Key Points
Fanca−/− megakaryocytes accumulate genomic instability through endomitotic cycles.
Defective endomitosis induces senescence of Fanca−/− megakaryocytes.
Abstract
Fanconi anemia (FA) is an inherited chromosomal instability syndrome that is characterized by progressive bone marrow failure. One of the main causes of morbidity and mortality in FA is a bleeding tendency, resulting from low platelet counts. Platelets are the final products of megakaryocyte (MK) maturation. Here, we describe a previously unappreciated role of Fanconi anemia group A protein (Fanca) during the endomitotic process of MK differentiation. Fanca deficiency leads to the accumulation of MKs with low nuclear ploidy and to decreased platelet production. We show, for the first time, that Fanca−/− mice are characterized by limited number and proliferative capacity of MK progenitors. Defective megakaryopoiesis of Fanca−/− cells is associated with the formation of nucleoplasmic bridges and increased chromosomal instability, indicating that inaccurate endoreplication and karyokinesis occur during MK polyploidization. Sustained DNA damage forces Fanca−/− MKs to enter a senescence-like state. Furthermore, inhibition of the Rho-associated kinase, a regulator of cytokinesis, improves the polyploidization of Fanca−/− MKs but greatly increases their genomic instability and diminishes their differentiation potential, supporting the notion that accumulation of DNA damage through endomitotic cycles affects MK maturation. Our study indicates that Fanca expression during endomitosis is crucial for normal megakaryopoiesis and platelet production.
Introduction
Platelets, the blood cell fragments involved in hemostasis, arise from the cytoplasmic blebbing and fragmentation of mature megakaryocytes (MKs). MKs originate from hematopoietic stem cells and progenitors (megakaryocyte-erythroid progenitors [MEPs]), leading to committed MK progenitors. The full maturation of MK progenitors to platelet-producing cells requires a unique process of polyploidization, termed endomitosis, by which a MK increases its cellular volume and DNA content up to 128N.1 Megakaryocytic development is unique in its requirement for a high number of nuclear divisions as opposed to the development of other committed progenitors, in which terminal differentiation is strictly dependent on cell cycle arrest.2
Fanconi anemia (FA) is an inherited chromosomal instability disorder characterized by progressive bone marrow (BM) failure, pancytopenia, and high susceptibility to cancer. One of the main causes of mortality and morbidity in FA is the well-known decrease in the platelet counts. The molecular process underlying the pathogenesis of this abnormality remains elusive. FA differs from other inherited BM failure syndromes for its unique cellular and chromosomal hypersensitivity to DNA interstrand cross-links (ICLs). FA is defined by mutations in 16 genes identified thus far, with FANCA mutations accounting for 60% to 70% of the patients.3 Eight FANC proteins (FANCA, -B, -C, -E, -F, -G, -L, and -M) constitute the FANCcore complex, which possesses E3-ubiquitin ligase activity and monoubiquitinates FANCD2 and FANCI, a step required for their relocalization to subnuclear repair foci.4 The other FANC proteins are subunits of endonuclease complexes (FANCP/SLX4 and FANCQ/XPF) and factors involved in homologous recombination (FANCJ/BRIP1, FANCO/RAD51C, FANCD1/BRCA2, and FANCN/PALB2). The primary function of the FANC proteins is to protect the cell against the genetic instability generated by intrinsic and extrinsic replication stress. We have shown that loss of function of the FANC pathway results in the accumulation of high levels of anaphase bridges and micronuclei and revealed a specific role of this pathway during mitosis.5,6 Some chromosomal loci, notably common fragile sites (CFSs), are particularly susceptible to replication stress, which leaves these regions with unresolved replication intermediates generating sister chromatid interlinkage during mitosis. The FANC pathway interacts with the helicase BLM (mutated in Bloom syndrome) during both replication and mitosis to promote proper resolution of replication intermediates and to maintain CFS stability.5,6 We recently described the presence of FANCD2 and BLM-associated anaphase bridges during MK endomitosis, suggesting that MKs are subjected to replicative stress during polyploidization.7 Consequently, we hypothesized that the FANC pathway is involved in genomic stability maintenance during the endomitotic process and that its failure results in decreased platelet counts in FA.
To test our hypothesis, we analyzed megakaryopoiesis in a Fanca−/− mouse model.8 Fanca−/− mice, similarly to other FA mice, present cellular and chromosomal hypersensitivity to ICLs and reduced fertility without evidence of BM failure or anemia.9 In agreement with previous reports,8,10 we confirmed that Fanca−/− mice present a decreased number of circulating platelets. In addition, we uncovered a significant reduction in the number and colony-forming potential of MK progenitors in Fanca−/− BM. Finally, we demonstrated that Fanca is essential to prevent nucleoplasmic bridge formation and contributes to the maintenance of chromosomal stability during MK endomitoses. Consequently, Fanca is required to ensure the progression of the differentiation process to yield MKs able to produce functional platelets, and its loss of function leads to MK progenitors depletion and endomitosis deregulation resulting in decreased platelet counts, a major FA hematopoietic dyscrasia.
Methods
Mice, cell culture, flow cytometry, and quantitative reverse transcriptase-polymerase chain reaction
Fanca−/− mice with the 129Ola/FVB background were described previously.8 Fanca+/− mice were backcrossed with wild-type (WT) FVB/N mice (>10 generations). As Fanca−/− mice show severely reduced fertility, WT and Fanca−/− mice used for analysis (8-10 weeks) correspond to siblings derived from crossbreeding of heterozygous mice. The project was officially approved by the Animal Experimentation Ethics Committee (registered under no. 26 by the French Department of Research) and conducted in accordance with French laws and regulations. BM was harvested by flushing tibias and femurs. Lin− progenitors were identified using biotinylated antibodies from a lineage cell depletion kit (Miltenyi Biotech, Cologne, Germany). LSK and various myeloid progenitors were analyzed by immunophenotyping using fluorescein isothiocyanate (FITC)- or allophycocyanin (APC)-streptavidin and phycoerythrin (PE)-Cy7-anti-Sca-1, PE-anti-c-Kit, AlexaFluor700-anti-FcγRII/III (CD16/32), eFluor450-anti-CD34, FITC-anti-CD150, or eFluor450-anti-CD41 antibodies. MKs were stained using FITC-anti-CD41 or PE-anti-CD41 antibodies. All antibodies were purchased from eBioscience (San Diego, CA). BrdU analysis was performed using APC BrdU Flow Kit (BD Pharmingen, San Diego, CA), according to the manufacturer’s protocol. For ploidy analysis, cells were fixed with 1% paraformaldehyde (PFA) prior to staining with anti-CD41 antibody. Subsequently, cells were incubated in a 0.1% sodium citrate hypotonic solution containing 50 μg/mL RNase and 50 μg/mL propidium iodide. In some cases, cells were stained with anti-CD41 antibody after incubation with Hoechst 33342 (Molecular Probes, Eugene, OR). Cells were sorted using an ARIA3 cell sorter (BD, Franklin Lakes, NJ). Analysis was performed using an LSRII flow cytometer or BD Accuri C6 together with FACSDiva (BD Biosciences, San Diego, CA) or FlowJo (TreeStar Inc, Ashland, OR) software.
