Abstract
Background
Fluticasone furoate (FF) is a novel, once-daily inhaled corticosteroid (ICS) that has been shown to improve lung function vs. placebo in asthma patients. This study evaluated the efficacy and safety of FF 50 mcg compared with placebo in asthma patients uncontrolled by non-ICS therapy.
Methods
This 12-week, multicentre, randomized, double-blind, placebo-controlled, parallel-group, phase III study randomized 248 patients (aged ≥12 years) to once-daily FF 50 mcg administered via the ELLIPTA™a dry powder inhaler or placebo. The primary endpoint was change from baseline in pre-dose evening trough forced expiratory volume in one second (FEV1). Secondary endpoints were change from baseline in percentage of rescue-free 24-h periods (powered), evening and morning peak expiratory flow, symptom-free 24-h periods and withdrawals due to lack of efficacy. Other endpoints included Asthma Control Test™, Asthma Quality of Life Questionnaire and ELLIPTA ease of use questions. Safety was assessed throughout the study.
Results
There was a significant difference in evening trough FEV1 between FF 50 mcg and placebo (treatment difference: 120 mL; p = 0.012). There was also a significant difference in rescue-free 24-h periods (11.6%; p = 0.004) vs. placebo. There were numerically greater improvements with FF vs. placebo for all remaining secondary endpoints. The incidence of adverse events was lower with FF (31%) than with placebo (38%); few were treatment-related (FF 50 mcg: n = 1, <1%; placebo: n = 4, 3%).
Conclusion
FF 50 mcg once daily significantly improved FEV1 and percentage of rescue-free 24-h periods experienced over 12 weeks vs. placebo, and was well tolerated.
Trial registration
www.clinicaltrials.gov, registration number: NCT01436071
Keywords: Fluticasone furoate, Inhaled corticosteroid, Lung function, Once daily, Safety
Background
Failure to achieve asthma control can impact patients’ daily lives and results in persistent symptoms, more frequent exacerbations and absenteeism from work and school [1,2]. Inhaled corticosteroids (ICS) are the most effective anti-inflammatory treatments for all severities of persistent asthma [3–5]. Patient adherence is a key component to the overall success of asthma treatment, and it has been demonstrated that compliance with a once-daily ICS is better than with a twice-daily regimen [6].
Fluticasone furoate (FF) is a novel once-daily ICS treatment for asthma [7–11], which is also used in combination with the long-acting β2-agonist (LABA) vilanterol (VI) for the once-daily treatment of asthma and COPD [12–14]. Animal and human pharmacology studies show that FF has a long duration of action and prolonged retention in the lung, suggesting it is appropriate for once-daily dosing [15,16]. As part of the overall FF clinical development program, a dose-ranging study (25–200 mcg doses of FF) showed that FF 50 mcg administered over 8 weeks was the minimum dose required to achieve significant improvements in evening trough forced expiratory volume in 1 s (FEV1) and the percentage of rescue-free 24-h periods compared with placebo [7].
This 12-week study sought to evaluate the efficacy and safety of once-daily FF 50 mcg dosed in the evening in asthma patients aged ≥12 years who were uncontrolled on short-acting β2-agonists (SABA) and/or leukotriene modifying agent. One other study with FF 50 mcg has been published [7], which was an 8-week dose ranging study. Two phase III studies of longer duration (of which this is one) comparing FF 50 mcg with placebo have been conducted in SABA only patients, to determine whether FF 50 mcg is a suitable starting dose for asthma patients not already using a controller medication. Preliminary results have been presented in abstract form [17].
Methods
Patients
Patients were aged ≥12 years with a diagnosis of asthma [4] made at least 12 weeks prior to screening and being treated with non-ICS controllers (a SABA alone or in combination with a leukotriene modifying agent); the use of ICS or LABA was not permitted for at least 4 weeks prior to the initial screening visit. Patients had to demonstrate a best FEV1 of ≥60% of the predicted normal value and ≥12% and 200 mL reversibility of FEV1 within 10–40 minutes following 2–4 inhalations of albuterol/salbutamol. Eligible patients also had no evidence of oral/oropharyngeal candidiasis.
At the end of a 2-week run-in period, patients were randomized if they had an evening pre-dose FEV1 ≥ 60% of the predicted normal value and, on at least 4 of the last 7 consecutive days of the run-in period, had documented use of albuterol/salbutamol and/or exhibited asthma symptoms and completed all morning and evening eDiary entries. Written informed consent was obtained from each patient.
