Microtubules participate in endoplasmic reticulum (ER) network formation by anchoring ER to cell cortex and supporting slow ER tubules extension.
Abstract
The endoplasmic reticulum (ER) is a network of tubules and sheet-like structures in eukaryotic cells. Some ER tubules dynamically change their morphology, and others form stable structures. In plants, it has been thought that the ER tubule extension is driven by the actin-myosin machinery. Here, we show that microtubules also contribute to the ER tubule extension with an almost 20-fold slower rate than the actin filament-based ER extension. Treatment with the actin-depolymerizing drug Latrunculin B made it possible to visualize the slow extension of the ER tubules in transgenic Arabidopsis (Arabidopsis thaliana) plants expressing ER-targeted green fluorescent protein. The ER tubules elongated along microtubules in both directions of microtubules, which have a distinct polarity. This feature is similar to the kinesin- or dynein-driven ER tubule extension in animal cells. In contrast to the animal case, ER tubules elongating with the growing microtubule ends were not observed in Arabidopsis. We also found the spots where microtubules are stably colocalized with the ER subdomains during long observations of 1,040 s, suggesting that cortical microtubules contribute to provide ER anchoring points. The anchoring points acted as the branching points of the ER tubules, resulting in the formation of multiway junctions. The density of the ER tubule junction positively correlated with the microtubule density in both elongating cells and mature cells of leaf epidermis, showing the requirement of microtubules for formation of the complex ER network. Taken together, our findings show that plants use microtubules for ER anchoring and ER tubule extension, which establish fine network structures of the ER within the cell.
The endoplasmic reticulum (ER) is a complex network composed of tubules and sheet structures. The ER network’s morphology changes dynamically by elongation and shrinkage of tubules, sheet expansion, and sliding junctions. For example, an ER tubule elongates straight forward from a cisterna and subsequently, fuses to another cisterna, producing a linkage between two cisternae. If an elongating tubule fails to fuse to another cisterna, the tubule contracts into the original cisterna. However, the ER has stable anchoring points that associate with other cellular structures, such as the plasma membrane or cytoskeleton. When an elongating ER tubule reaches an association point, it forms a stable ER anchor (i.e. establishment of the ER anchoring points forms stable ER tubules). Hence, increasing the number of ER anchoring points produces fine ER meshwork.
ER dynamics in eukaryotes depend on the cytoskeleton. In plants, major contributors for ER organization are actin filaments (Quader et al., 1989; Knebel et al., 1990; Lichtscheidl and Hepler, 1996; Sparkes et al., 2009a) and the actin-associated motor proteins (myosins; Prokhnevsky et al., 2008; Peremyslov et al., 2010; Ueda et al., 2010). However, it had generally been thought that microtubules are not involved in ER organization in plants, because microtubule-depolymerizing drugs do not induce obvious changes in the ER network (Quader et al., 1989; Knebel et al., 1990; Lichtscheidl and Hepler, 1996; Sparkes et al., 2009a). Nevertheless, involvement of microtubules in plant ER organization has been suspected from several electron microscopy observations that showed microtubules located close to the ER membrane in Vicia faba guard cells, Nicotiana alata pollen tubes, and Funaria hygrometrica caulonemata (Lancelle et al., 1987; Hepler et al., 1990; McCauley and Hepler, 1992).
Foissner et al. (2009) have suggested that microtubules are involved in motility and orientation of cortical ER in Characean algae (Nitella translucens, Nitella flexilis, Nitella hyalina, and Nitella pseudoflabellata) internodal cells. Characean cortical ER is spatially separated from inner cytoplasmic streaming by the middle layer of fixed chloroplasts. The cortical ER forms a tight meshwork of predominantly transverse ER tubules that frequently coalign with microtubules, and microtubule depolymerization reduces the transverse ER tubules and increases mesh size (Foissner et al., 2009). Consistently, Hamada et al. (2012) have shown in Arabidopsis (Arabidopsis thaliana) that microtubule depolymerization increases mesh size in young elongating cells. In addition, stable ER tubule junctions are often colocalized with cortical microtubules (Hamada et al., 2012), suggesting that microtubules stabilize ER tubule junctions to form fine ER meshes. Oryzalin-induced ER nodulation (Langhans et al., 2009) was not observed in our experimental conditions.
Here, we showed that ER tubules elongate along microtubules in plant cells. In addition, we revealed that the ER is stably anchored to defined points on cortical microtubules. The stable anchoring points are the basis of various ER shapes, such as three-way, two-way, or dead-end ER tubules. These microtubule-ER interactions, together with the actin-myosin system, contribute to ER network organization.