BM cells were cultured in RPMI medium with 10% fetal calf serum (Gibco-Life Technologies, Carlsbad, CA) and 50 ng/mL thrombopoietin (TPO) (Peprotech, Rocky Hill, NJ) with or without 10 μM Rho-associated kinase (ROCK) inhibitor (Y27632; Sigma-Aldrich, Lyon, France). MK progenitors were cultured in StemPro (Gibco-Life Technologies) supplemented with TPO (50 ng/mL), stem cell factor (50 ng/mL), interleukin (IL)-3 (10 ng/mL), and IL-6 (20 ng/mL) (all from Peprotech). Total RNA of sorted 2N, 4N, and ≥8N MKs was isolated using the RNeasy Plus Micro kit (Qiagen, Hilden, Germany) and reverse-transcribed using an Affinity Script Multi Temperature cDNA synthesis kit (Agilent Technologies, Santa Clara, CA). All reactions were performed using Fast Start Universal SYBR Green Master mix (Roche, Penzberg, Germany) and a 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA) with primers as previously reported.11
Pro-platelets production in vitro
Pro-platelets production from mouse BM was performed as described.12 Briefly, Lin− cells were isolated from BM and precultured for 24 hours in Dulbecco’s modified Eagle medium supplemented with fetal calf serum 10%, TPO (50 ng/mL; Peprotech), and heparin (20 U/mL; Sigma-Aldrich). Lin−CD41+ cells were then sorted and cultured in the same conditions. MKs producing pro-platelets were scored between 250 alive MKs at days 4 and 7 of culture.
Immunofluorescence
Immunofluorescence was performed as previously described13 using cells cytospun on poly-l-lysine–coated slides or on glass Laboratory-Tek slides (Thermo Scientific, Waltham, MA) coated with SuperFibronectin (Sigma-Aldrich). The antibodies used were mouse anti-lamin A/C (Cell Signaling, Beverly, MA), rabbit anti-53BP1 (Abcam, Cambridge, UK), and mouse anti-α-tubulin (Abcam). Secondary antibodies were as follows: donkey anti-mouse Alexa488 and donkey anti-rabbit Alexa594 (Molecular Probes, Eugene, OR). Slides were mounted using Fluoromount G (Southern Biotech, Birmingham, AL) and visualized using an Axio Observer Z1 (Carl Zeiss, Oberkochen, Germany) microscope equipped with a 63×1 oil-immersion objective and a charge coupled device camera AxioCam MRm (Carl Zeiss). Pictures were analyzed using the ImageJ software.
Colony forming unit–MK assay and peripheral blood counts
A total of 105 mononuclear BM cells were seeded into MegaCult-C medium (StemCell Technologies, Vancouver, Canada) supplemented with IL-3, IL-6, and TPO (Peprotech). In rescue experiments, LSK cells were infected with a retroviral vector expressing FANCA–internal ribosome entry site–enhanced green flourescent protein (EGFP) (kindly provided by Dr M. Milsom, German Cancer Research Center, Heidelberg, Germany) or an EGFP control vector, and cells expressing EGFP were sorted. Colonies (≥3 MKs) were scored on day 6 after fixation and staining according to the manufacturer’s instructions. Peripheral blood was collected in EDTA-containing tubes, and cell counts were determined using a Hemavet (HV950FS; Drew Scientific, Dallas, TX).
Genomic instability
After 3 days of culture in the presence of TPO, BM cells were incubated with colcemid for 3 hours, and metaphase spreads were prepared as previously described.5 Briefly, cells were incubated in hypotonic solution (KCl 0.075 M, 37°C, 15 minutes) and fixed with ethanol:acetic acid (3:1), followed by spreading on SuperFrost Plus slides (Thermo Scientific). Metaphases from each sample were blindly scored for chromosome deletions, gaps, fragments, and fusions. Fluorescence in situ hybridization (FISH) analysis was performed as previously described, using BAC clones 216B5 and 463E17 from the murine RP23 library (kindly provided by Prof M. Debatisse, Curie Institute, Paris, France).14
Statistical analysis
All experimental data are the results of observations made by investigators blinded to the mice genotype. Unless otherwise stated, results were analyzed by unpaired or paired Student t test using the GraphPad Prism software, version 6.0 (GraphPad Software, San Diego, CA). Data are shown as means and standard error of the mean (SEM), and P values are reported as follows: *P < .05, **P < .01, and ***P < .001.
Results
Fanca deficiency affects megakaryopoiesis
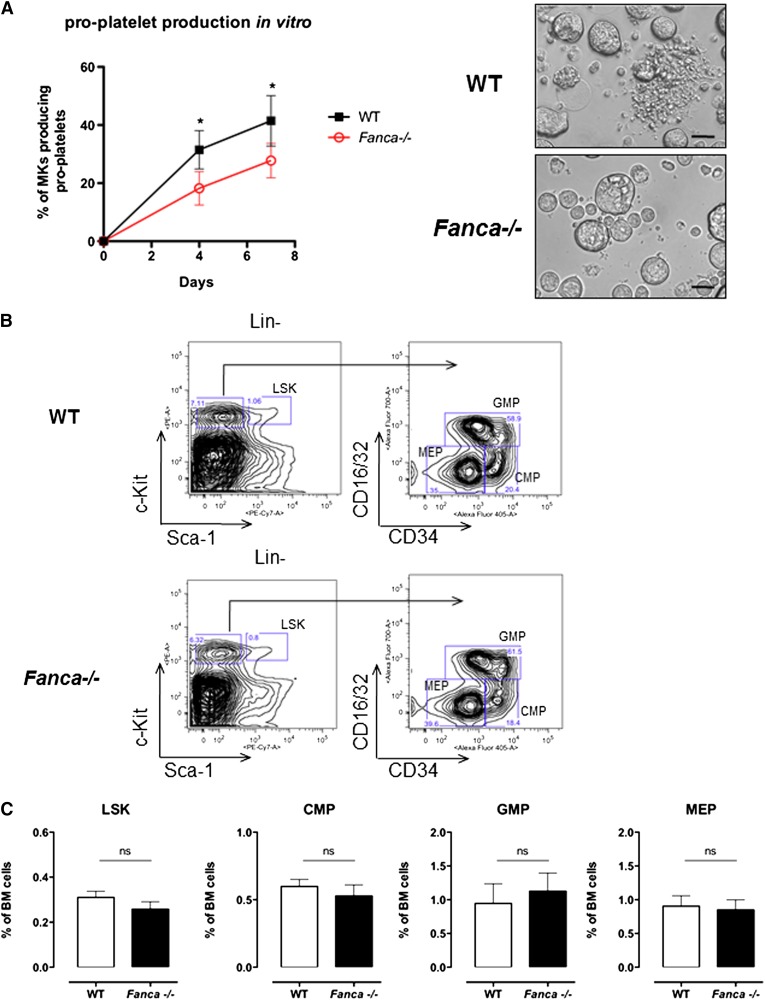
Adult Fanca−/− mice did not show gross hematological abnormalities or overt signs of anemia; the red blood cell counts and hematocrit were similar between knockout and WT mice (Table 1). However, Fanca−/− mice demonstrated a significant decrease in platelet counts in the circulating blood (Table 1). Fanca−/− Lin−CD41+ cells isolated from BM showed a reduced capacity to produce pro-platelets in vitro (Figure 1A).
Table 1.