Study design and treatments
This was a phase III, multicentre, randomized, placebo-controlled, double-blind, parallel-group study conducted between 12th September 2011 and 7th August 2012 at 19 centers in four countries (Mexico, Peru, Russia, United States) (GSK study number FFA115283; www.clinicaltrials.gov registration number NCT01436071). The study was approved by local ethics committees and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Patients were randomized (1:1) to receive FF 50 mcg or placebo for a period of 12 weeks; both treatments were administered by the ELLIPTA dry powder inhaler once daily in the evening. Patients were randomized in accordance with a central randomization schedule generated by the sponsor using a validated computerized system (RandAll [GlaxoSmithKline, UK]), after a telephone call to the Registration and Medication Ordering System (RAMOS [GlaxoSmithKline, UK]). Both patients and investigators were blinded to treatment allocations. Treatment compliance was assessed by reviewing the dose counter on the ELLIPTA device. All patients received albuterol/salbutamol, to be used as needed throughout the run-in and treatment periods; no other asthma medications were permitted. The following non-asthma medications were permitted during the study: decongestants; intranasal and topical corticosteroids; immunotherapy; short- and long-acting antihistamines; and antihistamine eye drops.
Outcome measurements
The primary efficacy endpoint was change from baseline in pre-dose evening (trough) FEV1 at Week 12. FEV1 measurements were performed in the clinic at Weeks 2, 4, 8 and 12 and were made within 1 h of the time FEV1 was measured at baseline and approximately 24-h after the last evening dose of medication.
The powered secondary endpoint was change from baseline in the percentage of rescue-free 24-h periods during the 12-week treatment period. Other secondary endpoints were: change from baseline in daily evening and morning peak expiratory force (PEF) averaged over the 12-week treatment period; change from baseline in the percentage of symptom-free 24-h periods during the 12-week treatment period; and number of withdrawals from study due to lack of efficacy. PEF measurements, symptoms and use of rescue medication were recorded daily using an eDiary.
Other selected endpoints included: change from baseline in Asthma Control TestTM (ACT) score at Week 12; percentage of patients controlled (defined as having an ACT score ≥20) at Week 12; change from baseline in Asthma Quality of Life Questionnaire (AQLQ) + 12 Total score at Week 12; and ease of use questions on the ELLIPTA dry powder inhaler at the end of 4 weeks of treatment.
Safety evaluations
Safety endpoints included the incidence of adverse events (AEs; coded using the Medical Dictionary for Regulatory Activities dictionary) and severe asthma exacerbations throughout the 12-week treatment period. A severe exacerbation was defined as deterioration of asthma requiring the use of systemic/oral corticosteroids for at least 3 days or an inpatient hospitalization or emergency department visit due to asthma that required systemic corticosteroids. Oropharyngeal examination was performed throughout the duration of the treatment period.
Statistical analysis
A total of 220 randomized patients were expected to provide 104 evaluable patients per arm, giving 94% power to detect a difference of 200 mL between FF 50 mcg and placebo groups in evening trough FEV1, with significance declared at the two-sided 5% level. This also provided 95% power to detect a difference of 15% between FF 50 mcg and placebo groups in the change from baseline in percentage of rescue-free 24-h periods, with significance declared at the two-sided 5% level. The overall power of the study to detect treatment differences between FF 50 mcg and placebo for the primary and powered secondary endpoints was 90%.
The primary efficacy endpoint was the change from baseline in pre-dose evening (trough) FEV1 at the end of treatment (Week 12). This was analysed using an Analysis of Covariance (ANCOVA) model, which allowed for effects due to baseline FEV1, region, sex, age and treatment group. Last Observation Carried Forward was used to impute missing data. A supporting analysis was performed using a repeated measures model. Powered secondary, secondary and other endpoint comparisons were also analyzed using ANCOVA, with the exception of withdrawals due to lack of efficacy (analyzed using Fisher’s Exact test) and the percentage of patients controlled (ACT score ≥20; analyzed using logistic regression).
The safety population comprised all patients randomized to treatment and who received at least one dose of study medication. The intent-to-treat (ITT) population comprised all patients in the safety population except for 20 (10 from each treatment arm) patients excluded due to data quality issues identified through a site audit during a previous study. The per protocol population comprised all ITT patients who did not have any full protocol deviations.
