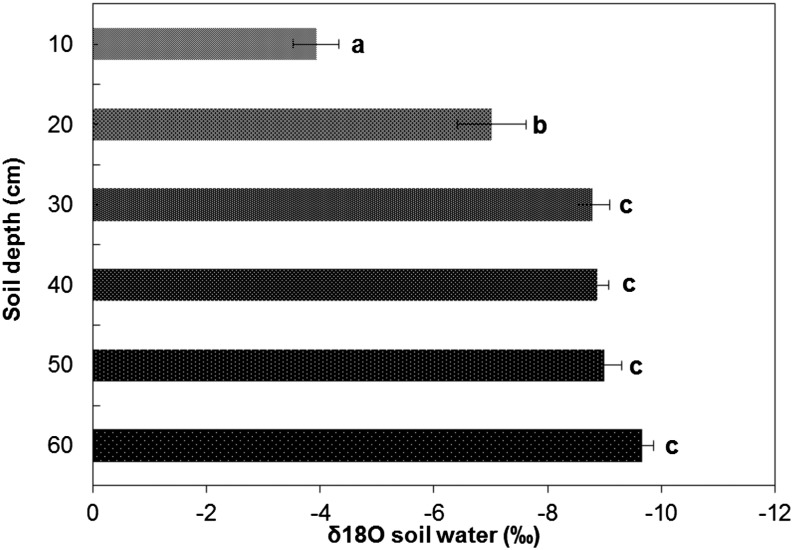
Analysis of soil water 18O to 16O isotope ratios (δ18O) showed a progressively lighter isotopic signature of water with increasing depth in water stress conditions (Fig. 9). However, the majority of change in this signature was in the top two layers: 0 to 10 and 10 to 20 cm depth (approximately 2.09‰). The values of soil water signature below 30 cm depth showed no significant difference with depth (Fig. 9) and were aggregated as deep water for further analysis. The average values of xylem water δ18O for genotypes varied by 3.19‰ (Table IV). Genotypes with reduced CCFN had a collective xylem water signature that was 28% lighter than that of genotypes with many cell files (Table IV). Soil water δ18O values were used in an isotopic mixing model to determine water sources contributing to the δ18O signature for xylem water, assuming that any water acquired below 30 cm depth was deep water. Genotypes with reduced CCFN had greater average reliance on deep water and were the least reliant on shallow water from the top two soil layers than genotypes with many cell files (Table IV). The proportion of deep water acquired by genotypes with reduced CCFN ranged from 21% to 81%, while for two genotypes with many cell files this value was zero. The only exception was genotype 181, which was classified as having many cell files but had relatively greater dependency on deep water of 32%.
Figure 9.
Mean oxygen isotope composition ± se of soil water along the soil profile in the rainout shelters (PA2011). Sampling was done 65 d after planting. Values are means ± se of three observation points in the rainout shelters.
Table IV. Means of δ18O of xylem water ± se measured for six genotypes contrasting in CCFN under water stress 65 d after planting.
Proportional water use by depth is shown from different soil layers, where deep is the aggregate of three deep soil layers (Fig. 8) calculated using multisource mixing model analysis (Phillips et al., 2005).
Water stress reduced shoot biomass by 30% (PA2011) and 33% (PA2012) and reduced yield from 26% to 68% (PA2011) and from 33% to 75% (PA2012) compared with well-watered plants (Table III; Fig. 8, C–F). Genotypes with reduced CCFN had 35% (PA2011) and 45% (PA2012) greater shoot biomass and 38% (PA2011) and 114% (PA2012) greater yield than lines with many cell files under water stress (Table III; Fig. 8, C–F).
In the field across two maize growing environments in Malawi, water stress reduced leaf RWC by 22% (BU2012) and 25% (CH2012), shoot biomass by 43% (BU2012) and 54% (CH2012), and grain yield by 59% (BU2012) and 53% (CH2012; Fig. 8). Under water stress, genotypes with reduced CCFN had 20% (BU2012) and 19% (CH2012) greater leaf RWC, 70% (BU2012) and 57% (CH2012) greater shoot biomass, and 93% (BU2012) and 33% (CH2012) greater yield than genotypes with many cell files under water stress (Table V; Fig. 10)
Table V. Summary of ANOVA for root depth (D95), leaf RWC (%), shoot biomass, and yield as influenced by soil moisture regime (treatment) and genotype in the field in Malawi (Bunda and Chitala).
The associated F values and probabilities (**P < 0.01 and ***P < 0.001) are shown.
Figure 10.
Performance of maize lines contrasting in CCFN in the field in water-stressed (WS) and well-watered (WW) conditions at two field sites in Malawi. Leaf RWC is shown at 60 d after planting for Bunda (A) and Chitala (B), shoot biomass per plant is shown at 70 d after planting for Bunda (C) and Chitala (D), and yield per plant is shown for Bunda (E) and Chitala (F). Bars show means ± se (n = 16–18) of four replicates per treatment and trait. Bars with the same letters are not significantly different within the same frame (P < 0.05).
DISCUSSION
We hypothesized that reduced CCFN would reduce root respiration per unit of root length, permitting greater root growth and exploration at depth, thereby enhancing water acquisition and improving plant growth and yield under drought. Our results extend from observations of young plants in greenhouse mesocosms to mature plants in the field in the United States and two environments in Malawi. Our results entirely support our hypotheses: CCFN varied substantially among maize genotypes, and genotypes with reduced CCFN had lower specific root respiration; under water stress, genotypes with reduced CCFN had greater rooting depth, greater acquisition of deep soil water, better plant water status, greater leaf photosynthesis, better growth, and better yield.
The utility of CCFN was evaluated using diverse sets of genotypes contrasting in CCFN in greenhouse mesocosms, in the field using moveable rainout shelters, and with differential irrigation in Malawi. The greenhouse mesocosms and movable rainout shelters in the field allowed us to simulate terminal drought by the progressive reduction of soil water content (Fig. 10). The mesocosms also permit a detailed analysis of root distribution by depth and root respiration, since entire root systems can be recovered. The field environments in Malawi were natural drought environments in which rainfall varied but was insufficient to meet plant water requirements. The combination of results from the field and mesocosms lends credence to our conclusions, as the field includes variable environmental factors such as soil temperature, biota, and soil physical properties, while mesocosms permit greater environmental control and more detailed measurement of root properties. RILs sharing the same genetic lineage were employed to minimize the effects of genetic interaction, epistasis, and pleiotropy, which may confound the interpretation of results from comparison of unrelated lines (Zhu and Lynch, 2004). CCFN is a quantitative trait associated with multiple genetic loci in maize (Saengwilai, 2013). For evaluation of the utility of quantitative traits such as CCFN, RILs are useful because they permit the comparison of lines differing in CCFN expression among a set of genotypes sharing common parents.