Hematologic parameters obtained from WT and Fanca−/− adult mice (8-10 weeks of age)
| WT (n = 12) | Fanca−/− (n = 10) | P value | |
|---|---|---|---|
| RBC, count ×106/μL | 8.5 ± 0.3 | 8.5 ± 0.3 | .9605 |
| Hb, concentration g/dL | 16.3 ± 0.5 | 17.4 ± 0.7 | .2153 |
| HCT, % | 48.5 ± 1.5 | 48.5 ± 1.9 | .9867 |
| WBC, count ×103/μL | 7.0 ± 0.5 | 5.2 ± 0.6 | .0315* |
| PLT, count ×103/μL | 635.3 ± 56.3 | 383.9 ± 30.8 | .0014** |
Data are shown as means ± SEM. Hb, hemoglobin; HCT, hematocrit; PLT, platelets; RBC, red blood cell; WBC, white blood cells. *P < .05; **P < .01.
Figure 1.
Fanca deficiency affects pro-platelet formation. (A) Pro-platelet production in vitro and representative images presenting CD41+Lin− WT and Fanca−/− cells at day 4 of culture (n = 4 mice per group, 250 alive MKs per point were scored, *P < .05, Student t test; scale bar = 50 μm). (B) Representative flow cytometry plots showing the gating strategy to identify different progenitors. (C) Number of Lin−Sca-1+c-Kit+ (LSK) cells and myeloid progenitors (CMPs, GMPs, and MEPs) in the BM of Fanca−/− and WT mice (n = 5 per group, results presented as means ± SEM; LSK, P = .2605; CMP, P = .4895; GMP, P = .6598; MEP, P = .8014).
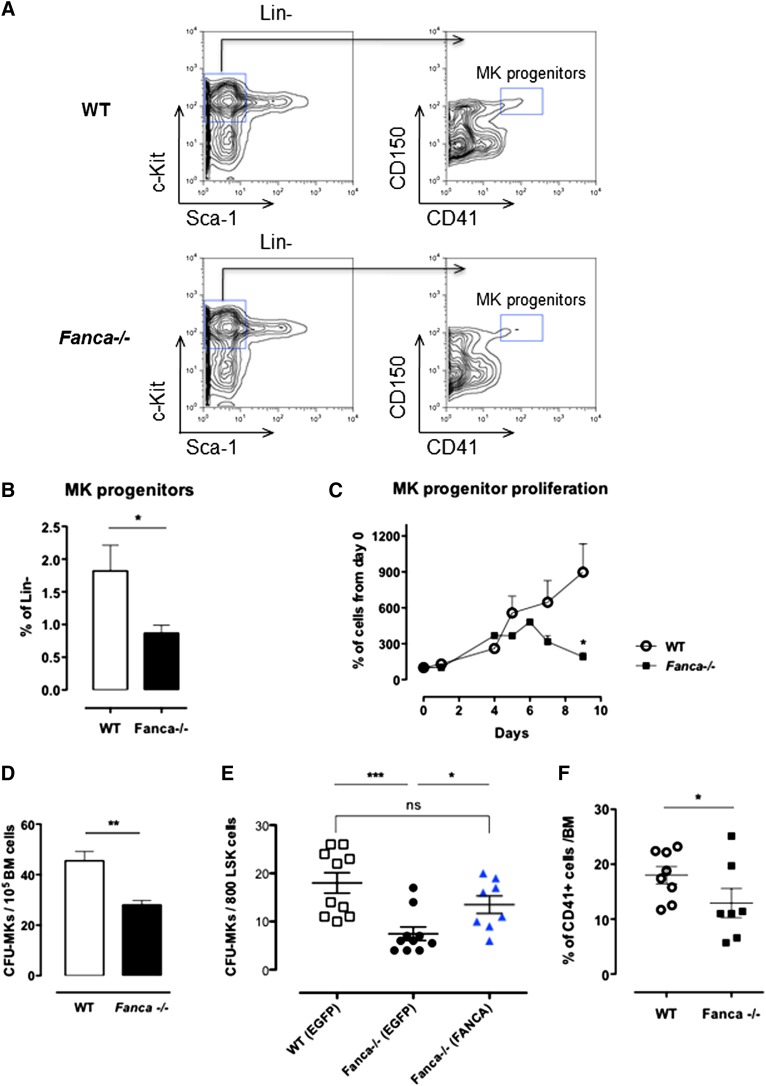
Despite a diminished amount of BM mononuclear cells in Fanca−/− mice (supplemental Figure 1A), analysis of cell populations enriched among early progenitors and hematopoietic stem cells (HSPCs) showed no difference in the LSK (Lin−Sca+c-Kit+) BM population between Fanca−/− and WT mice (Figure 1B-C). Furthermore, this equal repartition of cell populations was sustained at the level of committed myeloid progenitors (CMPs) and granulocyte/macrophage progenitors (GMPs). Finally, no difference was observed in the frequency of MEPs in the BM of Fanca−/− and WT mice (Figure 1B-C). This observation prompted us to examine in further detail the maturation process of MKs downstream of the MEP stage. Analysis of the MK progenitors phenotypically defined as Lin−c-Kit+Sca−CD150+CD41+15 revealed a significant lower quantity of MK progenitors in the BM of Fanca−/− mice than in WT littermates (Figure 2A-B). To define their proliferative potential, we cultured sorted MK progenitors under conditions stimulating their growth and differentiation (stem cell factor, TPO, IL-3, and IL-6). Fanca−/− MK progenitors underwent a first phase of proliferation comparable to WT cells, followed by an abrupt decline of growth that was not observed in the WT population (Figure 2C). At day 6, ∼50% of cells in both populations were still mononuclear, suggesting that Fanca deficiency accelerated the exhaustion of MK progenitors. Next, we evaluated the MK progeny potential by clonogenic assay of nonfractionated BM cells using the MegaCult-C test (colony forming unit [CFU]-MK). Fanca−/− displayed a reduced number and a smaller size of CFU-MK–derived colonies in comparison with WT after 6 days of in vitro culture (Figure 2D), indicating the presence of a defect in MK differentiation. Noticeably, this defect was rescued when Fanca−/− LSK cells were transduced with a retroviral vector expressing FANCA (Figure 2E). To further characterize the process of megakaryopoiesis, we cultured BM cells from WT and Fanca−/− mice in the presence of TPO for 3 days. Both groups of animals presented a similar basal percentage of CD41+ cells in the BM (2.2%; supplemental Figure 1B). However, liquid cultures of BM cells in the presence of TPO resulted in augmentation of the MK population up to 18 ± 1.58% and 12.98 ± 2.66% for WT and Fanca−/− mice, respectively (Figure 2F). Together with the results obtained from the CFU-MK test, our data suggest that a functional defect occurs during megakaryopoiesis in Fanca-deficient mice.
Figure 2.
Proliferation and differentiation are altered in Fanca−/− MK progenitors. (A) Representative flow cytometry plots showing the gating strategy for MK progenitors. (B) Percentage of MK progenitors in Lin− cells of the BM of Fanca−/− and WT mice. (C) Proliferation curve (WT, n = 3; Fanca−/−, n = 4; results presented as means ± SEM, *P < .05, Student t test). (D) Number of MK-CFU colonies scored at day 6 of MegaCult-C from WT and Fanca−/− BM. (E) Number of MK-CFU scored at day 7 of MegaCult-C from LSKs transduced with an EGFP expressing vector (WT EGFP, Fanca−/− EGFP) or FANCA-EGFP vector (Fanca−/− FANCA) (n = 5 mice per group WT EGFP and Fanca−/− EGFP; n = 4 per group Fanca−/− FANCA, ***P < .001, *P < .05, Student t test). (F) Percentages of MKs obtained from nonfractionated nuclear WT and Fanca−/− BM cells after 3 days of culture with TPO (50 ng/mL, *P < .05, Student t test).