In order to account for multiplicity across the key endpoints, a step-down closed testing procedure was applied for the primary and secondary endpoints whereby failure to achieve significance (p < 0.05) for the primary treatment comparison (FF 50 mcg vs. placebo), at any point in the hierarchy, meant that all tests lower down in the hierarchy were interpreted as descriptive only. The hierarchy was as follows: (1) evening trough FEV1; (2) rescue-free 24-h periods; (3) evening PEF; (4) morning PEF; (5) symptom-free 24-h periods; and (6) withdrawals due to lack of efficacy. If significance was achieved at each stage of the hierarchy, then all other efficacy endpoints were tested without further multiplicity adjustment.
Results
Study population
Of 449 patients screened, 248 were randomized, of whom 242 comprised the safety population and 222 comprised the ITT population. Within the ITT population, 90 (81%) placebo and 100 (90%) FF 50 mcg patients completed the study (Figure 1). Mean age, percentage of female patients and screening/baseline characteristics of lung function were similar between treatment groups (Table 1), with a baseline mean% predicted FEV1 of 81.58% in the ITT population. The majority of patients were of Hispanic/Latino ethnicity (73% placebo; 64% FF 50 mcg). Overall compliance with study medication (measured during the study using the dose counter on the DPI) was high and similar between treatment groups, at 97.7% in the placebo group and 99.0% in the FF 50 mcg group.
Figure 1.
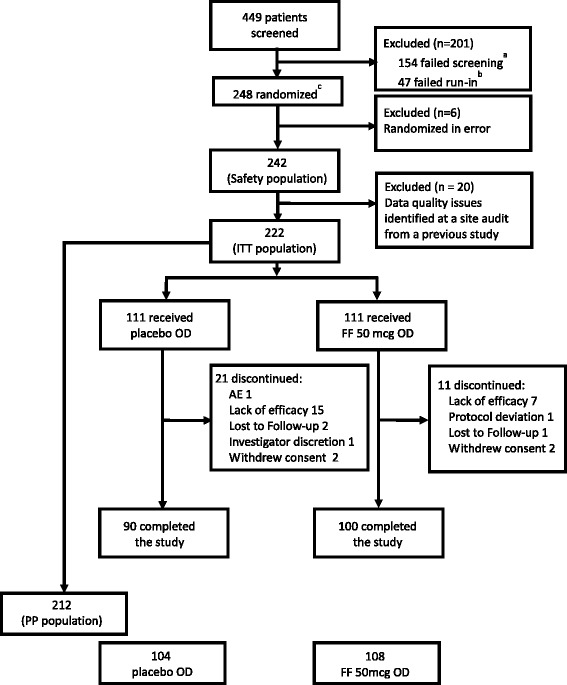
Patient disposition. aMain reason was patients did not meet inclusion/exclusion criteria: 151(34%); bmain reason was patients did not meet continuation criteria: 36(8%); AE = adverse event; FF = fluticasone furoate; ITT = intent-to-treat; OD = once daily; PP = per protocol.
Table 1.
Patient demographics and lung function at screening/baseline (intent-to-treat population)
| Placebo OD PM (N = 111) | FF 50 mcg OD PM (N = 111) | |
|---|---|---|
| Age, mean (SD) | 33.8 (13.90) | 36.7 (16.16) |
| Age range, years | 12–68 | 12–77 |
| Female, n (%) | 70 (63) | 63 (57) |
| Race, n (%) | ||
| American Indian or Alaska Native | 59 (53) | 45 (41) |
| White | 29 (26) | 42 (38) |
| American Indian or Alaska Native and White | 21 (19) | 24 (22) |
| Other | 2 (2) | 0 |
| Screening characteristics, mean (SD) | ||
| Pre-bronchodilator FEV1 (L) | 2.527 (0.6940) | 2.452 (0.6843) |
| Percent predicted FEV1, % | 77.33 (12.884) | 74.71 (9.493) |
| Post-bronchodilator FEV1 (L) | 3.139 (0.8784) | 3.047 (0.8443) |
| Percent reversibility FEV1, % | 24.32 (10.368) | 24.77 (9.906) |
| Baseline characteristics, mean (SD) | ||
| Pre-bronchodilator FEV1* (L) | 2.712 (0.8305) | 2.669 (0.8172) |
| Percent predicted FEV1*, % | 82.30 (14.115) | 80.85 (12.277) |
| Rescue-free 24-h periods, % | 7.5 (21.04) | 10.2 (21.49) |
| Symptom-free 24-h periods, % | 3.2 (12.73) | 5.0 (14.53) |
| PM PEF (L/min) | 356.8 (120.53) | 359.0 (121.14) |
| AM PEF (L/min) | 350.3 (115.89) | 349.2 (117.21) |
*Assessed in 110 patients from each treatment arm; FEV1 = forced expiratory volume in one second; FF = fluticasone furoate; OD = once daily; PEF = peak expiratory flow PM = evening; SD = standard deviation.