Maize has substantial genetic variation for root architectural and anatomical phenes (Hochholdinger, 2009; Bayuelo-Jiménez et al., 2011; Trachsel et al., 2011; Burton et al., 2013, 2014; Lynch, 2013). The genotypic variability observed here was consistent with previous studies, which found that CCFN varies in the range from seven to 16 for CCFN in Zea spp. (including maize landraces and wild Zea spp.; Burton et al., 2013). Cortical cell files are formed by several successive asymmetric periclinal divisions in the root apical meristem (Baum et al., 2002; Chapman et al., 2003; Lux et al., 2004). It has been documented that the number of such periclinal divisions varies among species, genotypes, and root types (Lux et al., 2004; Coudert et al., 2010), which might generate differences in CCFN as observed here. Maize, like other monocots, has no secondary growth in its roots (Esau, 1965). Hence, CCFN variation along the longitudinal axis of a root represents the radial patterning in the root apical meristem. Therefore, the CCFN variation observed in this study was largely due to genotypic differences. However, the genetic and physiological mechanisms of this variation in maize are not yet known and deserve further exploration.
CCFN can be observed easily with a microscope and, therefore, is amenable to direct phenotypic selection in crop improvement programs. In this study, we showed that CCFN measured on young plants from greenhouse mesocosms 30 d after planting was an accurate reflection of CCFN measured on mature plants in the field 70 d after planting. We also observed correlation between CCFN measured in the field across two contrasting maize growing environments in Malawi. These results indicate that CCFN was stable across environments in this study.
We have proposed that reduced CCFN may be a useful adaptation to drought by reducing the metabolic costs of soil exploration (Lynch, 2013). Previous studies have associated a reduction of root respiration with RCA formation (Fan et al., 2003; Zhu et al., 2010). Jaramillo et al. (2013) found that reduced LCA substantially reduces root respiration in maize. In that study, it was concluded that LCA is a stronger predictor of root respiration than either RCA or root diameter, since it takes into account the differing cortical areas among root classes. Two key determinants of LCA are cell file number and cell size, and altering either one may affect the size of LCA, consequently affecting root metabolic costs. We have recently shown that large cortical cell size in maize is associated with reduced root respiration and greater root depth, water acquisition, plant growth, and yield under drought (Chimungu et al., 2014). As shown in this study, decreasing CCFN from 16 to eight was associated with a 57% reduction of root respiration (Fig. 3). This respiratory pattern may reflect the effect of decreasing the proportion of metabolically active cells in the cortex and increasing the proportion of nonrespiring tissues such as sclerenchyma and xylem vessels.
Root respiration along with growth, maintenance, and ion uptake are major components of root metabolic costs (Lambers, 1979; Van der Werf et al., 1988; Peng et al., 1993; Lambers et al., 2002; Lynch and Ho, 2005). In this study, root respiration was measured in the mature region of the root; therefore, total respiration in this region is primarily the respiration for tissue maintenance. Root construction cost is assumed to be a one-time cost that occurs when the root is formed (Yanai et al., 1995). In contrast, maintenance costs accumulate over time and can quickly exceed initial construction costs; therefore, maintenance costs are important determinants of root metabolic cost (Eissenstat and Yanai, 1997; Lynch and Ho, 2005; Lynch and Brown, 2008). For example, Postma and Lynch (2011b) reported that maize plants without maintenance respiration had up to 72% greater growth under nutrient-limiting conditions than plants with root maintenance respiration. The importance of maintenance costs is clearly shown by the case of RCA, which reduces the maintenance respiration and nutrient content of mature root tissue by converting living cortical cells to air space. Differential RCA formation among maize genotypes is associated with reduced maintenance respiration of root tissue, which, when plants are stressed by suboptimal availability of water, nitrogen, phosphorus, or potassium, results in greater root growth, greater acquisition of soil resources, greater plant growth, and greater yield (Fan et al., 2003; Zhu et al., 2010; Postma and Lynch 2011a, 2011b; Saengwilai et al., 2014a). Since RCA is formed in mature regions of the cortex, it affects root maintenance costs rather than root construction costs. Although CCFN affects both construction and maintenance costs, we believe that, by analogy with RCA, the effects of CCFN on maintenance costs are more important for plant adaptation to stress than effects on construction costs. A more detailed analysis of this issue would be possible using the structural-functional plant model SimRoot (Lynch et al., 1997). SimRoot is a structural-functional plant model that simulates the three-dimensional architecture and soil resource acquisition of a root system as it develops over time. It is difficult to quantify both construction and maintenance costs in greenhouse and field studies, because of the tightly coupled integration between the two costs. SimRoot may provide useful insights in this context by allowing the quantification and independent manipulation of maintenance and construction costs in plants contrasting for CCFN. SimRoot has provided such insights in the context of the effects of RCA on maintenance and construction costs in maize (Postma and Lynch 2011a, 2011b)
CCFN was a stronger predictor than SRL for root segment respiration, with a slightly greater coefficient of determination (Table II). Generally, greater SRL permits more efficient soil exploration (Eissenstat, 1992). SRL is influenced by root diameter as well as root anatomy, or tissue mass density (Wahl and Ryser, 2000). However, specific root length varies widely with environmental conditions, and the direction of change in SRL is not always predictable based on resource supply (Eissenstat et al., 2005). In addition, SRL is a coarse metric that aggregates many distinct phenes to provide an overall estimate of mass per unit of root length, without indicating how mass varies or the composition and hence energy content of the mass. SRL also does not indicate whether the root mass is living or dead tissue; therefore, it is not well correlated with variation for maintenance respiration among root classes and ages. Therefore, CCFN should be a more direct predictor of root respiratory costs than SRL (Table II), since it takes into account the differing cortical areas that generally have high metabolic rates. For example, Hall et al. (1971), working with maize, showed that fresh isolated cortex had greater respiration than fresh steles. Therefore, CCFN is an important determinant of root metabolic cost. Lynch (2013) proposed that large cortical cells may also substantially reduce root respiration, since larger cells have a higher ratio of vacuolar to cytoplasmic volume and, hence, reduced respiration per unit of tissue volume. For this reason, we propose that the benefit of reduced CCFN should be strongest in roots with small cortical cells.