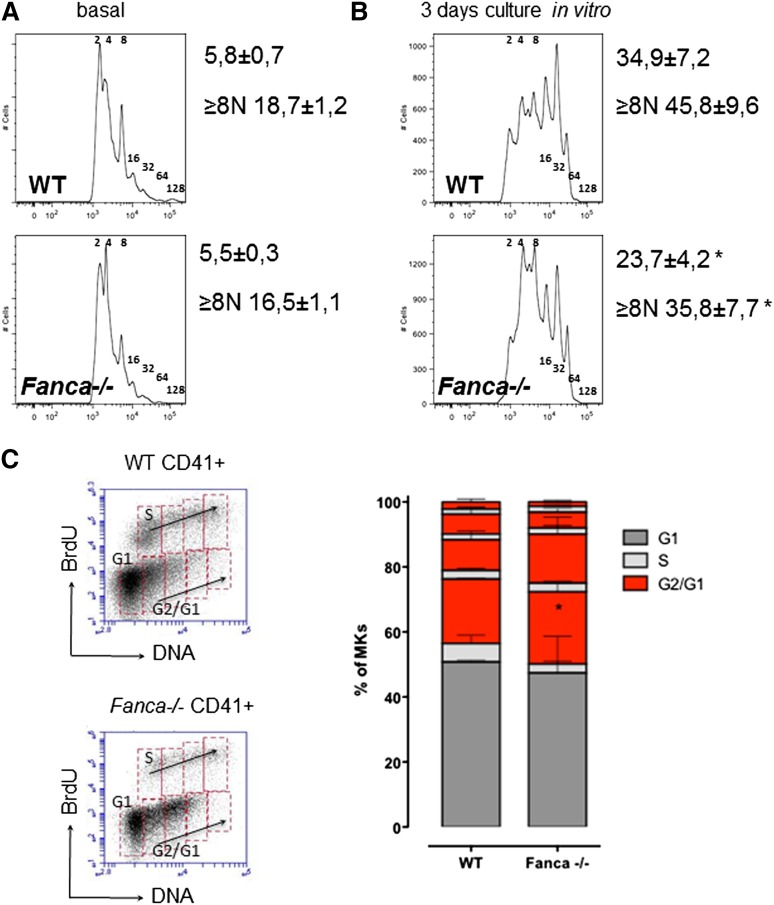
Fanca−/− CD41+ cell populations in BM showed a slight, although not significant, decrease in ploidy (Figure 3A). However, analysis of Fanca−/− CD41+ cells after TPO stimulation showed decreased levels of MKs with high ploidy (≥8N; Figure 3B) compared with controls. Similar results were observed after TPO stimulation in vivo (supplemental Figure 1C-D). Analysis of endomitotic cycling after BrdU incorporation showed that accumulation of cells with low ploidy was accompanied by more pronounced cell cycle accumulation after each replication phase during megakaryopoiesis in Fanca−/− cells compared with WT (Figure 3C). Collectively, these data indicate that the thrombocytopenic phenotype of the Fanca−/− mice is associated with a reduced number of MK progenitors showing limited differentiation capacity.
Figure 3.
Fanca−/− MKs present defects in ploidy and endoreduplication. Graphic representation of MKs ploidy and calculated mean of ploidy (A) at baseline (n = 5) and (B) after 3 days of differentiation in vitro (n = 6, *P < .05, Student t test). (C) Representative plots of CD41-positive cells and BrdU analysis of endomitotic cycles (left); percentages of WT and Fanca−/− MKs at different phases of endomitosis (right; G1 in black; S in gray; G2/G1 in red; n = 3 per group). The results are presented as means ± SEM. *P < .05, 2-way analysis of variance test.
Fanca−/− MKs enter a senescence-like state more readily than WT MKs
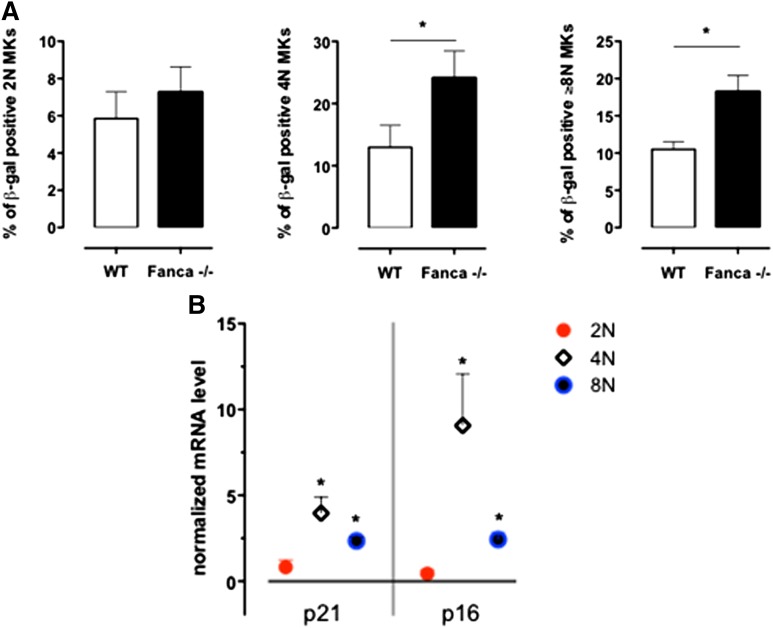
Based on a recent report that MKs enter a programmed senescence-like state during differentiation,16 we performed a β-galactosidase assay on MKs sorted according to their ploidy at day 3 of culture with TPO. We observed significantly increased levels of 4N and ≥8N Fanca−/− SA-β-gal–positive MKs in comparison with WT cells (Figure 4A). Therefore, in the 2N, 4N, and ≥8N sorted populations of WT and Fanca−/− CD41+ cells, we measured by quantitative reverse transcriptase-polymerase chain reaction analysis the expression of the well-established mediators of senescence p21 and p16.17-19 An increased expression of both p21 and p16 was observed in Fanca−/− polyploid MKs (Figure 4B), which confirmed that the observed cycle arrest in those cells resulted, at least partially, from the induction of a senescence program.
Figure 4.
Senescence is increased in Fanca−/− MKs. (A) Frequency of SA-β-galactosidase-positive 2N, 4N, and ≥8N MKs per mouse evaluated in a minimum of 200 cells sorted at day 3 of differentiation in vitro (n = 3, *P < .05, Student t test). (B) Quantitative reverse transcriptase-polymerase chain reaction analysis of p21 and p16 expression in sorted 2N, 4N, and ≥8N Fanca−/− MKs. The results are calculated relative to the WT and normalized against the level of Gapdh (n = 3 each from a pool of 2 mice).
In addition, we assessed the viability of cultured cells using Annexin V staining in the 2N, 4N, and ≥8N cell populations. We observed a slight but significant increase in the percentage of apoptotic Fanca−/− MKs in the 4N population, whereas the percentage of apoptotic cells increased in parallel with ploidy in both Fanca−/− and WT MKs (supplemental Figure 2).
Altogether, our findings indicate that Fanca is required for efficient terminal differentiation of MKs and that loss of its function results in defective polyploidization accompanied by increased levels of apoptotic and senescent MKs. In addition, the increased senescence of Fanca−/− MKs suggests that the accumulation we observed after each endomitotic cycle results from G1 arrest after each nuclear division.