RESULTS
Actin Filament-Independent ER Tubule Extension
We generated Arabidopsis plants that expressed both lifeact-venus and tag Red Fluorescent Protein (RFP) with a signal peptide (SP) at the N-terminus and an ER retention signal at the C-terminus (SP-tagRFP-HDEL) to visualize actin filaments and the ER, respectively. We took time-lapse images to analyze dynamic ER organization based on actin filaments. Orientation of actin filaments and ER morphology dramatically changed every 2 s (Fig. 1A; Supplemental Movie S1). From 4 to 6 s, an ER tubule elongated along an actin filament bundle (Fig. 1A, arrowheads). Next, we treated the transgenic plants with Latrunculin B (Lat B) for 30 min to depolymerize actin filaments. After Lat B treatment, all of the fine actin filaments disappeared, but immobile actin filament bundles still remained (Fig. 1B). It seems that these bundles were stabilized by the excess stabilization effects of lifeact-venus (van der Honing et al., 2011). We found that ER tubule extension still occurred in the presence of Lat B, although most ER movements were abolished (Fig. 1B). Interestingly, the ER tubule extension was observed in the area that lacked bundled actin filaments (Fig. 1B, arrowheads; Supplemental Movie S2). The elongating ER tubule is shown by red lines in Figure 1B. In addition, the velocity of the tubule extension in the presence of Lat B was obviously slower than that in the absence of Lat B (Fig. 1B; Supplemental Movie S2). We named the actin filament-independent ER tubule extension as slow ER tubule extension.
Figure 1.
Actin filament-independent ER tubule extension in the presence of Lat B. ER and actin filaments were observed in hypocotyl epidermal cells of 5-d-old Arabidopsis seedlings expressing SP-tagRFP-HDEL and lifeact-venus. A, ER tubule extension along actin filaments. B, ER tubule extension without actin filaments. Seedlings were treated with 0.1% dimethyl sulfoxide (DMSO; A) or 2 µm Lat B (B) for 30 min. Inactive bundled actin filaments that did not have normal actin dynamics were left in Lat B treatment. Photos were taken every 2 (A) or 5 s (B; corresponding to Supplemental Movies S1 and S2, respectively). Arrowheads show elongating ER tubules. The DSLT and drawing columns show illustrations of ER and actin filament dynamics corresponding to the AF and ER columns. Actin filaments and ER are shown in magenta and green, respectively, in the DSLT column and black and gray in the drawing column. Red lines in the drawing column indicate growing ER tubules. AF, Actin filament.
Slow ER Tubule Extension Occurs along Microtubules
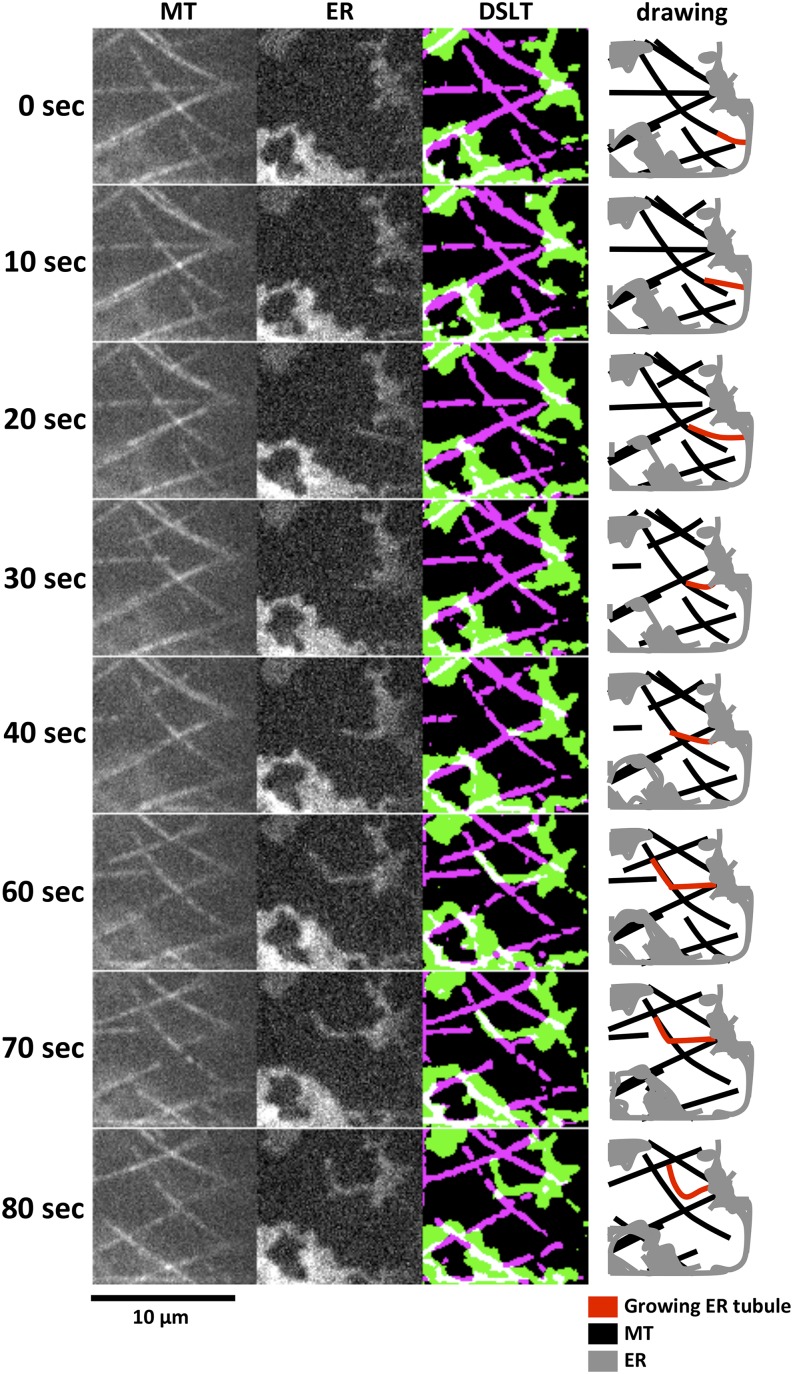
The straightforward movement of ER tubule extension implied involvement of other cytoskeletal components. Microtubules, which are abundant in the cortical region of plant cells, are a top candidate for the base of the slow ER tubule extension. To examine the relationship between microtubules and the slow ER tubule extension, we used Arabidopsis seedlings expressing both GFP-tubulin and SP-tagRFP-HDEL to visualize microtubules and the ER, respectively. In the presence of Lat B, an ER tubule emerged from an ER cisterna (0 s in Fig. 2; Supplemental Movie S3), elongated straightforward along microtubules (15 s in Fig. 2), and finally reached another ER cisterna (30 s in Fig. 2). Other ER tubules and sheets were mostly immobile during 50 s (Supplemental Movie S3). Importantly, all of the slow ER tubule extensions (as many as 67) in our 90-min observation period were along microtubules. For example, 11 ER tubule extensions occurred during a 10-min observation, and all of them were along microtubules (Supplemental Movie S4). The slow ER tubule extension along microtubules was occasionally observed, even in the absence of Lat B (Supplemental Fig. S1), suggesting that the slow ER tubule extension occurs in intact cells.