Efficacy
For the primary endpoint, the change from baseline evening trough FEV1 at Week 12 was 157 mL with FF 50 mcg and 38 mL with placebo; the treatment difference of 120 mL (p = 0.012) was statistically significant (Table 2; Figure 2). Repeated measures analysis demonstrated that trough FEV1 was consistently greater with FF 50 mcg vs. placebo throughout the study period (Figure 3). Supporting analysis of the per protocol population was similar (treatment difference in favor of FF 50 mcg: 131 mL [95% CI: 38, 224]; p = 0.006).
Table 2.
Results for primary, secondary and selected other endpoints (intent-to-treat population)
| Endpoint | Placebo OD PM (N = 111) | FF 50 mcg OD PM (N = 111) | ||||
|---|---|---|---|---|---|---|
| n | Mean (SE) | n | Mean (SE) | Difference (95% CI) | ||
| Pre-dose evening FEV1 a, L | Primary | 106 | 0.038 (0.0333) | 108 | 0.157 (0.0330) | 0.120* (0.026, 0.213) |
| Rescue-free 24-h periodsb, % | Secondary (powered) | 110 | 17.1 (2.78) | 111 | 28.7 (2.77) | 11.6*(3.8, 19.4) |
| Evening PEFb, L/min | Secondary | 110 | 19.5 (3.72) | 111 | 22.8 (3.70) | 3.3 (−7.2, 13.7) |
| Morning PEFb, L/min | Secondary | 110 | 22.9 (3.65) | 111 | 34.5 (3.64) | 11.6 (1.4, 21.9) |
| Symptom-free 24-h periodsb, % | Secondary | 110 | 14.0 (2.49) | 111 | 22.6 (2.47) | 8.6 (1.6, 15.6) |
| Number of patients | Number of patients | |||||
| Withdrawal due to lack of efficacyb, % | Secondary | 14 | 6 | |||
| n | Mean (SE) | n | Mean (SE) | Difference (95% CI) | ||
| Asthma Control Test™ (ACT) scorea | Other | 92 | 4.0 (0.39) | 100 | 6.2 (0.38) | 2.2 (1.1, 3.3) |
| n | Number of patients | n | Number of patients | Odds Ratio (95% CI) | ||
| Patients with ACT score ≥20a, % | Other | 92 | 55 | 100 | 69 | 1.88 (0.97, 3.65) |
| n | Mean (SE) | n | Mean (SE) | Difference (95% CI) | ||
| Total Asthma Quality of Life Questionnaire (AQLQ) + 12 scorea | Other | 92 | 0.84 (0.097) | 100 | 1.30 (0.093) | 0.45 (0.18, 0.72) |
Change from baseline: aat Week 12, bduring or averaged over Weeks 1–12. *statistically significant. FEV1 = forced expiratory volume in one second; PEF = peak expiratory flow; FF = fluticasone furoate; OD = once daily; PM = evening; SE = standard error; CI = confidence interval.
Figure 2.
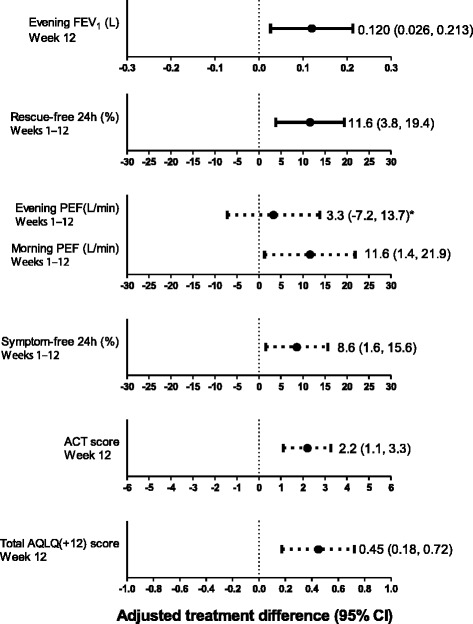
Adjusted treatment differences for the change from baseline for primary, and selected secondary and other endpoints (intent-to-treat population). *Data were analysed using a closed step-down statistical hierarchy, whereby failure to achieve significance at any point in the hierarchy meant that statistical significance could not be inferred for subsequent endpoints in the hierarchy. Solid lines indicate a significant treatment difference; dashed lines indicate differences which are not significant (evening PEF) or for which significance cannot be inferred (all remaining endpoints); FEV1 = forced expiratory volume in one second; PEF = peak expiratory flow; ACT = Asthma Control Test; AQLQ = Asthma Quality of Life Questionnaire; CI = confidence interval.