Fanca−/− MKs present high levels of nucleoplasmic bridges
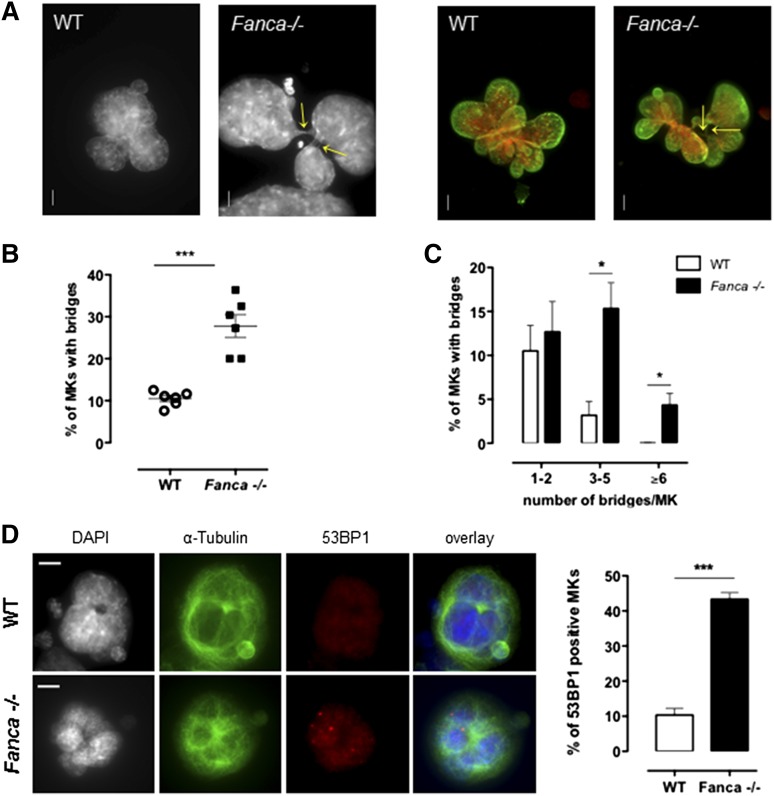
It is clearly established that FANC pathway deficiency results in an increased frequency of anaphase bridges in somatic cells.5,20 Indeed, in FANC pathway-proficient cells, these structures, which are characteristic of incomplete replication or incomplete resolution of replication intermediates, are targeted by FANCD2, which favors their resolution by the end of mitosis, thus ensuring proper chromosome segregation.5 Consequently, we evaluated whether the observed defect in the progression of polyploidization of Fanca−/− MKs was accompanied by an increase in the number of cells with nucleoplasmic bridges (NPBs) and/or in the number of bridges per cell. Indeed, MKs of Fanca-deficient mice displayed higher levels of NPBs than controls (Figure 5A-B), indicating abnormal karyokinesis following the endoreplication process. In addition, we observed a greater than fourfold increase in the number of Fanca−/− MKs with 3 to 5 NPBs compared with the WT; 4.3 ± 1.3% of Fanca−/− MKs presented ≥6 bridges per cell, whereas we did not find any WT MKs with this abnormality (346 scored cells from 4 WT mice; Figure 5C). The presence of higher levels of chromatin bridges indicates the occurrence of replication abnormalities that remain unresolved in FANC pathway-deficient MKs. Accumulation of replication-associated DNA abnormalities accounts for the growth arrest of MKs after each endomitosis and the accelerated entry of Fanca-deficient cells into a senescence-like condition. Accordingly, MKs from Fanca−/− mice demonstrated an enhanced accrual of 53BP1-positive nuclei and increased 53BP1 nuclear foci formation, a hallmark of replication stress-induced DNA damage (Figure 5D; supplemental Figure 3).
Figure 5.
Fanca−/− MKs present high level of nucleoplasmic bridges and DNA damage. (A) Representative view of multilobulated nuclei of WT and Fanca−/− MKs after DAPI (left) and DAPI (red) plus Lamin A/C (green) staining (right; bar = 10 μm). Arrows indicate the location of NPBs. (B,C) Percentages of WT and Fanca−/− MKs with NPBs (B, minimum of 80 cells scored per point, ***P < .001, Student t test) with the indicated number of NPBs per cell (C, n = 3, minimum of 100 cells scored per point, *P < .05, Student t test). (D) Representative images of MKs stained for 53BP1 (left) and percentages of 53BP1-positive MKs (right; n = 3, *P < .05, Student t test, ≥50 cells analyzed per point). Means ± SEM are shown; bar = 10 μm.
Polyploid Fanca−/− MKs accumulate chromosomal breaks
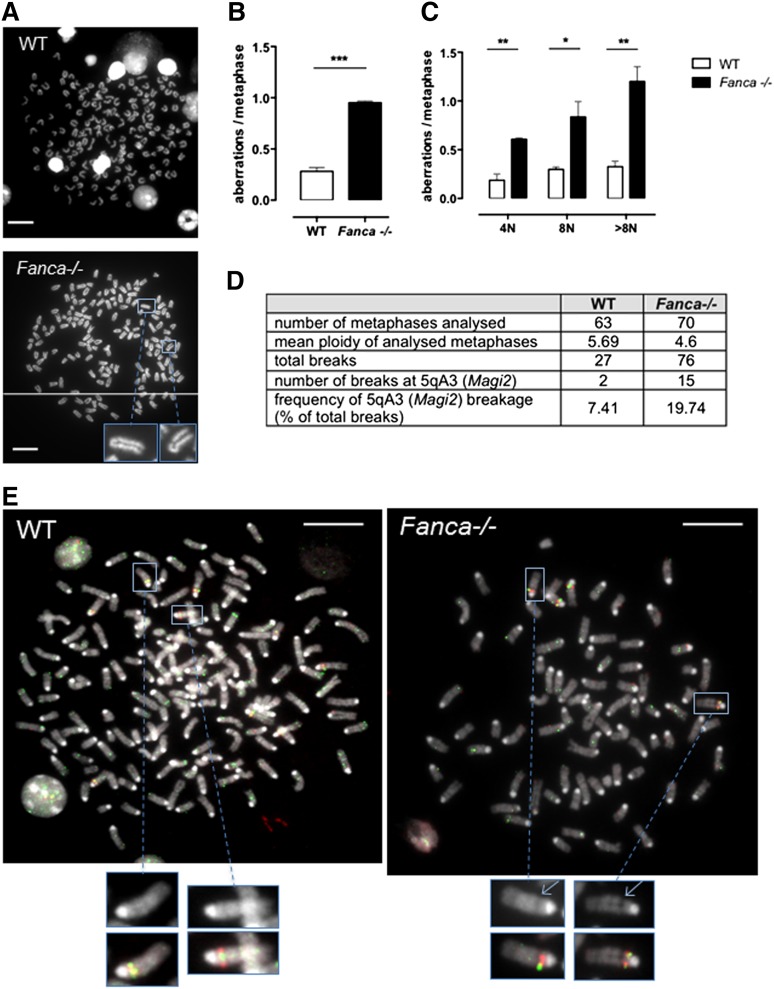
Although we demonstrated that increased arrest of the cell cycle followed by senescence beginning with 4N ploidy is characteristic of MKs of Fanca−/− mice, some cells were able to proceed further through endomitosis. We tested these cells for the presence of chromosomal aberrations. Metaphase spreads were scored for chromatid breaks, fusions, or fragments in differentiating MKs starting from 4N. Polyploid Fanca−/− MKs at day 3 after TPO stimulation presented 3 times more aberrations than WT cells (Figure 6A-B), indicating a progressive accumulation of genomic instability. The frequency of aberrations per metaphase was augmented at each stage of ploidy in Fanca-deficient MKs, and a particularly high level of damage was present in >8N Fanca−/− cells (Figure 6C).