Figure 2.
ER tubule extension along cortical microtubules in the presence of Lat B. ER and microtubules were observed in hypocotyl epidermal cells of 5-d-old Arabidopsis seedlings expressing SP-tagRFP-HDEL and GFP-tub6. Seedlings were treated with 2 µm Lat B for 30 min. Most of the ER was immobile, but some was elongated along microtubules (arrowheads). Note that the tip of an elongating ER tubule was associated with a microtubule from 10 to 25 s. The speed of elongation was dramatically slower compared with the actin-driven ER tubule elongation. Still images corresponding to Supplemental Movie S3 are shown. The DSLT and drawing columns of ER dynamics correspond to the MT and ER columns. Microtubules and ER are shown in magenta and green, respectively, in the DSLT column and black and gray in the drawing column. Red lines in the drawing column indicate growing ER tubules. MT, Microtubule.
We tested the possibility of involvement of factors other than microtubules in the slow ER tubule extension. Seedlings were treated with Lat B and oryzalin (a microtubule-depolymerizing drug) to depolymerize both actin filaments and microtubules. ER tubules and sheets vibrated and changed their shape slowly; however, directional ER tubule elongation did not occur anymore (Supplemental Fig. S2; Supplemental Movie S5). Next, we checked the involvement of Golgi bodies in the slow ER tubule extension, because movements of Golgi bodies associated with ER can induce ER tubule extension (Sparkes et al., 2009b). We used transgenic Arabidopsis expressing both Endoplasmic Reticulum Retention Defective2 (ERD2)-GFP (a cis-Golgi/ER marker) and SP-tagRFP-HDEL (an ER marker). No Golgi body was found at the tip of elongating ER tubules in the presence of Lat B, indicating that the slow ER tubule extension occurred independent of Golgi body movement (Supplemental Fig. S3; Supplemental Movie S6). These results show that the driving force for slow ER tubule extension is generated in a microtubule-dependent manner.
Characterization of the Slow ER Tubule Extension
To characterize the microtubule-dependent ER tubule extension, we carefully analyzed the time-lapse images. An ER tubule started to elongate along a microtubule (0 s in Fig. 3; Supplemental Movie S7). The tip of the ER tubule moved along the microtubule, but the lateral side of the following ER tubule did not align with the microtubule in this case (from 10 to 40 s in Fig. 3; Supplemental Movie S7). The elongating ER tubule shrank one time and reelongated (Supplemental Movie S7). Interestingly, the tip of the elongating ER tubule on one microtubule track transferred to another microtubule track (80 s in Fig. 3; Supplemental Movie S7). In contrast, we did not find an ER tubule extension that synchronized with the growing ends of the microtubules (Supplemental Movie S4).
Figure 3.
The tip of the ER tubule associates with microtubules during tubule extension. The tip of an ER tubule slid along a microtubule, but the following part of the ER tubule was apart from the microtubule. From 70 to 80 s, the tip of an ER tubule changed direction of elongation with a different microtubule. Seedlings were treated with 2 µm Lat B for 30 min before observation. Still images corresponding to Supplemental Movie S7 are shown. The DSLT and drawing columns of ER dynamics correspond to the MT and ER columns. Microtubules and ER are shown in magenta and green, respectively, in the DSLT column and black and gray in the drawing. Red lines in the drawing column indicate growing ER tubules. MT, Microtubule.
Next, we examined the elongation direction of ER tubules against microtubule polarity. Microtubule polarity is distinguishable by observing microtubule dynamics; plus ends of microtubules show faster growing and shortening than minus ends. Figure 4A is an example image that shows the elongation direction of ER tubules (Fig. 4A, red arrows) and the plus-end direction of microtubules (Fig. 4A, blue arrows; Supplemental Movie S4). During a 10-min observation, ER tubule extension occurred 11 times in total, consisting of 4 times bound to the plus end, 5 times bound to the minus end, and 2 times that were uncharacterized extensions (Fig. 4A; Supplemental Movie S4). We analyzed the elongation direction of 64 ER tubules in Supplemental Movies S1 to S8, and each is composed of time-lapse images over 10 min (Fig. 4D). The frequencies of ER tubule extensions bound to plus and minus ends of microtubules were 0.25 ± 0.05 and 0.46 ± 0.07 times per minute, respectively, indicating that minus end-directed ER tubule extensions are nearly 2 times higher than plus end-directed ER tubule extension.