Figure 3.
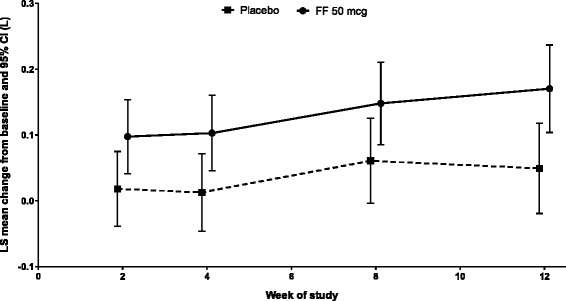
Repeated measures analysis of change from baseline in trough FEV 1 (L) (intent-to-treat population). FF = fluticasone furoate; OD = once daily; LS = least squares; CI = confidence interval.
For the powered secondary endpoint, there was a statistically significant increase from baseline in the percentage of rescue-free 24-h periods (28.7%) compared with placebo (17.1%; treatment difference of 11.6%; p = 0.004) (Table 2, Figure 2); this improvement equated to an additional 0.8 rescue-free 24-h periods per week with FF 50 mcg treatment. Rescue-free 24-h periods were consistently greater over the course of the study with FF 50 mcg compared with placebo (Figure 4A).
Figure 4.
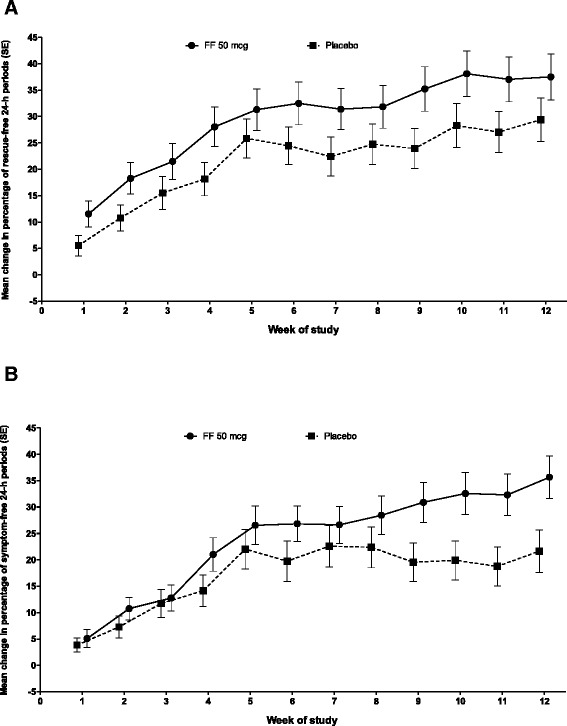
Change from baseline in percentage of rescue-free (A) and symptom-free (B) 24-h periods during the 12-week study period (intent-to-treat population). FF = fluticasone furoate; OD = once daily; SE = standard error.
The results for selected secondary and other endpoints are summarized in Table 2 and Figure 2. The change from baseline in evening PEF over the 12-week treatment period was increased with FF 50 mcg (22.8 L/min) and placebo (19.5 L/min), but the treatment difference (3.3 L/min) was not statistically significant (p = 0.536). In accordance with the pre-defined statistical hierarchy of endpoints, significance could not be inferred for the remaining endpoints. Increase from baseline in morning PEF was numerically greater for FF 50 mcg (34.5 L/min) compared with placebo treatment (22.9 L/min; treatment difference of 11.6 L/min). Likewise, the increase from baseline in the percentage of symptom-free 24-h periods was also numerically greater for FF 50 mcg (22.6%) compared with placebo treatment (14.0%; treatment difference of 8.6%), which equates to an additional 0.6 symptom-free 24-h periods per week with FF 50 mcg treatment. The greatest improvement in symptom-free 24-h periods with FF 50 mcg compared with placebo was observed during the last 4 weeks of the study (Figure 4B). A numerically greater proportion of patients in the placebo group withdrew due to lack of efficacy (14%) compared with patients in the FF 50 mcg group (6%) (Figure 5).
Figure 5.
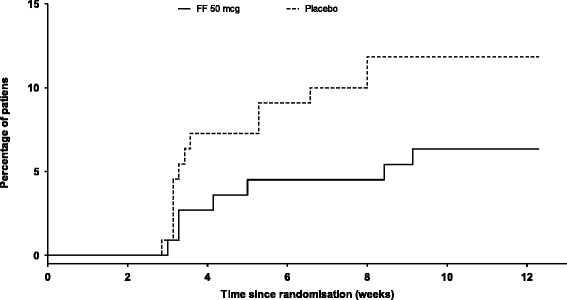
Time to withdrawal due to lack of efficacy (intent-to-treat population). FF = fluticasone furoate; OD = once daily.