Figure 6.
Fanca−/− MKs accumulate chromosomal aberrations. (A) Representative examples of WT (upper) and Fanca−/− (lower) 8N metaphase spreads. (B) Frequencies of chromosomal breaks, gaps, fusions, and deletions scored in ≥50 metaphases per point (n = 3 mice per group, ***P < .001, Student t test) or (C) scored in 4N, 8N, or > 8N metaphase spreads (minimum of 30 metaphases analyzed per point, n = 3 mice per group, *P < .05, **P < .01, Student t test). Means ± SEM are shown; bar = 10 μm. (D) Frequency of 5qA3 breakage in WT and Fanca−/− (pool of 2 mice WT and 3 mice Fanca−/−; 2 separate experiments shown) metaphases analyzed by FISH, with representative images (E).
Perturbation of DNA replication in somatic cells may result in breaks at CFSs in mitotic chromosomes. To determine whether the chromosomal aberrations we observed in MKs of Fanca−/− mice involved conserved CFSs, we performed a FISH analysis to detect one of the most frequently expressed CFSs in mouse cells, which is localized at 5qA3.14 We evaluated the frequency of gaps and breaks at this CFS and found specific increased breakage at 5qA3 in Fanca−/− MKs compared with their WT counterparts (Figure 6D-E). The latter results corroborate the importance of FANC pathway in the maintenance of chromosomal stability, notably at CFSs, and extend the notion of this central activity to the endomitotic process.
Inhibition of RhoA signaling improves the ploidy of Fanca−/− MKs but increases their genomic instability
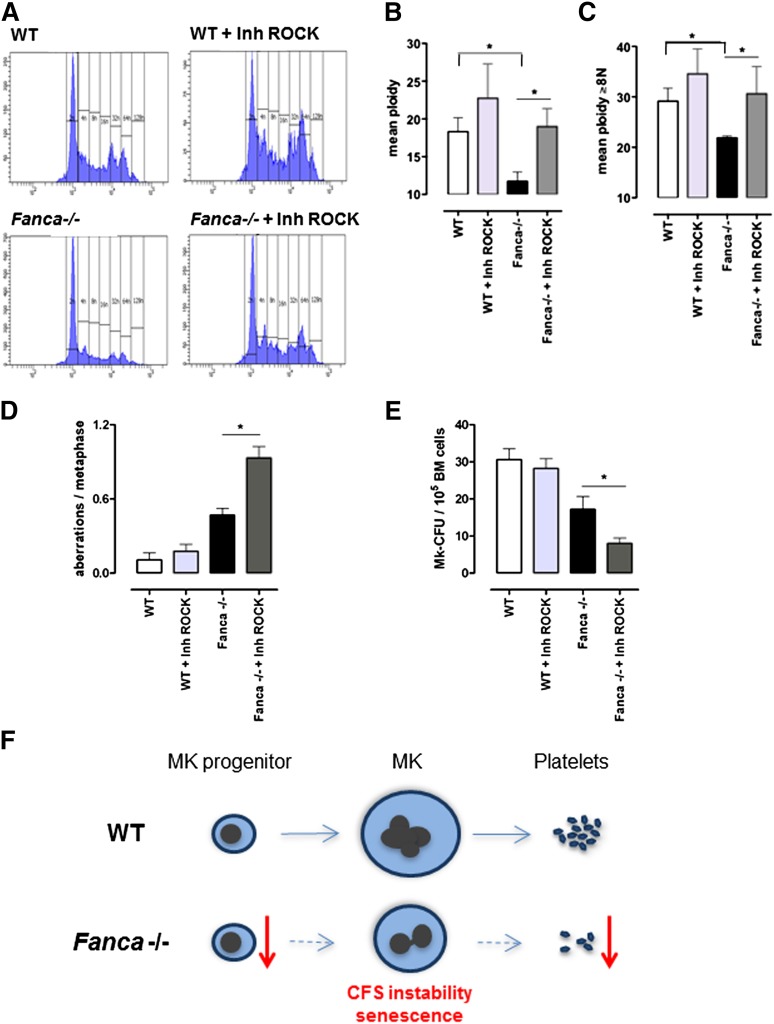
Endomitosis is characterized by repeated cycles of nuclear replication without cytokinesis. During cytokinesis, RhoA signaling is required for assembly of the actomyosin ring at the cleavage furrow to produce contractile force and complete cell division. One of the key effectors of RhoA is the RhoA-associated kinase ROCK, a protein involved in the regulation of cytokinesis by phosphorylation of the myosin light chain. RhoA activity is greatly diminished during the first endomitotic division of differentiating MKs and stays undetectable during the endomitosis of higher-ploidy MKs, contributing to late cytokinesis failure and polyploidization of MKs.21,22 To test whether the polyploidization defect of Fanca-deficient MKs could be rescued by inhibition of the RhoA-ROCK signaling pathway, we used Y27632, a well-characterized and highly specific inhibitor of Rho-associated kinases. It has been shown already that exposure to Y27632 prevents normal cytokinesis and increases ploidy in different types of cells, including MKs.23,24 Strikingly, addition of a ROCK inhibitor significantly attenuated the polyploidization defect of Fanca-deficient MKs (Figure 7A-C). A slight increase of the mean ploidy was also observed in WT cells, supporting the notion that residual RhoA-ROCK activity is present in both Fanca−/− and WT cells and that its pharmacological inhibition improves the polyploidization process in mouse cells. We therefore analyzed the consequences of rescuing polyploidization defects in Fanca-deficient MKs.
Figure 7.
Downregulation of ROCK improves ploidy but increases genomic instability in Fanca−/− MKs. Ploidy analysis of CD41+ cells after 3-day culture with TPO, with or without the ROCK inhibitor Y27632: (A) representative flow cytometry histograms; (B) mean ploidy of all CD41+ cells; and (C) mean ploidy of CD41+ cells with ≥8N (n = 4 per group of WT; n = 5 per group of Fanca−/−, *P < .05, Student t test). (D) Frequency of chromosomal aberrations scored in a minimum of 30 metaphases per point (n = 3 per group, *P < .05, Student t test), with or without ROCK inhibitor. (E) Number of colonies obtained at day 6 of MegaCult-C under the same conditions described above (n = 5 per group, *P < .05, Student t test). Means ± SEM are shown. (F) Proposed model for development of thrombocytopenia in Fanca−/− mice. See discussion for details.
Metaphase spread analysis did not show significant differences in the frequency of chromosomal aberrations after incubation of WT BM cells with the ROCK inhibitor. In contrast, the same treatment further increased the frequency of aberrations in Fanca−/− MKs compared with the already high level found in untreated Fanca−/− MKs (Figure 7D; supplemental Figure 4A-B). Interestingly, we also observed greatly aberrant metaphase spreads with high number of fragmented chromosomes in Fanca−/− cells treated with the ROCK inhibitor, whereas similar metaphases were never observed in WT cells (supplemental Figure 4C).