The benefits of the reduced metabolic cost of soil exploration were greater under water stress (Figs. 3–7, 9, and 10). The greater utility of reduced CCFN under drought is associated with the fact that the genotypes with less costly root tissue had deeper rooting, better access to water, and therefore extra carbon gain through photosynthesis, which in turn will increase root growth further, creating a positive feedback for plant growth under water stress. We found that reduced CCFN was associated with increased rooting depth (D95) in the field under water stress but did not affect rooting depth in well-watered conditions (Fig. 7; Supplemental Fig. S1, A and B). In addition, our results show that genotypes with reduced CCFN and deeper D95 were able to maintain greater RWC in the field and stomatal conductance in mesocosms under water stress than genotypes with many cell files (Figs. 5, 8, A and B, and 10, A and B). These results suggest that increased availability of carbon from reduced respiration allows the plant to grow more roots under drought. Root growth in deep soil domains under water stress resulted in increased water acquisition, greater plant water status, and greater photosynthesis, which benefits overall plant growth and yield.
Figure 5.
Carbon dioxide exchange rate (A) and stomatal conductance (B) of six genotypes with contrasting CCFN 28 d after planting in well-watered (WW) and water-stressed (WS) conditions in greenhouse mesocosms (GH3). Bars represent means ± se of four replicates per treatment. Bars with the same letters are not significantly different (P < 0.05).
Xylem water reflects the oxygen isotopic composition of water acquired by the plant from the soil, as no isotopic fractionation occurs during water uptake and transport (Ehleringer and Dawson, 1992; Dawson and Pate, 1996; Ehleringer et al., 2000). In this study, we used natural variation in the isotopic signature of soil water to provide insight into the potential between root depth and water acquisition (Figs. 4, 7, and 9). The isotopic signature of soil water observed in this study is determined by evaporation from the soil, precipitation, and irrigation. Because soil cores were collected 30 d after the last irrigation or rainfall, the surface soil water was isotopically enriched due to evaporation. For the subsoil water, the isotope signature could be attributed to the combination of the evaporation effect and the isotopic signatures of irrigation water and rainfall, resulting in a gradient of δ18O with soil depth (Fig. 9). Xylem water δ18O signatures showed that genotypes with reduced CCFN had lighter isotope signatures and greater dependency on deep soil water than genotypes with many cell files (Table III). The difference in the depths of root water acquisition between genotypes with reduced CCFN and many cell files could be attributed to their rooting depth (Figs. 4 and 7).
The additional benefit of reducing root costs in annual crops like maize is that extra resources from reduced root metabolic demand can contribute to crop yield by enhancing plant reproductive growth, since reproduction and roots are competing sinks for current photosynthate. In this study, we found that, irrespective of maize population, environment, soil type, and trial management, genotypes with reduced CCFN had both greater shoot biomass and grain yield than genotypes with many cell files under water stress (Figs. 6, 9, C–F, and 10, C–F). These results support the hypothesis that genotypes with less costly root tissue could develop the extensive, deep root systems required to fully utilize soil water resources in drying soil without as much yield penalty.
The physiological utility of a phene may depend on interactions with other phenes in integrated phenotypes (York et al., 2013). These interactions among phenes could result in synergistic or antagonistic effects on resource acquisition. Understanding phene synergisms is essential in developing ideotypes for breeding crops with greater tolerance of edaphic stress. We also recognize that a phene can be beneficial for multiple stresses. Root phenes, such as reduced CCFN, that influence the metabolic cost of soil exploration may be important to plants in low-input systems by increasing rooting depth. Rooting depth is important for the acquisition of mobile nutrients, including nitrate and sulfate, particularly in soils with high leaching potential. Evidence for this comes from modeling studies, where it has been shown that deeper roots could significantly improve the acquisition of nitrogen (Dunbabin et al., 2004; Postma and Lynch, 2011b; Dathe et al., 2013). Reduced CCFN also may affect root hydraulic conductivity, because a smaller radial path is associated with greater hydraulic conductivity and, consequently, greater water acquisition (Rieger and Litvin, 1999). We anticipate that root radial hydraulic conductivity would increase with reduced CCFN. Reduced CCFN may exhibit tradeoffs when soil hardness restricts root penetration, since the capacity to penetrate hard soil is associated with larger root diameter (Materechera et al., 1992; Bengough et al., 2006, 2011). Further research is needed to understand these potential tradeoffs and synergisms before the deployment of this trait in crop improvement programs.
In this study, we have demonstrated the utility of reduced CCFN for maize under water stress. We propose that the utility of reduced CCFN may be applicable to the acquisition of nitrogen in leaching environments and should be applicable to other plant species, especially graminaceous species lacking secondary root growth, including rice (Oryza sativa), wheat (Triticum aestivum), barley (Hordeum vulgare), oat (Avena sativa), sorghum (Sorghum bicolor), and millet (Pennisetum glaucum).
These results support the hypothesis that phenes and phene states that reduce the metabolic cost of soil exploration improve the acquisition of limiting soil resources (Lynch and Ho, 2005; Lynch, 2014; Lynch et al., 2014). Such phenes include the production of an optimal number of root axes, biomass allocation to metabolically efficient root classes, and reduced tissue respiration (Miller et al., 2003; Jaramillo et al., 2013; Lynch, 2014; Saengwilai et al., 2014b). CCFN is an example of the third category (i.e. an anatomical phene that affects the metabolic costs of soil exploration by affecting tissue respiration). Another example in this category is RCA, which destroys living cortical cells. Maize genotypes with abundant RCA have reduced root respiration, greater rooting depth, greater water acquisition under drought (Zhu et al., 2010), greater nitrogen acquisition under nitrogen limitation (Postma and Lynch, 2011b; Saengwilai et al., 2014a), and greater P and K acquisition in soils with suboptimal availability of those resources (Postma and Lynch, 2011b). Similarly, maize genotypes with greater cortical cell size have less root respiration, greater rooting depth, and greater water acquisition under drought (Chimungu et al., 2014). The deployment of root phenotypes with greater metabolic efficiency of soil exploration represents a novel, unexploited paradigm to develop crops with greater resource efficiency and resilience (Lynch, 2014).