Numerically greater increases in ACT score, proportion of patients with an ACT score ≥20 and change from baseline in Total AQLQ(+12) score were observed for FF 50 mcg compared with placebo (Table 2; Figure 2). At baseline, most patients were able to use the ELLIPTA inhaler correctly after being instructed once (98% FF 50 mcg; 96% placebo). At Week 4, most patients rated the ELLIPTA inhaler as ‘easy/very easy’ to use (97%) and ‘easy/very easy’ to see how many doses of medication were left in the inhaler (95%).
Safety assessments
Within the safety population, the overall incidence of on-treatment AEs was similar for placebo (38%) and FF 50 mcg (31%); the most frequently occurring on-treatment AEs were headache, nasopharyngitis, pharyngitis and influenza (Table 3). The incidence of treatment-related AEs was low in both treatment groups: 3% (n = 4) with placebo (pharyngitis, n = 1; upper respiratory tract infection, n = 1; dysgeusia and headache, n = 1; urticaria, n = 1); <1% (n = 1) with FF 50 mcg (contusion, n = 1). One patient in the placebo group was withdrawn from the study following an episode of urticaria. Additionally, three other AEs of special interest were reported: hypersensitivity (placebo), oropharyngeal pain (FF 50 mcg) and skeletal injury (FF 50 mcg). However, none of these were considered related to study medication or resulted in study withdrawal.
Table 3.
Summary of most frequent on-treatment AEs and serious AEs (safety population)
| n, % | Placebo OD PM (N = 121) | FF 50 mcg OD PM (N = 121) |
|---|---|---|
| AEs | ||
| On treatment | 46 (38) | 37 (31) |
| On treatment, treatment-related | 4 (3) | 1 (<1) |
| On treatment, leading to withdrawal | 1 (<1)a | 0 |
| Post treatment | 1 (<1) | 1 (<1) |
| Serious AEs | ||
| On treatmentb | 1 (<1) | 1 (<1) |
| On treatment AEs occurring in ≥3% patients in either treatment group | ||
| Headache | 14 (12) | 6 (5) |
| Nasopharyngitis | 5 (4) | 7 (6) |
| Pharyngitis | 6 (5) | 6 (5) |
| Influenza | 4 (3) | 1 (<1) |
aAn incidence of urticaria in one placebo-treated patient; btraffic accident, placebo group; perforated appendix, FF 50 mcg group; AE = adverse event; FF = fluticasone furoate; OD = once daily; PM = evening.
Two serious AEs were reported (traffic accident, placebo; perforated appendix, FF 50 mcg); neither was fatal nor considered to be treatment related, and both patients completed the study. Three patients in the placebo group experienced severe asthma exacerbations, while no patient in the FF 50 mcg group reported or was treated for a severe asthma exacerbation. There were no reports of pneumonia or oral/oropharyngeal candidiasis.
Discussion
In patients with persistent asthma uncontrolled by non-ICS medications, FF 50 mcg administered once daily in the evening for 12 weeks significantly improved evening trough FEV1 compared with placebo. Patients who received FF 50 mcg also showed a significant increase in the percentage of rescue-free 24-h periods compared with placebo. The safety profile for FF 50 mcg was acceptable and similar to that of placebo. The patient population was chosen as the most appropriate for once-daily treatment with a low dose of FF.
The statistically significant improvement in evening trough FEV1 observed with FF 50 mcg compared with placebo (120 mL) is similar to findings from a recent dose-ranging study that investigated the potential of low doses of once-daily FF (25–200 mcg) for the treatment of persistent asthma over 8 weeks [7]. The current study was performed in a similar population of asthma patients (i.e., those who required a step-up to Step 2 of asthma treatment guidelines) and, although lung function at baseline was different, those authors found that FF 50–200 mcg statistically significantly improved lung function compared with placebo; the treatment difference between FF 50 mcg and placebo in trough FEV1 was 129 mL (p < 0.033). The present data for FF, generated from a larger cohort of patients and over a longer period of time, support these findings [7]. The study was powered based on a change in evening trough FEV1 of 200 mL. However, in another study comparing morning and evening dosing of FF/VI whose findings were reported after our study design was finalized, smaller treatment differences vs. placebo were seen for evening FEV1 when compared with morning FEV1 – this was probably due to the known diurnal variation in lung function [18]. Despite this, the effect of FF 50 mcg on evening FEV1 in our study was significant. However, in another phase III study the improvement for FF 50 mcg over placebo was not statistically significant, although improvement with the active comparator fluticasone propionate (FP) 100 mcg was significant (102 mL; p = 0.030) [14]. The reason for the findings of Busse et al. not being consistent with those of the current and previous studies of FF 50 mcg [7] is unclear, as the patient population enrolled was very similar to that of the current study. Finally, for the powered secondary endpoint in this study, the statistically significant increase in the percentage of rescue-free 24-h periods with FF 50 mcg compared with placebo is consistent with results from the 8-week dose-ranging study that involved FF 50 mcg [7]. Collectively, the findings suggest that FF 50 mcg results in meaningful improvements in trough FEV1 and percentage of rescue-free 24-h periods.