We did not observe changes in CFU-MKs between WT cells cultured with or without the ROCK inhibitor, which confirmed the lack of toxic side effects. In contrast, we observed an even more pronounced defect in MK differentiation resulting from Fanca deficiency and inhibition of ROCK activity. Fanca−/− cells incubated in the presence of the ROCK inhibitor developed >2 times fewer colonies than cells cultured without the inhibitor (Figure 7E).
Collectively, these data show that, despite its clear role in the improvement of polyploidization, ROCK inhibition leads to exacerbation of genomic instability and fails to improve pro-platelet production capacity of Fanca−/− MKs (supplemental Figure 5).
Discussion
Thrombocytopenia is commonly observed in FA, but the underlying molecular defects are incompletely understood. Here, we describe a previously unappreciated function of Fanca in the differentiation of MKs and in the maintenance of their polyploidization, in which Fanca limits genomic instability and senescence.
Although FA mice are characterized by cellular hypersensitivity to genotoxic agents and reduced fertility, they rarely exhibit the strong hematologic hallmarks of the human disease.9 A defect in maintaining the pool of HSPCs, with a subsequent diminution of LSK cells followed by reduction in pools of CMPs and MEPs, was observed in Fancd2−/− and Fancg−/− mice.25,26 Moreover, Fancd2-Aldh2 double-knockout mice may develop aplastic anemia because of aldehyde-mediated genotoxicity restricted to the HSPC population.27 Finally, it has been reported that tumor necrosis factor-α and CD95 activation suppress erythropoiesis in Fancc−/− mice.28
Similar to other FA mouse models, adult Fanca−/− mice did not present overt characteristics of anemia. No significant diminution in the pools of LSKs, CMPs, or MEPs were found (Figure 1).29 However, Fanca−/− mice clearly presented thrombocytopenia (this work),8,10 a defect that has also been reported in Fancp−/− mice.30 The observed reduction in platelet counts (Table 1) and pro-platelet production in vitro (Figure 1A) results from a specific defect affecting the differentiation of committed MK progenitors. Polyploidization of MKs depends on sequential, repeated rounds of DNA replication and endomitosis separated by very short gap phases. Fanca-deficient MKs present a spontaneously high level of DNA damage, and accumulate nucleoplasmic bridges, likely as a result of an incapability to overcome replication difficulties safely. We observed a progressive accumulation of genomic instability throughout the endomitotic cycles and cell cycle arrest/delay after each replication phase. We suggest that the absence of a true G2 phase increases the risk of naturally occurring, difficult-to-replicate genomic regions (such as CFSs) being left incompletely replicated at the onset of endomitosis. Indeed, certain chromosomal aberrations observed in the MKs of Fanca−/− mice involved conserved fragile sites (Figure 6). CFSs per se may then represent a relevant endogenous source of replication stress-driven DNA damage in Fanca-deficient cells in vivo. It would be interesting to determine whether this particular location of breaks impacts the expression of genes located close to CFSs and involved in megakaryocyte development or function.
Unrepaired DNA lesions and bridges can trigger programmed cell death or permanent cell cycle arrest/senescence. We observed a slightly augmented level of cell death in Fanca−/− 4N MKs; in addition, polyploid cells presented an increased level of β-galactosidase activity and transcription of p21 and p16, indicating a strong induction of the senescence program in Fanca−/− MKs. Interestingly, an exacerbated p53/p21 response has also been observed in HSPC of FA patients.31 The authors suggested unresolved DNA damage as the likely basis for p53/p21 activation. Here we show that replication stress occurring in physiological conditions is able to induce defective karyokinesis and accumulation of chromosome instability in FANC-deficient MKs, leading to an increased senescence response. However, some cells were able to escape the blockade of senescence and undergo further rounds of endomitosis. The mechanism underlying this senescence bypass needs further evaluation. Endomitosis is characterized by a late failure of cytokinesis, coupled to decreased activity of RhoA-associated kinases.21,32 Previous studies reported that inhibitors of the RhoA-ROCK pathway, notably Y27632, increase polyploidization of MKs.16,21 Interestingly, in the present study, treatment with Y27632 significantly restored the ploidy distribution in Fanca−/− MKs, but not pro-platelet production capacity (supplemental Figure 5). Inhibition of the RhoA-ROCK signaling pathway in Fanca−/− MKs resulted in massive chromosome fragmentation, and clonogenic potential was even more strongly affected than in Fanca−/− MKs cultured without the inhibitor. This observation strongly supports our hypothesis that augmentation of genomic instability could contribute to the decreased differentiation potential of Fanca−/− MKs, thus resulting in decreased colony formation and ultimately limited platelet production.
Collectively, our data clearly demonstrate a requirement of Fanca for efficient megakaryopoiesis. First, we showed that Fanca−/− mice present a highly decreased number and reduced proliferative potential of BM-derived MK progenitors. We suggest that the Fanca−/− MK progenitors are forced to proliferate to compensate for the normal number of MKs. This effect could induce endogenous replicative stress, leading to exhaustion of progenitors. Fanca serves as a guardian of genomic integrity during endomitosis and its deficiency increases the frequency of unresolved replication intermediates and defective karyokinesis, which forces the cell to enter persistent cell cycle arrest. It is worth noting that with every new round of endomitosis, the frequency of chromosomal aberrations increases, which finally prevents further maturation of megakaryocytes, thereby affecting the production of platelets (Figure 7F). Our results suggest that the senescence-like program entered after each endomitotic cycle could represent a barrier preventing cells with damaged DNA to progress, thereby protecting cellular fitness. Elucidation of the molecular mechanisms underlying the replication stress response during endomitotic cycles could open new avenues for understanding and treating FA.
Acknowledgments
The authors thank members of the F. Rosselli team for helpful discussion and advice, P. Rameau and Y. Lécluse (Imaging and Cytometry Platform of IGR) for cell sorting, and F. Porteu for critical reading of the manuscript and valuable suggestions. The authors also thank M. Debatisse, B. LeTallec, and T. Wilhelm for kindly providing the mouse 5qA3 probe and for help with FISH experiments. The authors are grateful to M. Milsom for the retroviral vector for expression of FANCA.
This work was supported by grants from La Ligue Contre le Cancer, Agence Nationale de la Recherche (ANR-08-GENO-0013), and INCa-DGOS-Inserm 6043.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: P.P. designed and performed experiments, analyzed data, performed statistical analysis, and wrote the manuscript; P.F. provided Fanca−/− mice; W.V. contributed to research design and data interpretation; F.R. conceived the project and contributed to data interpretation and manuscript writing; and V.N. conceived the project, planned research, interpreted data, and contributed to manuscript writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Valeria Naim, Université Paris Sud, Laboratoire Stabilité Génétique et Oncogenèse, CNRS UMR 8200, Institut de Cancérologie Gustave Roussy, 114 rue Edouard Vaillant, 94805 Villejuif, France; e-mail: valeria.naim@gustaveroussy.fr.