MATERIALS AND METHODS
Plant Materials
Maize (Zea mays) genotypes from IBM, NyH populations, and advanced lines from the Malawi maize breeding program were utilized in this study. The IBM lines are from the intermated population of B73 × Mo17 and were obtained from Shawn Kaeppler at the University of Wisconsin and designated as Mo (Supplemental Table S1). The NyH lines are from the Ny821 × H99 population (also obtained from Shawn Kaeppler). Based on previous experiments carried out under optimum growing conditions (Burton et al., 2014), a subset of 15 IBM lines and 11 NyH lines was selected to assess the phenotypic variation of CCFN and its impact on root respiration (GH1). A set of six IBM lines contrasting in CCFN was used in 2011 experiments (GH2 and PA2011), and another set of six IBM lines also contrasting in CCFN was used for 2012 experiments (GH3 and PA2012), to evaluate the utility of CCFN under water-limited conditions (Supplemental Table S1). In Malawi, a set of 70 breeding lines was used to assess the phenotypic variation of CCFN in Malawian germplasm (MW2011). These lines originated from the Malawi national maize breeding program and were selected to represent a broad range of gene pools. A subset of 33 lines contrasting in CCFN was used in two field experiments (BU2012 and CH2012) to evaluate the utility of CCFN under water-limited conditions and across sites in Malawi (Supplemental Table S1). In all experiments, genotypes were classified as reduced CCFN (10 or fewer cell files) and as many CCFN (13 or more cell files; Supplemental Table S1).
Greenhouse Experiments
Three experiments were conducted under the same conditions in 2 consecutive years (GH1–GH3; Supplemental Table S1). The experiments were conducted in a greenhouse at University Park, Pennsylvania (40°48′N, 77°51′W), under constant conditions (14-h/10-h day/night, 23°C/20°C day/night, 40%–70% relative humidity) with maximum illumination of 1,200 μmol photons m−2 s−1; additional light was provided when necessary with 400-W metal-halide bulbs (Energy Technics). Plants were grown in mesocosms (Supplemental Fig. S2) consisting of polyvinyl chloride cylinders 1.5 m in height by 0.15 m in diameter, with plastic liners made of 4-mil (0.116 mm thick) transparent high-density polyethylene film, which was used to facilitate root sampling. The growth medium consisted of 50% (v/v) commercial grade sand (Quikrete), 35% (v/v) vermiculite (Whittemore), 5% (v/v) perlite (Whittemore), and 10% (v/v) topsoil (Hagerstown silt loam top soil [fine, mixed, mesic Typic Hapludalf]). Mineral nutrients were provided by mixing the medium with 70 g per column of Osmocote Plus fertilizer consisting of (in w/w) 15% nitrogen, 9% phosphorus, 12% potassium, 2.3% sulfur, 0.02% boron, 0.05% copper, 0.68% iron, 0.06% manganese, 0.02% molybdenum, and 0.05% zinc (Scotts-Sierra Horticultural Products) for each column. The seeds were germinated by placing them in darkness at 28°C ± 1°C in a germination chamber for 2 d prior to transplanting two seedlings per mesocosm and thinned to one uniform seedling per mesocosm 5 d after planting.
At harvest, the shoot was removed, and the plastic liner was pulled out of the polyvinyl chloride column and placed on a washing bench. The plastic liner was cut open, and the roots were washed carefully by rinsing the medium away with water. This allowed us to recover the entire plant root system. Samples for root respiration measurement were collected 10 to 20 cm from the base of three representative second whorl crown roots per plant. Root respiration (CO2 production) was measured using an infrared gas analysis system (LI-6400; LI-COR) equipped with a custom 56-mL closed chamber of plastic tubing (1.5-cm diameter) having connection points sealed with silicon grease. The change in CO2 concentration in the chamber was monitored for 3 min. During the time of measurement, the chamber was placed in a temperature-controlled water bath at 27°C ± 1°C to maintain constant temperature. Following respiration measurements, root segments were preserved in 75% ethanol for anatomical analysis as described below.
Root length distribution was measured by cutting the root system into seven segments of 20-cm depth increments. Roots from each increment were spread in a 5-mm layer of water in transparent plexiglass trays and imaged with a flatbed scanner equipped with top lighting (Epson Perfection V700 Photo; Epson America) at a resolution of 23.6 pixels mm−1 (600 dots per inch). Total root length for each segment was quantified using WinRhizo Pro (Regent Instruments). Following scanning, the roots were dried at 70°C for 72 h and weighed. To summarize the vertical distribution of the root length density, we used the D95 (Schenk and Jackson, 2002). Specific root length was calculated by dividing root length by corresponding weight.
Root segments were ablated using laser ablation tomography (B. Hall and J.P. Lynch, unpublished data) to obtain images for anatomical analysis. In brief, laser ablation tomography is a semiautomated system that uses a laser beam (Avia 7000; 355-nm pulsed laser) to vaporize or sublimate the root at the camera focal plane ahead of an imaging stage. The sample is incremented, vaporized, or sublimated and imaged simultaneously. The cross-section images were taken using a Canon T3i camera with a 5× micro lens (MP-E 65 mm) on the laser-illuminated surface. Root images were analyzed using RootScan, an image-analysis tool developed for analyzing root anatomy (Burton et al., 2012). CCFN was determined from three different images per root segment. CCFN was obtained by counting the cell layers from the epidermis to the endodermis.
Experiment I (GH1)
The aim of this experiment was to assess the relationship between phenotypic variation for CCFN and root respiration. The experiment was laid out as a randomized complete block design, replicated three times with time of planting as a blocking factor. A set of 26 genotypes was planted in the mesocosms, and water stress was imposed by withholding water starting 14 d after planting. Plants were harvested for root respiration measurements and anatomical analysis 35 d after planting.
Experiments II (GH2) and III (GH3)
Two experiments were conducted, one in fall 2011 (GH2) and one in summer 2012 (GH3), and were laid out as a randomized complete block design, replicated four times with time of planting as a blocking factor. Planting was staggered by 7 d. A set of six genotypes contrasting in CCFN was planted in each experiment (Supplemental Table S1). These genotypes were selected based on phenotypes from previous experiments (Burton et al., 2014; Chimungu, 2014). In both experiments, the irrigated mesocosms (control) each received 200 mL of water every other day to replenish water lost by evapotranspiration; in stressed mesocosms, water application was withheld starting 5 d after planting to allow the plants to exploit residual moisture to simulate terminal drought. Leaf gas exchange of the third fully expanded leaves was measured with a LI-6400 infrared gas analyzer (LI-COR) using a red-blue light at photosynthetically active radiation intensity of 1,200 μmol photons m−2 s−1 and constant CO2 concentration of 400 µL L−1 28 d after planting. The measurements were done between 9 and 11 am. Plants were harvested 30 d after planting for root respiration measurements, root growth distribution, and shoot biomass. The dry matter of the shoot and root was measured after drying at 70°C for 72 h, and root length distribution was determined as described above.