Statistical significance was not achieved for the secondary endpoint of change from baseline in evening PEF for FF 50 mcg vs. placebo, meaning that significance could not be inferred for the remaining secondary and other endpoints. However, other studies investigating treatment with FF 50 mcg [7,14] or FF 100 mcg [8] have reported numerically greater improvements in evening PEF compared with placebo (treatment differences of 20.7 L/min, 17.2 L/min and 15.9 L/min, respectively). Lung function is generally improved in the evening due to diurnal variation [19], reducing the likelihood of detecting treatment benefit at that time point. However, trough FEV1, which was also assessed in the evening, did improve significantly following FF 50 mcg compared with placebo treatment. For the remaining endpoints of morning PEF, symptom-free 24-h periods, ACT score, percentage of patients controlled (defined by ACT score ≥20 at Week 12) and Total AQLQ score, numerically greater increases were observed over 12 weeks in patients who received FF 50 mcg compared with placebo. More patients in the placebo group were withdrawn due to lack of efficacy compared with the FF 50 mcg group.
The overall safety profile was favorable in both the FF 50 mcg and placebo groups, consistent with previous findings for this dose of FF [7,14]. Cortisol levels were not measured in this study, as the treatment was with a low dose of FF and no effect of cortisol had been seen with higher doses. The effect of FF on cortisol levels has been assessed in a separate meta-analysis, which is published elsewhere [20]. Common AEs experienced by asthma patients receiving ICS treatment included headache, nasopharyngitis, pharyngitis and influenza. Incidence of these AEs was similarly low between treatment groups in this study, although headache was more frequent in the placebo group.
A strength of our study is the inclusion of a statistical hierarchy of endpoints, which added robustness in validating the overall efficacy of FF 50 mcg compared with placebo. However, this might also be considered a weakness, as failure to achieve statistical significance for evening PEF meant that treatment differences between FF 50 mcg and placebo for the remaining endpoints could only be interpreted in descriptive terms. Another strength was the use of electronic diary cards, which meant that all entries were date and time stamped and which did not allow retrospective entries; this is likely to have increased the quality of daily recordings.
Conclusions
In summary, FF 50 mcg administered once daily in the evening significantly improved evening trough FEV1 and the percentage of rescue-free 24-h periods compared with placebo. Improvements with FF 50 mcg were numerically greater than with placebo for all efficacy endpoints. Fewer patients receiving FF 50 mcg treatment withdrew due to lack of efficacy compared with patients receiving placebo treatment and there were no safety concerns. Overall, the efficacy/tolerability profile of FF 50 mcg was acceptable, suggesting that treatment with FF 50 mcg is potentially suitable for patients ≥12 years of age with persistent asthma that is uncontrolled by non-ICS therapy.
Endnote
aELLIPTA™ is a trademark of the GlaxoSmithKline group of companies.
Acknowledgements and role of the funding source
This study was funded by GlaxoSmithKline (GSK study number FFA115283; www.clinicaltrials.gov registration number NCT01436071). Employees of the sponsor were involved in the conception and design of the study and in the analysis and interpretation of the data. All authors, including authors employed by the sponsor, participated in the development of the manuscript, had access to the data from the study and assisted in the writing of the manuscript. The decision to submit for publication was also that of the authors alone.
Editorial support in the form of development of the draft outline and manuscript first draft in consultation with the authors, editorial suggestions to the outline and first draft of this paper, assembling tables and figures, collating author comments, copyediting, fact checking, referencing and graphic services was provided by Vikas Sharma, PhD at Gardiner-Caldwell Communications (Macclesfield, UK); editorial support in the form of developing the second draft and final drafts of this paper, in consultation with the authors, was provided by Laura Maguire, MChem at Gardiner-Caldwell Communications (Macclesfield, UK). Editorial support was funded by GlaxoSmithKline.