Figure 4.
Characterization of microtubule-dependent ER tubule extension. A, Bidirectional ER tubule extension along microtubules. The ER and microtubule dynamics in Supplemental Movie S4 were analyzed and are shown in a still image from time 0. Microtubules are indicated by blue arrows. The direction of the blue arrows indicates the plus ends of the microtubules. The direction of ER extensions is represented by red arrows. Slow ER tubule extension occurred in both directions of microtubule polarities. This figure does not have time information. Seedlings were treated with 2 µm Lat B for 30 min. B, Distributions of run distances of ER tubule extension. Numbers of samples are 23 and 41 for plus end- and minus end-directed ER tubule extensions, respectively. C, Distributions of velocities of ER tubule extension. Numbers of samples are 23 and 41 for plus end- and minus end-directed ER tubule extensions, respectively. D, Statistical analyses for average rate, frequency of ER extension, average of run distance, and proportions of switch-track movement. Numbers of samples are 23, 41, and 64 for plus-end directed, minus-end directed, and sum, respectively.
We estimated the velocities of ER tubule elongation (Fig. 4D). The average velocity of the slow ER tubule elongation in the presence of Lat B was 0.39 ± 0.07 µm s−1, although the average velocity of ER tubule elongation in the control was 7.8 ± 1.7 µm s−1. The velocity of the slow ER tubule elongation is similar to velocities reported for microtubule-driven elongation of ER tubules in animal cells (Waterman-Storer and Salmon, 1998). We also investigated the plus end- and minus end-directed slow ER tubule elongations. There are no significant differences of average velocities of tubule elongation between the plus-end direction (0.40 ± 0.05 µm s−1) and the minus-end direction (0.39 ± 0.08 µm s−1) and no differences of average lengths between plus-end direction (4.38 ± 1.91 µm) and minus-end direction (5.40 ± 2.41 µm; Fig. 4, B–D).
ER Anchoring Sites on Cortical Microtubules Are Maintained during ER Reorganization
During observations in the presence of Lat B, we found that dead end-shaped ER often associated with microtubules (Fig. 5; Supplemental Movie S4). In most cases, the tips of dead end-shaped ER were paused and stably anchored to microtubules. It has been reported that ER tubule junctions often colocalize with microtubules in young elongating cells under normal conditions (Hamada et al., 2012). However, ER anchoring on microtubules has not been observed previously.
Figure 5.
Dead end-shaped ER often associates with microtubules in the presence of Lat B. Four examples were taken from Supplemental Movie S4. Dead end-shaped (one-way) ER tubules were often observed in the presence of Lat B. Arrowheads indicate ER anchoring sites on the microtubules. Seedlings were treated with 2 µm Lat B for 30 min. MT, Microtubule.
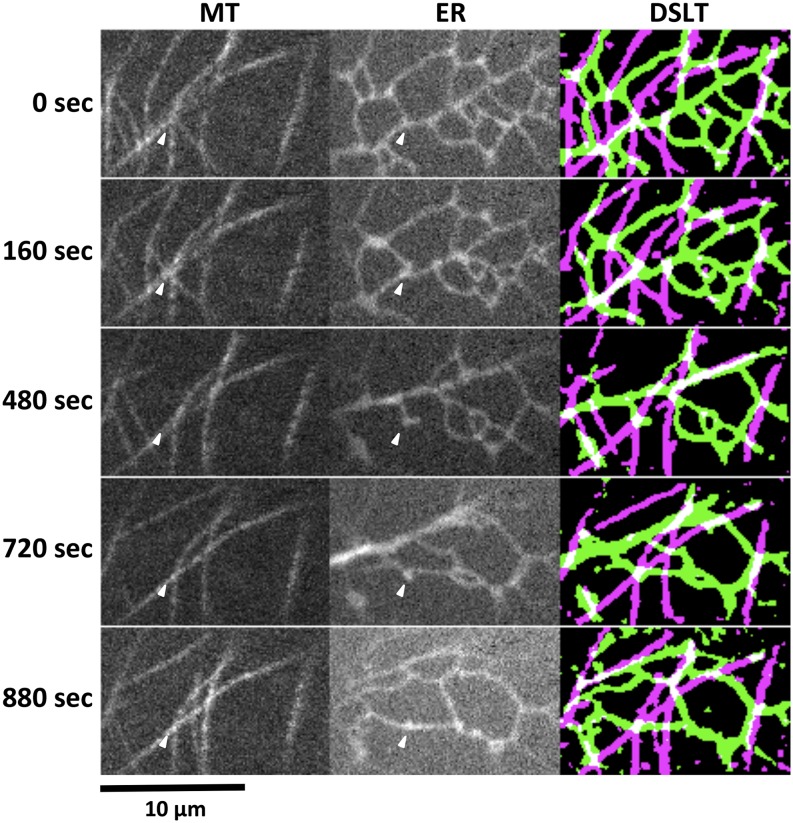
We investigated whether ER anchoring occurs under normal conditions (i.e. in the absence of Lat B). During long observations of 1,040 s, we found more than 10 ER anchoring points (Fig. 6; Supplemental Movie S8). An ER anchoring site at 0 s functioned as a three-way junction of ER tubules (0 s in Fig. 6, arrowheads). The ER tubule junction kept its shape for 240 s (160 s in Fig. 6; from 0 to 240 s in Supplemental Movie S8). The ER morphology surrounding the anchoring site dramatically changed by bulk ER flow; however, the ER anchoring site still held the ER cisterna and formed dead end-shaped ER (480 s in Fig. 6; from 320 to 640 s in Supplemental Movie S8). Other ER flow reached the anchoring site and formed the two-way linear ER tubule (from 720 to 880 s in Fig. 6 and Supplemental Movie S8). Interestingly, the ER tubule was thicker, especially at the ER anchoring site. Finally, new ER tubules elongated from the ER anchoring site and formed three-way ER tubule junctions again (from 960 to 1,040 s in Supplemental Movie S8). These ER anchoring sites on cortical microtubules were relatively stable during dynamic ER reorganization but were not permanent structures. These results suggest that cortical microtubules contribute to providing ER anchoring sites and forming stable junctions or dead-end ER tubules.