Field Experiments
Assessing Phenotypic Variation of CCFN (MW2011)
The experiment was conducted at Bunda College research farm in Lilongwe, Malawi (33°48′E, 14°10′S), in 2011 under optimal conditions (i.e. the plots were rainfed but only rarely were they severely moisture stressed). The soil is Lilongwe series sandy clay loam (Oxic Rhodustalf). The experiment was arranged as a randomized complete block design with three replications. Each plot consisted of a single 6-m-long row with 25 plants. Root crowns were excavated by shovelomics (Trachsel et al., 2011). In brief, roots were excavated by removing a soil cylinder 30 to 40 cm in diameter with the shoot at its center and a depth of 20 to 30 cm. The excavated root crowns were shaken briefly to remove a large fraction of the soil adhering to the root crown. The root crowns were immersed in soapy water for 5 to 10 min in order to facilitate removal of the remaining soil. Three 8-cm root segments were collected 10 to 20 cm from the base of a representative second whorl crown root of each plant and used to assess CCFN. The segments were preserved in 75% ethanol before being processed as described above.
Utility of CCFN under Water Stress
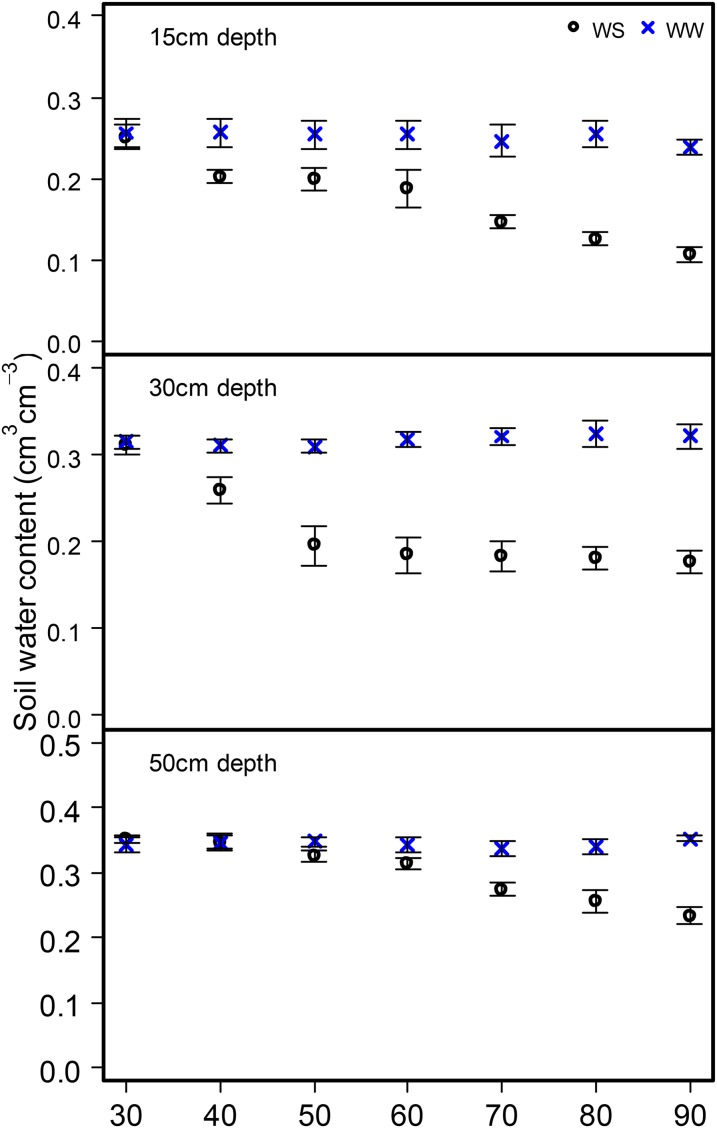
In the United States, two experiments were conducted in rainout shelters (Supplemental Fig. S3) located at the Russell E. Larson Agricultural Research Center in Rock Springs, Pennsylvania (40°42′N, 77°57′W), during the summers of 2011 (PA2011) and 2012 (PA2012). In Malawi, experiments were conducted at the Chitala Agriculture Research station of the Ministry of Agriculture in Salima (13°28′S, 33°59′E; CH2012) and the Bunda Research Farm of the Lilongwe University of Agriculture and Natural Resources (14°10′S, 33°48′E; BU2012). The soil is a Hagerstown silt loam (fine, mixed, mesic Typic Hapludalf) in Rock Springs and sandy clay loam (Oxic Rhodustalf) at both Chitala and Bunda. The experiments (PA2011, PA2012, CH2012, and BU2012) were arranged as a randomized complete block split-plot design with four replications. The main plots were composed of two moisture regimes, and the subplots contained genotypes contrasting in CCFN in each experiment. The plants were hand planted on June 15, 2011, and June 25, 2012, in Rock Springs and on September 3 and 4 in Bunda and Chitala, respectively. In Rock Springs, each subplot consisted of three rows, with each row being 2.5 m long, with 25 cm between plants and 75 cm between rows. In Malawi, the experiments were planted in single 6-m row plots with 25- and 75-cm spacing between planting stations and rows, respectively. The drought treatment was initiated starting 35 to 40 d after planting using an automated rainout shelter in Rock Springs and by withholding water application in Malawi. The shelters (10 × 30 m) were covered with a clear greenhouse plastic film (0.184 mm) and were automatically triggered by rainfall to cover the plots, excluding natural precipitation. Adjacent nonsheltered control plots were rainfed and drip irrigated when necessary to maintain the soil moisture close to field capacity throughout the growing season. At each location, the recommended fertilizer rate was applied before planting. Soil water content for both well-watered and water-stressed treatments was monitored regularly during the experiment (Fig. 11). Soil water content was monitored using the TRIME FM system (IMKO Micromodultechnik) at three depths (20, 35, and 50 cm) at six points inside the shelter and three points outside the shelter. Seven readings were taken between 30 and 120 d after planting.
Figure 11.
Effect of drought treatment on soil volumetric water content at 15-, 30-, and 50-cm depths both in well-watered (WW) and water-stressed (WS) conditions in the rainout shelters at Rock Springs, Pennsylvania (PA2012). Points are means ± se of six measurements in the rainout shelter and three measurements in well-watered plots. Terminal drought was imposed in water-stressed plots beginning at 30 d after planting. [See online article for color version of this figure.]