References
- 1.Deutsch VR, Tomer A. Megakaryocyte development and platelet production. Br J Haematol. 2006;134(5):453–466. doi: 10.1111/j.1365-2141.2006.06215.x. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RA. Cell cycle regulators and hematopoiesis. Oncogene. 2002;21(21):3403–3413. doi: 10.1038/sj.onc.1205325. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi H, Nakanishi K, Kojima S. Inherited bone marrow failure syndromes in 2012. Int J Hematol. 2013;97(1):20–29. doi: 10.1007/s12185-012-1249-9. [DOI] [PubMed] [Google Scholar]
- 4.Kee Y, D’Andrea AD. Expanded roles of the Fanconi anemia pathway in preserving genomic stability. Genes Dev. 2010;24(16):1680–1694. doi: 10.1101/gad.1955310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naim V, Rosselli F. The FANC pathway and BLM collaborate during mitosis to prevent micro-nucleation and chromosome abnormalities. Nat Cell Biol. 2009;11(6):761–768. doi: 10.1038/ncb1883. [DOI] [PubMed] [Google Scholar]
- 6.Naim V, Rosselli F. The FANC pathway and mitosis: a replication legacy. Cell Cycle. 2009;8(18):2907–2911. doi: 10.4161/cc.8.18.9538. [DOI] [PubMed] [Google Scholar]
- 7.Lordier L, Pan J, Naim V, et al. Presence of a defect in karyokinesis during megakaryocyte endomitosis. Cell Cycle. 2012;11(23):4385–4389. doi: 10.4161/cc.22712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng NC, van de Vrugt HJ, van der Valk MA, et al. Mice with a targeted disruption of the Fanconi anemia homolog Fanca. Hum Mol Genet. 2000;9(12):1805–1811. doi: 10.1093/hmg/9.12.1805. [DOI] [PubMed] [Google Scholar]
- 9.Bakker ST, de Winter JP, te Riele H. Learning from a paradox: recent insights into Fanconi anaemia through studying mouse models. Dis Model Mech. 2013;6(1):40–47. doi: 10.1242/dmm.009795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Río P, Segovia JC, Hanenberg H, et al. In vitro phenotypic correction of hematopoietic progenitors from Fanconi anemia group A knockout mice. Blood. 2002;100(6):2032–2039. [PubMed] [Google Scholar]
- 11.Santaguida M, Schepers K, King B, et al. JunB protects against myeloid malignancies by limiting hematopoietic stem cell proliferation and differentiation without affecting self-renewal. Cancer Cell. 2009;15(4):341–352. doi: 10.1016/j.ccr.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strassel C, Eckly A, Léon C, et al. Hirudin and heparin enable efficient megakaryocyte differentiation of mouse bone marrow progenitors. Exp Cell Res. 2012;318(1):25–32. doi: 10.1016/j.yexcr.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Pawlikowska P, Leray I, de Laval B, et al. ATM-dependent expression of IEX-1 controls nuclear accumulation of Mcl-1 and the DNA damage response. Cell Death Differ. 2010;17(11):1739–1750. doi: 10.1038/cdd.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Tallec B, Millot GA, Blin ME, Brison O, Dutrillaux B, Debatisse M. Common fragile site profiling in epithelial and erythroid cells reveals that most recurrent cancer deletions lie in fragile sites hosting large genes. Cell Reports. 2013;4(3):420–428. doi: 10.1016/j.celrep.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Pronk CJH, Rossi DJ, Månsson R, et al. Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell. 2007;1(4):428–442. doi: 10.1016/j.stem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Besancenot R, Chaligné R, Tonetti C, et al. A senescence-like cell-cycle arrest occurs during megakaryocytic maturation: implications for physiological and pathological megakaryocytic proliferation. PLoS Biol. 2010;8(9) doi: 10.1371/journal.pbio.1000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9(6):400–414. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmitt CA, Fridman JS, Yang M, et al. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell. 2002;109(3):335–346. doi: 10.1016/s0092-8674(02)00734-1. [DOI] [PubMed] [Google Scholar]
- 19.Baker DJ, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vinciguerra P, Godinho SA, Parmar K, Pellman D, D’Andrea AD. Cytokinesis failure occurs in Fanconi anemia pathway-deficient murine and human bone marrow hematopoietic cells. J Clin Invest. 2010;120(11):3834–3842. doi: 10.1172/JCI43391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lordier L, Jalil A, Aurade F, et al. Megakaryocyte endomitosis is a failure of late cytokinesis related to defects in the contractile ring and Rho/Rock signaling. Blood. 2008;112(8):3164–3174. doi: 10.1182/blood-2008-03-144956. [DOI] [PubMed] [Google Scholar]
- 22.Chang Y, Auradé F, Larbret F, et al. Proplatelet formation is regulated by the Rho/ROCK pathway. Blood. 2007;109(10):4229–4236. doi: 10.1182/blood-2006-04-020024. [DOI] [PubMed] [Google Scholar]
- 23.Avanzi MP, Chen A, He W, Mitchell WB. Optimizing megakaryocyte polyploidization by targeting multiple pathways of cytokinesis. Transfusion. 2012;52(11):2406–2413. doi: 10.1111/j.1537-2995.2012.03711.x. [DOI] [PubMed] [Google Scholar]
- 24.Kosako H, Yoshida T, Matsumura F, Ishizaki T, Narumiya S, Inagaki M. Rho-kinase/ROCK is involved in cytokinesis through the phosphorylation of myosin light chain and not ezrin/radixin/moesin proteins at the cleavage furrow. Oncogene. 2000;19(52):6059–6064. doi: 10.1038/sj.onc.1203987. [DOI] [PubMed] [Google Scholar]
- 25.Parmar K, Kim J, Sykes SM, et al. Hematopoietic stem cell defects in mice with deficiency of Fancd2 or Usp1. Stem Cells. 2010;28(7):1186–1195. doi: 10.1002/stem.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barroca V, Mouthon MA, Lewandowski D, et al. Impaired functionality and homing of Fancg-deficient hematopoietic stem cells. Hum Mol Genet. 2012;21(1):121–135. doi: 10.1093/hmg/ddr447. [DOI] [PubMed] [Google Scholar]
- 27.Garaycoechea JI, Crossan GP, Langevin F, Daly M, Arends MJ, Patel KJ. Genotoxic consequences of endogenous aldehydes on mouse haematopoietic stem cell function. Nature. 2012;489(7417):571–575. doi: 10.1038/nature11368. [DOI] [PubMed] [Google Scholar]
- 28.Otsuki T, Nagakura S, Wang J, Bloom M, Grompe M, Liu JM. Tumor necrosis factor-alpha and CD95 ligation suppress erythropoiesis in Fanconi anemia C gene knockout mice. J Cell Physiol. 1999;179(1):79–86. doi: 10.1002/(SICI)1097-4652(199904)179:1<79::AID-JCP10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 29.Milsom MD, Lee AW, Zheng Y, Cancelas JA. Fanca-/- hematopoietic stem cells demonstrate a mobilization defect which can be overcome by administration of the Rac inhibitor NSC23766. Haematologica. 2009;94(7):1011–1015. doi: 10.3324/haematol.2008.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crossan GP, van der Weyden L, Rosado IV, et al. Sanger Mouse Genetics Project. Disruption of mouse Slx4, a regulator of structure-specific nucleases, phenocopies Fanconi anemia. Nat Genet. 2011;43(2):147–152. doi: 10.1038/ng.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ceccaldi R, Parmar K, Mouly E, et al. Bone marrow failure in Fanconi anemia is triggered by an exacerbated p53/p21 DNA damage response that impairs hematopoietic stem and progenitor cells. Cell Stem Cell. 2012;11(1):36–49. doi: 10.1016/j.stem.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao Y, Smith E, Ker E, et al. Role of RhoA-specific guanine exchange factors in regulation of endomitosis in megakaryocytes. Dev Cell. 2012;22(3):573–584. doi: 10.1016/j.devcel.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]