Figure 6.
ER anchoring to cortical microtubule is retained during ER reorganization. Three-way ER tubule junctions (arrowheads at 0 s) change to one-way (dead end; at 480–640 s) and subsequently, two-way (linear; 720–880 s) junctions, while keeping microtubule-ER interaction. Arrowheads point to an ER anchoring site on a cortical microtubule. Still images corresponding to Supplemental Movie S8 are shown. The DSLT images of ER dynamics corresponding to the MT and ER columns. Microtubules and ER are shown in magenta and green, respectively, in the DSLT column. MT, Microtubule.
Density of ER Tubule Junctions Positively Correlates with Microtubule Density in Both Elongating and Expanded Cells
We examined the contribution of microtubules to the architecture of the ER network under different developmental conditions of cells. Both tubule-tubule and sheet-tubule junctions were associated with microtubules in expanded cells (Fig. 7A, right) as well as elongating cells (Hamada et al., 2012; Fig. 7A, left). We analyzed the correlation between microtubule density and ER junction number in 80 independent regions of interest of 10 × 10 µm from both elongating and expanded cells. The number of ER junctions positively correlated with microtubule density (Fig. 7B). The correlation was observed even in a single cell (Fig. 7B). Taken together with previous reports that microtubule depolymerization induced coarse ER meshwork (Foissner et al., 2009; Hamada et al., 2012), these results clearly show that microtubules contribute to the increasing density of the ER meshwork throughout cell development.
Figure 7.
Association of ER tubule junctions with cortical microtubules in expanded cells. A, Left, Elongating epidermal leaf cells of 5-d-old Arabidopsis seedlings were used. Small ER tubule patches and crowded microtubules are shown. ER tubule junctions were often associated with microtubules. A, Right, Expanded epidermal leaf cells of 10-d-old Arabidopsis seedlings were used. The density of microtubules and the size of ER tubule patches decreased. ER tubule junctions still associated with microtubules. ER and microtubules are shown in green and magenta, respectively. Yellow and red dots represent microtubule-associated ER junctions and free ER junctions, respectively. B, Correlation between the number of ER tubule junctions and microtubule density. The microtubule density and the number of ER tubule junctions were measured in 80 regions of interest of 10 × 10 µm from both elongating and expanded cells. Blue dots and red squares correspond to data from elongating and expanded cells, respectively. Microtubule densities were calculated by area of threshold images converting from microtubule images. MT, Microtubule.
DISCUSSION
Mechanism of Microtubule-Dependent ER-Tubule Extension and ER Anchoring
The ER tubule extension mediated by microtubules is relatively slow (0.14 µm s−1) compared with the velocity of ER tubule extension mediated by actin filaments (7.8 µm s−1). In animals, ER tubule extension occurs along microtubules with a velocity of approximately 0.1 µm s−1 (Waterman-Storer and Salmon, 1998), which is similar to our observations. In animals, two different mechanisms of ER tubule extension along microtubules have been identified: a sliding mechanism driven by kinesin/dynein motor proteins and a Tip Attachment Complex mechanism through microtubule plus end-accumulating protein END BINDING1 (EB1; Friedman and Voeltz, 2011). In plants, EB1-like protein is reported to link microtubule dynamics to endomembrane organization (Mathur et al., 2003). However, our observations revealed that slow ER tubule extension did not synchronize with the microtubule growing plus ends (Supplemental Movie S4). In addition, ER tubule tips are associated with microtubules during the slow ER tubule extensions. These results suggest that the slow ER tubule extension in plants is generated by the motor protein-dependent sliding mechanism but not the EB1-dependent Tips Attachment Complex mechanism. It should be noted that the tip association of ER tubules with microtubules is necessary and sufficient for slow ER tubule extension, although the lateral association of the ER tubules that is occasionally observed is not required for ER tubule extension. A similar manner was reported for the actin-mediated ER tubule extension (Yokota et al., 2011).