Plant Measurements
In all field experiments, midday leaf RWC was measured and used as a physiological indicator of plant water status. To measure leaf RWC, fresh leaf discs (3 cm in diameter) were collected from the third fully expanded leaf for three representative plants per plot 60 d after planting and weighed immediately to determine fresh weight (FW). After this, the discs were immediately hydrated to full turgidity (6 h) by soaking them in distilled water. Following soaking, the discs were blotted dry and again weighed to determine turgid weight (TW). Discs were then oven dried at 70°C for 72 h, and dry weight (DW) was determined. Leaf RWC was calculated according to the following equation:
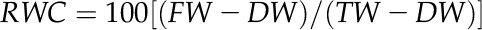 |
In PA2011 and PA2012, soil cores were collected 80 d after planting to determine root distribution in the soil profile. A soil coring tube (Giddings Machine) 5.1 cm in diameter and 60 cm long was used for sampling; the core was taken midway between the plants within a row. The cores were sectioned into six segments of 10 cm depth increments and washed. Subsequently, the washed roots were scanned using a flatbed scanner (Epson Perfection V700 Photo; Epson America) at a resolution of 23.6 pixels mm−1 (600 dots per inch) and analyzed using the image-processing software WinRhizo Pro (Regent Instruments). Root distribution in the soil profiles was calculated as described above.
Shoots and roots were evaluated 75 d after planting. To accomplish this, three representative plants in each plot were cut at soil level. The collected shoot material was dried at 70°C for 72 h and weighed. Root crowns were excavated by shovelomics (Trachsel et al., 2011). Three 8-cm root segments were collected 10 to 20 cm from the base of a representative second whorl crown root of each plant for anatomical analysis. The segments were preserved in 75% alcohol before being processed as described above. At physiological maturity, grain yield was collected for each plot.
Soil and Plant Sampling of δ18O Analysis
In PA2011, soil samples were collected adjacent to plants in the rainout shelter 65 d after planting using a 5-cm diameter soil core. Soil cores were taken to the maximum achievable depth of 60 cm. The cores were immediately separated into 10-cm increments: 10, 20, 30, 40 50, and 60 cm. The corresponding maize stems were collected at the same time when soil was sampled, approximately 8 to 10 cm of the stem was collected just above ground level, and the epidermis was immediately removed. Soil and maize stem samples were put in snap vials, sealed with Parafilm to prevent evaporation, and refrigerated immediately. Cryogenic vacuum distillation (West et al., 2006; Koeniger et al., 2010) was used to extract soil water and crop stem water. In cryogenic vacuum distillation, two glass tubes were attached to a vacuum pump. The sample was placed in one tube and frozen by submerging the tube in liquid nitrogen, and then both tubes were evacuated by vacuum pump to create a closed U-shape configuration. After that, the tube containing the sample was heated, while the collection tube was still immersed in liquid nitrogen to catch the vapor. Samples were weighed and oven dried after extraction to ensure that the extraction time was sufficient to vaporize all the water in the samples. The water samples were analyzed at the Pennsylvania State Institutes of Energy and the Environment. Stable isotopic analyses were performed using the L2130-i δD/δ18O Ultra High Precision Isotopic Water Analyzer (Picarro). Results were expressed as parts per thousand deviations from the Vienna Standard Mean Ocean Water. To determine the percentage contribution of soil water at depth to the signature of water within the plant’s xylem, an isotopic mixing model was used (Phillips et al., 2005). IsoSource version 1.3.1 (Phillips and Gregg, 2003) was used to evaluate the relative contribution of each soil layer to plant xylem water signature. The fractional increment was set at 1%, and tolerance was set at 0.1.
Data Analysis
The data from each year were analyzed separately, considering that different sets of genotypes were used. For greenhouse data, for comparisons of genotypes, irrigation levels, and their interaction effects, a two-way ANOVA was used. Field data were analyzed as randomized complete block split-plot designs to determine the presence of significant effects due to irrigation level, genotype, and interaction effects on the measured and calculated parameters. Mean separation of genotypes for the different parameters was performed by Tukey’s honestly significant difference test. Unless noted otherwise, honestly significant difference 0.05 values were only reported when the F test was significant at P ≤ 0.05. Linear regression analysis was used to establish relationships between CCFN and measured and calculated parameters.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Root length density at different soil depths.
Supplemental Figure S2. Greenhouse mesocosms.
Supplemental Figure S3. Automated rainout shelter facility.
Supplemental Table S1. Explanation of experiments, treatments, and genotypes.
Supplementary Material
Acknowledgments
We thank Jennifer Yang and Eric Nord for critical reading of the article and Karol E. Confer, Robert Synder, Moses Maliro, Patson Nalivata, and Andrew Mkopoliwa for technical assistance in the course of this study.