In addition, we found that ER tubules elongated in both directions of microtubule polarity (Fig. 4; Supplemental Movie S4). In animal cells, bidirectional ER tubule extension has also been observed. Two different proteins (kinesins and cytoplasmic dynein) that direct to the plus and minus ends of microtubules, respectively, mediate ER tubule extension (Woźniak et al., 2009). Many kinesin homologs occur widely in eukaryotes, including plants (Richardson et al., 2006), although plants lack cytoplasmic dynein. It has been suggested that plants have special minus end-directed kinesins, such as some of the kinesin-14 family (Chen et al., 2002; Marcus et al., 2002, 2003; Ambrose et al., 2005). These members are candidate drivers for ER tubule extension to the minus-end direction. In animals, kinesins require adapter proteins, such as kinectin, to induce ER tubule elongation (Toyoshima et al., 1992). Plants lack obvious kinectin homologs. Identification of ER-related kinesins and adapter proteins is required.
Animal cells have ER binding microtubules-associated proteins, such as STROMAL INTERACTION MOLECULE1 and CYTOSKELETON-LINKING MEMBRANE PROTEIN63 (Friedman and Voeltz, 2011), for ER anchoring to microtubules. However, plants have no obvious homologs of these animal proteins. In plants, overexpression of Arabidopsis formin14 induced the alignment of ER tubules along cortical microtubules (Deeks et al., 2010). This finding seems to be a secondary effect of the microtubule-actin filaments interaction, because formin could bind to both actin filaments and microtubules. Recently, the ER membrane-associated protein VAMP/SYNAPTOBREVIN-ASSOCIATED27 (VAP27) directly bound to microtubules in vitro (Wang et al., 2014). VAP27 may be involved in ER anchoring to microtubules.
Contribution of Microtubules to ER Network Organization
Based on the slow ER tubule extension and ER anchoring, microtubules provide fine ER tubule meshwork at the cortex in plant cells. Although the exact functions of the fine ER meshwork remain unclear, the fine ER tubule meshwork has at least two advantages compared with the coarse ER tubule meshwork and ER sheet. First, the fine ER tubule meshwork has a large surface area per volume. Increasing the ER surface area may strengthen the protein synthesis ability and signal transduction between the cytoplasm and ER lumen. Second, the fine ER tubule meshwork provides lots of high-curvature faces on the ER. The ER exit site, which mediates protein transport from the ER to Golgi bodies, accumulates, especially at high-curvature faces of the ER (Okamoto et al., 2012). Plants may enhance ER functions by forming fine ER tubule meshwork with regulation of cortical microtubules.
At the cortex of plant cells, the slow ER tubule extension along cortical microtubules may be insignificant, because most of the ER tubule extensions are actin-myosin-dependent. Considering the stability of microtubule-dependent ER tubules, microtubules may contribute to the stabilization of the ER network. In the case of mitotic cells, the ER tubule extension along microtubules may significantly contribute to the organization of ER structures. ER tubules are associated with the spindle and early phragmoplast from metaphase to early telophase (Hepler and Wolniak, 1984; Gupton et al., 2006). Future analyses based on identification of microtubule-ER-connecting proteins and their mutants’ analyses will shed light on the importance of microtubules to ER organization in plant cells.
CONCLUSION
ER-microtubule interactions in plant cells were suggested by electron microscopy observations more than two decades ago (Hepler et al., 1990). However, the functions of microtubules in ER network organization are unclear compared with those of actin filaments. In this study, we found that microtubules engage slow ER tubule extension and ER anchoring in plant cells. Our findings confirm the contribution of microtubules to ER network organization in plant cells.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
All material was Arabidopsis (Arabidopsis thaliana) ‘Heynh’ on Columbia-0 background. For labeling microtubules and ER, plants expressing both GFP-β-TUBULIN6 (TUB6) and SP-TagRFP-HDEL were used as described by Hamada et al. (2012). For labeling actin filaments, plants expressing lifeact-venus were used (Era et al., 2009). For labeling Golgi bodies, plants expressing ERD2-GFP were used (Takeuchi et al., 2000). Seeds were sterilized in 5% (w/v) sodium hypochlorite and 1% (w/v) Triton X-100 for 5 min. After sterilization, seeds were rinsed five times with sterile water and plated on agar containing 1% (w/v) Suc, 1% (w/v) agar, and one-half-strength nutrient solution as described by Haughn and Somerville (1986). Plates were set in a near-vertical position at 22°C with constant light. Five-day-old plants were used for analyses of elongating cells and drug treatments. Ten-day-old plants were used for analyses of expanded cells.
Imaging Analyses and Drug Treatments
Observations were performed on a fluorescence microscope (Axio Observer; Zeiss) equipped with a confocal spinning disc unit (CSU-X1; Yokogawa) with a dual band-pass filter (Semrock) and a twin electron-multiplying CCD cameras (Andor) system. Images were taken with IQ software (Andor) and analyzed with Image J (National Institutes of Health; http://rsbweb.nih.gov/ij/). For drug treatments, plants were soaked in small tubes containing 2 µm Lat B (Sigma) or 50 µm oryzalin (Sigma) and incubated for 30 min at 22°C in light. Direction-selective local threshold (DSLT) is a modified local threshold method (T. Kawase, S.S. Sugano, T. Shimada, and I. Hara-Nishimura, unpublished data). In brief, DSLT measures the neighboring regions connected to the central point by line segment kernels in various orientations rather than weighing the cubic or spherical neighboring regions. The threshold at each pixel is the minimal weighed sum calculated by convolving the original images with a line segment kernel.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Slow ER tubule extension in the absence of Lat B.