Glossary
- RCA
root cortical aerenchyma
- LCA
living cortical area
- CCFN
cortical cell file number
- RIL
recombinant inbred line
- SRL
specific root length
- D95
depth above which 95% of total root length is located in the soil profile
- RWC
relative water content
Footnotes
This work was supported by the National Science Foundation/Basic Research to Enhance Agricultural Development (grant no. 4184–UM–NSF–5380) and the Agriculture and Food Research Initiative of the U.S. Department of Agriculture National Institute of Food and Agriculture (grant no. 2014–67013–2157).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Araus JL, Slafer GA, Reynolds MP, Royo C (2002) Plant breeding and drought in C3 cereals: what should we breed for? Ann Bot (Lond) 89: 925–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araus JL, Slafer GA, Royo C, Serret MD (2008) Breeding for yield potential and stress adaptation in cereals. CRC Crit Rev Plant Sci 27: 377–412 [Google Scholar]
- Battisti DS, Naylor RL (2009) Historical warnings of future food insecurity with unprecedented seasonal heat. Science 323: 240–244 [DOI] [PubMed] [Google Scholar]
- Baum SF, Dubrovsky JG, Rost TL (2002) Apical organization and maturation of the cortex and vascular cylinder in Arabidopsis thaliana (Brassicaceae) roots. Am J Bot 89: 908–920 [DOI] [PubMed] [Google Scholar]
- Bayuelo-Jiménez JS, Gallardo-Valdéz M, Pérez-Decelis VA, Magdaleno-Armas L, Ochoa I, Lynch JP (2011) Genotypic variation for root traits of maize (Zea mays L.) from the Purhepecha Plateau under contrasting phosphorus availability. Field Crops Res 121: 350–362 [Google Scholar]
- Bengough AG, Bransby MF, Hans J, McKenna SJ, Roberts TJ, Valentine TA (2006) Root responses to soil physical conditions: growth dynamics from field to cell. J Exp Bot 57: 437–447 [DOI] [PubMed] [Google Scholar]
- Bengough AG, McKenzie BM, Hallett PD, Valentine TA (2011) Root elongation, water stress, and mechanical impedance: a review of limiting stresses and beneficial root tip traits. J Exp Bot 62: 59–68 [DOI] [PubMed] [Google Scholar]
- Burke MB, Lobell DB, Guarino L (2009) Shifts in African crop climates by 2050, and the implications for crop improvement and genetic resources conservation. Glob Environ Change 19: 317–325 [Google Scholar]
- Burton AL, Brown KM, Lynch JP (2013) Phenotypic diversity of root anatomical and architectural traits in Zea species. Crop Sci 53: 1042–1055 [Google Scholar]
- Burton AL, Johnson J, Foerster J, Hanlon MT, Kaeppler SM, Lynch JP, Brown KM (October 19, 2014) QTL mapping and phenotypic variation of root anatomical traits in maize (Zea mays L.). Theor Appl Genet. http://dx.doi.org/10.1007/s00122-014-2414-8 [DOI] [PubMed] [Google Scholar]
- Burton AL, Williams MS, Lynch JP, Brown KM (2012) RootScan: software for high-throughput analysis of root anatomical traits. Plant Soil 357: 189–203 [Google Scholar]
- Chapman K, Groot EP, Nichol SA, Rost TL (2003) Primary root growth and the pattern of root apical meristem organization are coupled. J Plant Growth Regul 21: 287–295 [Google Scholar]
- Chimungu JG (2014) Root anatomical traits for efficient water acquisition under drought in maize (Zea mays L.). PhD thesis. Pennsylvania State University, University Park [Google Scholar]
- Chimungu JG, Brown KM, Lynch JP (2014) Large root cortical cell size improves drought tolerance in maize. Plant Physiol 166: 2166–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudert Y, Périn C, Courtois B, Khong NG, Gantet P (2010) Genetic control of root development in rice, the model cereal. Trends Plant Sci 15: 219–226 [DOI] [PubMed] [Google Scholar]
- Dathe A, Postma JA, Lynch JP (2013) Modeling resource interactions under multiple edaphic stresses. In D Timlin, LR Ahuja, eds, Enhancing Understanding and Quantification of Soil–Root Growth Interactions. ASA-CSSA-SSSA, Madison, WI, pp 273–294 [Google Scholar]
- Dawson TE, Pate JS (1996) Seasonal water uptake and movement in root systems of Australian phraeatophytic plants of dimorphic root morphology: a stable isotope investigation. Oecologia 107: 13–20 [DOI] [PubMed] [Google Scholar]
- Dunbabin V, Rengel Z, Diggle AJ (2004) Simulating form and function of root systems: efficiency of nitrate uptake is dependent on root system architecture and the spatial and temporal variability of nitrate supply. Funct Ecol 18: 204–211 [Google Scholar]
- Ehleringer JR, Dawson TE (1992) Water uptake by plants: perspectives from stable isotope composition. Plant Cell Environ 15: 1073–1082 [Google Scholar]
- Ehleringer JR, Roden J, Dawson TE (2000) Assessing ecosystem-level water relations through stable isotopes ratio analyses. In OE Sala, RB Jackson, HA Mooney, RW Howarth, eds, Methods in Ecosystem Science. Springer-Verlag, Berlin, pp 181–198 [Google Scholar]
- Eissenstat DM. (1992) Costs and benefits of constructing roots of small diameter. J Plant Nutr 15: 763–782 [Google Scholar]
- Eissenstat DM, Volder A, Caldwell MM, Heldmaier G, Jackson RB, Lange OL, Mooney HA, Schultze ED, Sommer U (2005) The efficiency of nutrient acquisition over the life of a root. In H BassiriRad, ed, Nutrient Acquisition by Plants: An Ecological Perspective. Springer-Verlag, Berlin, pp 185–220 [Google Scholar]
- Eissenstat DM, Yanai RD (1997) The ecology of root lifespan. Adv Ecol Res 27: 1–60 [Google Scholar]
- Esau K (1965) Plant Anatomy. John Wiley, New York [Google Scholar]
- Evans DE. (2004) Aerenchyma formation. New Phytol 161: 35–49 [Google Scholar]
- Fan M, Zhu J, Richards C, Brown KM, Lynch JP (2003) Physiological roles for aerenchyma in phosphorus-stressed roots. Funct Plant Biol 30: 493. [DOI] [PubMed] [Google Scholar]
- Hall JL, Sexton R, Baker DA (1971) Metabolic changes in washed, isolated steles. Planta 96: 54–61 [DOI] [PubMed] [Google Scholar]
- Hammer GL, Dong Z, McLean G, Doherty A, Messina C, Schussler J, Zinselmeier C, Paszkiewicz S, Cooper M (2009) Can changes in canopy and/or root system architecture explain historical maize yield trends in the U.S. corn belt? Crop Sci 49: 299 [Google Scholar]
- Ho MD, Rosas JC, Brown KM, Lynch JP (2005) Root architectural trade-offs for water and phosphorus acquisition. Funct Plant Biol 32: 737–748 [DOI] [PubMed] [Google Scholar]
- Hochholdinger F (2009) The maize root system: morphology, anatomy, and genetics. In JL Bennetzen, SC Hake, eds, Handbook of Maize: Its Biology. Springer, New York, pp 145–160 [Google Scholar]
- Jaramillo RE, Nord EA, Chimungu JG, Brown KM, Lynch JP (2013) Root cortical burden influences drought tolerance in maize. Ann Bot (Lond) 112: 429–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeniger P, Leibundgut C, Link T, Marshall JD (2010) Stable isotopes applied as water tracers in column and field studies. Org Geochem 41: 31–40 [Google Scholar]