Supplemental Figure S2. Removal of both microtubules and actin filaments repressed ER tubule extension.
Supplemental Figure S3. ER tubule extension in the presence of Lat B is independent of Golgi bodies.
Supplemental Movie S1. Dynamics of ER and actin filaments.
Supplemental Movie S2. Dynamics of ER and actin filaments in the presence of 2 µm Lat B.
Supplemental Movie S3. ER tubule extension along cortical microtubules in the presence of Lat B.
Supplemental Movie S4. Dynamics of ER and microtubules for 10 min in the presence of 2 µm Lat B.
Supplemental Movie S5. Dynamics of ER and microtubules in the presence of 50 µm oryzalin and 2 µm Lat B.
Supplemental Movie S6. Dynamics of ER and Golgi bodies in the presence of 2 µm Lat B.
Supplemental Movie S7. Dynamics of an ER tip and microtubules in the presence of 2 µm Lat B.
Supplemental Movie S8. Dynamics of ER and microtubules.
Supplementary Material
Acknowledgments
We thank Atsuko Era, Akihiko Nakano, and Takashi Ueda (University of Tokyo) for donation of the seeds of the Arabidopsis line expressing 35S:lifeact-venus or 35S:ERD2-GFP.
Glossary
- DSLT
direction-selective local threshold
- ER
endoplasmic reticulum
- Lat B
Latrunculin B
Footnotes
This work was supported by the Japan Society for the Promotion of Science (research fellowship no. 11J01084 to T.H., Grants-in-Aid for Scientific Research nos. 21200065 and 25440132 to H.U., and Grant-in-Aid for Specially Promoted Research no. 22000014 to I.H-.N.).
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Ambrose JC, Li W, Marcus A, Ma H, Cyr R (2005) A minus-end-directed kinesin with plus-end tracking protein activity is involved in spindle morphogenesis. Mol Biol Cell 16: 1584–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Marcus A, Li W, Hu Y, Calzada JPV, Grossniklaus U, Cyr RJ, Ma H (2002) The Arabidopsis ATK1 gene is required for spindle morphogenesis in male meiosis. Development 129: 2401–2409 [DOI] [PubMed] [Google Scholar]
- Deeks MJ, Fendrych M, Smertenko A, Bell KS, Oparka K, Cvrcková F, Zársky V, Hussey PJ (2010) The plant formin AtFH4 interacts with both actin and microtubules, and contains a newly identified microtubule-binding domain. J Cell Sci 123: 1209–1215 [DOI] [PubMed] [Google Scholar]
- Era A, Tominaga M, Ebine K, Awai C, Saito C, Ishizaki K, Yamato KT, Kohchi T, Nakano A, Ueda T (2009) Application of Lifeact reveals F-actin dynamics in Arabidopsis thaliana and the liverwort, Marchantia polymorpha. Plant Cell Physiol 50: 1041–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foissner I, Menzel D, Wasteneys GO (2009) Microtubule-dependent motility and orientation of the cortical endoplasmic reticulum in elongating characean internodal cells. Cell Motil Cytoskeleton 66: 142–155 [DOI] [PubMed] [Google Scholar]
- Friedman JR, Voeltz GK (2011) The ER in 3D: a multifunctional dynamic membrane network. Trends Cell Biol 21: 709–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupton SL, Collings DA, Allen NS (2006) Endoplasmic reticulum targeted GFP reveals ER organization in tobacco NT-1 cells during cell division. Plant Physiol Biochem 44: 95–105 [DOI] [PubMed] [Google Scholar]
- Hamada T, Tominaga M, Fukaya T, Nakamura M, Nakano A, Watanabe Y, Hashimoto T, Baskin TI (2012) RNA processing bodies, peroxisomes, Golgi bodies, mitochondria, and endoplasmic reticulum tubule junctions frequently pause at cortical microtubules. Plant Cell Physiol 53: 699–708 [DOI] [PubMed] [Google Scholar]
- Haughn GW, Somerville C (1986) Sulfonylurea resistant mutants of Arabidopsis thaliana. Mol Gen Genet 204: 430–434 [Google Scholar]
- Hepler PK, Palevitz BA, Lancelle SA, McCauley MM, Lichtscheidl I (1990) Cortical endoplasmic reticulum in plants. J Cell Sci 96: 355–373 [Google Scholar]
- Hepler PK, Wolniak SM (1984) Membranes in the mitotic apparatus: their structure and function. Int Rev Cytol 90: 169–238 [DOI] [PubMed] [Google Scholar]
- Knebel W, Quader H, Schnepf E (1990) Mobile and immobile endoplasmic reticulum in onion bulb epidermis cells: short- and long-term observations with a confocal laser scanning microscope. Eur J Cell Biol 52: 328–340 [PubMed] [Google Scholar]
- Lancelle SA, Cresti M, Hepler PK (1987) Ultrastructure of the cytoskeleton in freeze-substituted pollen tubes of Nicotiana alata. Protoplasma 140: 141–150 [Google Scholar]
- Langhans M, Niemes S, Pimpl P, Robinson DG (2009) Oryzalin bodies: in addition to its anti-microtubule properties, the dinitroaniline herbicide oryzalin causes nodulation of the endoplasmic reticulum. Protoplasma 236: 73–84 [DOI] [PubMed] [Google Scholar]
- Lichtscheidl IK, Hepler PK (1996) Endoplasmic reticulum in the cortex of plant cells. InSmallwood M, Knox P, Bowles D, eds, Membranes: Specialised Functions in Plants. BIOS Scientific Publishers, Wallingford, United Kingdom, pp 383–402 [Google Scholar]
- Marcus AI, Ambrose JC, Blickley L, Hancock WO, Cyr RJ (2002) Arabidopsis thaliana protein, ATK1, is a minus-end directed kinesin that exhibits non-processive movement. Cell Motil Cytoskeleton 52: 144–150 [DOI] [PubMed] [Google Scholar]
- Marcus AI, Li W, Ma H, Cyr RJ (2003) A kinesin mutant with an atypical bipolar spindle undergoes normal mitosis. Mol Biol Cell 14: 1717–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur J, Mathur N, Kernebeck B, Srinivas BP, Hülskamp M (2003) A novel localization pattern for an EB1-like protein links microtubule dynamics to endomembrane organization. Curr Biol 13: 1991–1997 [DOI] [PubMed] [Google Scholar]
- McCauley MM, Hepler PK (1992) Cortical ultrastructure of freeze-substituted protonemata of the moss Funaria hygrometrica. Protoplasma 169: 168–178 [Google Scholar]
- Okamoto M, Kurokawa K, Matsuura-Tokita K, Saito C, Hirata R, Nakano A (2012) High-curvature domains of the ER are important for the organization of ER exit sites in Saccharomyces cerevisiae. J Cell Sci 125: 3412–3420 [DOI] [PubMed] [Google Scholar]
- Peremyslov VV, Prokhnevsky AI, Dolja VV (2010) Class XI myosins are required for development, cell expansion, and F-Actin organization in Arabidopsis. Plant Cell 22: 1883–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokhnevsky AI, Peremyslov VV, Dolja VV (2008) Overlapping functions of the four class XI myosins in Arabidopsis growth, root hair elongation, and organelle motility. Proc Natl Acad Sci USA 105: 19744–19749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quader H, Hofmann A, Schnepf E (1989) Reorganization of the endoplasmic reticulum in epidermal cells of onion bulb scales after cold stress: involvement of cytoskeletal elements. Planta 177: 273–280 [DOI] [PubMed] [Google Scholar]
- Richardson DN, Simmons MP, Reddy ASN (2006) Comprehensive comparative analysis of kinesins in photosynthetic eukaryotes. BMC Genomics 7: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkes I, Runions J, Hawes C, Griffing L (2009a) Movement and remodeling of the endoplasmic reticulum in nondividing cells of tobacco leaves. Plant Cell 21: 3937–3949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkes IA, Ketelaar T, de Ruijter NC, Hawes C (2009b) Grab a Golgi: laser trapping of Golgi bodies reveals in vivo interactions with the endoplasmic reticulum. Traffic 10: 567–571 [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Ueda T, Sato K, Abe H, Nagata T, Nakano A (2000) A dominant negative mutant of sar1 GTPase inhibits protein transport from the endoplasmic reticulum to the Golgi apparatus in tobacco and Arabidopsis cultured cells. Plant J 23: 517–525 [DOI] [PubMed] [Google Scholar]
- Toyoshima I, Yu H, Steuer ER, Sheetz MP (1992) Kinectin, a major kinesin-binding protein on ER. J Cell Biol 118: 1121–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H, Yokota E, Kutsuna N, Shimada T, Tamura K, Shimmen T, Hasezawa S, Dolja VV, Hara-Nishimura I (2010) Myosin-dependent endoplasmic reticulum motility and F-actin organization in plant cells. Proc Natl Acad Sci USA 107: 6894–6899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Honing HS, van Bezouwen LS, Emons AMC, Ketelaar T (2011) High expression of Lifeact in Arabidopsis thaliana reduces dynamic reorganization of actin filaments but does not affect plant development. Cytoskeleton (Hoboken) 68: 578–587 [DOI] [PubMed] [Google Scholar]
- Wang P, Hawkins TJ, Richardson C, Cummins I, Deeks MJ, Sparkes I, Hawes C, Hussey PJ (2014) The plant cytoskeleton, NET3C, and VAP27 mediate the link between the plasma membrane and endoplasmic reticulum. Curr Biol 24: 1397–1405 [DOI] [PubMed] [Google Scholar]
- Waterman-Storer CM, Salmon ED (1998) Endoplasmic reticulum membrane tubules are distributed by microtubules in living cells using three distinct mechanisms. Curr Biol 8: 798–806 [DOI] [PubMed] [Google Scholar]
- Woźniak MJ, Bola B, Brownhill K, Yang YC, Levakova V, Allan VJ (2009) Role of kinesin-1 and cytoplasmic dynein in endoplasmic reticulum movement in VERO cells. J Cell Sci 122: 1979–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota E, Ueda H, Hashimoto K, Orii H, Shimada T, Hara-Nishimura I, Shimmen T (2011) Myosin XI-dependent formation of tubular structures from endoplasmic reticulum isolated from tobacco cultured BY-2 cells. Plant Physiol 156: 129–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.