- Lambers H. (1979) Efficiency of root respiration in relation to growth rate, morphology and soil composition. Physiol Plant 46: 194–202 [Google Scholar]
- Lambers H, Atkin OK, Millenaar FF (2002) Respiratory patterns in roots in relation to their functioning. In Y Waisel, A Eshel, K Kafkaki, eds, Plant Roots, Hidden Half, Ed 3. Marcel Dekker, New York, pp 521–552 [Google Scholar]
- Lobell DB, Bänziger M, Magorokosho C, Vivek B (2011) Nonlinear heat effects on African maize as evidenced by historical yield trials. Nat Clim Chang 1: 42–45 [Google Scholar]
- Ludlow MM, Muchow RC (1990) A critical evaluation of traits for improving crop yields in water-limited environments. Adv Agron 43: 107–153 [Google Scholar]
- Lux A, Luxova M, Abe J (2004) Root cortex: structural and functional variability and responses to environmental stress. Root Res 13: 117–131 [Google Scholar]
- Lynch JP. (2007a) Roots of the second green revolution. Aust J Bot 55: 493–512 [Google Scholar]
- Lynch JP. (2007b) Rhizoeconomics: the roots of shoot growth limitations. HortScience 42: 1107–1109 [Google Scholar]
- Lynch JP. (2011) Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiol 156: 1041–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. (2013) Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann Bot (Lond) 112: 347–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. (November 17, 2014) Root phenes that reduce the metabolic costs of soil exploration: opportunities for 21(st) century agriculture. Plant Cell Environ http://dx.doi.org/10.1111/pce.12451 [DOI] [PubMed] [Google Scholar]
- Lynch JP, Brown KB (2008) Root strategies for phosphorus acquisition. In PJ White, JP Hammond, eds, The Ecophysiology of Plant-Phosphorus Interaction. Springer Science, New York, pp 83–116 [Google Scholar]
- Lynch JP, Chimungu JG, Brown KM (2014) Root anatomical phenes associated with water acquisition from drying soil: targets for crop improvement. J Exp Bot 65: 6155–6166 [DOI] [PubMed] [Google Scholar]
- Lynch JP, Ho MD (2005) Rhizoeconomics: carbon costs of phosphorus acquisition. Plant Soil 269: 45–56 [Google Scholar]
- Lynch JP, Nielsen KL, Davis RD, Jablokow AG (1997) SimRoot: modelling and visualization of root systems. Plant Soil 188: 139–151 [Google Scholar]
- Manschadi AM, Christopher J, deVoil P, Hammer GL (2006) The role of root architectural traits in adaptation of wheat to water-limited environments. Funct Plant Biol 33: 823. [DOI] [PubMed] [Google Scholar]
- Materechera SA, Alston AM, Kirby JM, Dexter AR (1992) Influence of root diameter on the penetration of seminal roots into a compacted subsoil. Plant Soil 144: 297–303 [Google Scholar]
- Miller CR, Ochoa I, Nielsen KL, Beck D, Lynch JP (2003) Genetic variation for adventitious rooting in response to low phosphorus availability: potential utility for phosphorus acquisition from stratified soils. Funct Plant Biol 30: 973–985 [DOI] [PubMed] [Google Scholar]
- Mishra V, Cherkauer KA (2010) Retrospective droughts in the crop growing season: implications to corn and soybean yield in the midwestern United States. Agric Meteorol 150: 1030–1045 [Google Scholar]
- Peng S, Eissenstat DM, Graham JH, Williams K, Hodge NC (1993) Growth depression in mycorrhizal citrus at high-phosphorus supply (analysis of carbon costs). Plant Physiol 101: 1063–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DL, Gregg JW (2003) Source partitioning using stable isotopes: coping with too many sources. Oecologia 136: 261–269 [DOI] [PubMed] [Google Scholar]
- Phillips DL, Newsome SD, Gregg JW (2005) Combining sources in stable isotope mixing models: alternative methods. Oecologia 144: 520–527 [DOI] [PubMed] [Google Scholar]
- Postma JA, Lynch JP (2011a) Theoretical evidence for the functional benefit of root cortical aerenchyma in soils with low phosphorus availability. Ann Bot (Lond) 107: 829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma JA, Lynch JP (2011b) Root cortical aerenchyma enhances the growth of maize on soils with suboptimal availability of nitrogen, phosphorus, and potassium. Plant Physiol 156: 1190–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger M, Litvin P (1999) Root system hydraulic conductivity in species with contrasting root anatomy. J Exp Bot 50: 201–209 [Google Scholar]
- Saengwilai P (2013) Root traits for efficient nitrogen acquisition and genome-wide association study of root anatomical traits in maize (Zea mays L.). PhD thesis. Pennsylvania State University, University Park [Google Scholar]
- Saengwilai P, Nord EA, Brown KM, Lynch JP (2014a) Root cortical aerenchyma enhances nitrogen acquisition from low nitrogen soils in maize. Plant Physiol 156: 1190–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saengwilai P, Tian X, Lynch JP (2014b) Low crown root number enhances nitrogen acquisition from low-nitrogen soils in maize. Plant Physiol 166: 581–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk HJ, Jackson RB (2002) The global biogeography of roots. Ecol Monogr 72: 311–328 [Google Scholar]
- Schmidhuber J, Tubiello FN (2007) Global food security under climate change. Proc Natl Acad Sci USA 104: 19703–19708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachsel S, Kaeppler SM, Brown KM, Lynch JP (2011) Shovelomics: high throughput phenotyping of maize (Zea mays L.) root architecture in the field. Plant Soil 314: 75–87 [Google Scholar]
- Van der Werf A, Kooijman A, Welschen R, Lambers H (1988) Respiratory energy costs for the maintenance of biomass, for growth and for ion uptake in roots of Carex diandra and Carex acudformis. Physiol Plant 72: 483–491 [Google Scholar]
- Wahl S, Ryser P (2000) Root tissue structure is linked to ecological strategies of grasses. New Phytol 148: 459–471 [DOI] [PubMed] [Google Scholar]
- West AG, Patrickson SJ, Ehleringer JR (2006) Water extraction times for plant and soil materials used in stable isotope analysis. Rapid Commun Mass Spectrom 20: 1317–1321 [DOI] [PubMed] [Google Scholar]
- Yanai RD, Fahey TJ, Miller SL (1995) Efficiency of nutrient acquisition by fine roots and mycorrhizae. In WK Smith, TM Hinckley, eds, Resource Physiology of Conifers. Academic Press, New York, pp 75–103 [Google Scholar]
- York LM, Nord EA, Lynch JP (2013) Integration of root phenes for soil resource acquisition. Front Plant Sci 4: 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Brown KM, Lynch JP (2010) Root cortical aerenchyma improves the drought tolerance of maize (Zea mays L.). Plant Cell Environ 33: 740–749 [DOI] [PubMed] [Google Scholar]
- Zhu J, Lynch JP (2004) The contribution of lateral rooting to phosphorus acquisition efficiency in maize (Zea mays L.) seedlings. Funct Plant Biol 31: 949–958 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.