Abbreviations
- ACT
Asthma control test™
- AE
adverse events
- ANCOVA
Analysis of covariance
- AQLQ
Asthma quality of life questionnaire
- FEV1
forced expiratory volume in one second
- FF
Fluticasone furoate
- ICS
Inhaled corticosteroid
- ITT
Intent-to-treat
- LABA
Long-acting β2-agonist
- PEF
Peak expiratory force
- SABA
Short-acting β2-agonists
- VI
Vilanterol
Footnotes
Competing interests
P.M.O’B. has served as a consultant to Almirall, AstraZeneca, Chiesi and Novartis; has served on advisory boards for AIM, Altair, Boehringer, GlaxoSmithKline, Medimmune and Merck; has received lecture fees from Chiesi; and has received research funding from AstraZeneca, Asmacure, Altair, Amgen, Genentech, Topigen and Wyeth. A.W. has served as consultant to Almirall, Cytos, Chiesi and GlaxoSmithKline; and has received lecture fees and research grants from GlaxoSmithKline. E.R.B. has served as a consultant for AstraZeneca, Boehringer Ingelheim, Genentech, GlaxoSmithKline, Johnson and Johnson and Merck; and has performed clinical trials for AstraZeneca, Boehringer Ingelheim, Cephalon, Forest, Genentech, GlaxoSmithKline, KalaBios, MedImmune, Novartis and Sanofi-Aventis, which have been administered by his employer Wake Forest University School of Medicine. E.D.B. has served as a consultant for Actelion, AlkAbello, Almirall, Cephalon, Hoffman la Roche, ICON, IMS Consulting Group, and Navigant Consulting; been on advisory boards for Almirall, AstraZeneca, Boehringer Ingelheim, Elevation Pharma, Forest, GlaxoSmithKline, Merck, Napp, Novartis, Pfizer and Takeda; and received lecture fees from AlkAbello, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Merck, Novartis, Pfizer, Nycomed and Takeda; and received payment for development of teaching materials for Indegene Lifesciences Ltd; and his institution has received remuneration for participation in clinical trials sponsored by Actelion, Aeras, Almirall, AstraZeneca, Boehringer Ingelheim, Cephalon, Chiesi, Forest, GlaxoSmithKline, Hoffman La Roche, Merck, Novartis, Nycomed, Takeda and TEVA. J.L. has served as a consultant to and received lecture fees from AstraZeneca, GlaxoSmithKline, Merck Sharpe and Dohme, Novartis and UCB Pharma; has been partly covered by some of these companies to attend previous scientific meetings including the ERS and the AAAAI; and has participated in clinical research studies sponsored by AstraZeneca, GlaxoSmithKline, Merck Sharpe and Dohme and Novartis. R.F., H.M.,and L.J. are employees of and hold stock in GlaxoSmithKline. W.W.B. has served as a consultant to Amgen, Genentech, GlaxoSmithKline, MedImmune, and Novartis; served on advisory boards for Boston Scientific, ICON, GlaxoSmithKline and Merck Sharpe and Dohme; received research funding from the National Institutes of Health; and receives royalties from Elsevier.
Authors’ contributions
PMO’B, AW, ERB, EDB, JL, RF, LJ and WWB were involved in the conception and design of the study; R.F was involved in data analysis; all authors were involved in the interpretation of data. All authors read and approved the final manuscript.
Contributor Information
Paul M O’Byrne, Email: obyrnep@mcmaster.ca.
Ashley Woodcock, Email: ashley.woodcock@manchester.ac.uk.
Eugene R Bleecker, Email: ebleeck@wakehealth.edu.
Eric D Bateman, Email: eric.bateman@uct.ac.za.
Jan Lötvall, Email: jan.lotvall@gu.se.
Richard Forth, Email: richard.6.forth@gsk.com.
Hilary Medley, Email: hilary.v.medley@gsk.com.
Loretta Jacques, Email: loretta.a.jacques@gsk.com.
William W Busse, Email: wwb@medicine.wisc.edu.
References
- 1.Rabe KF, Adachi M, Lai CK, Soriano JB, Vermeire PA, Weiss KB, Weiss ST. Worldwide severity and control of asthma in children and adults: the global asthma insights and reality surveys. J Allergy Clin Immunol. 2004;114(1):40–47. doi: 10.1016/j.jaci.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 2.Haselkorn T, Fish JE, Zeiger RS, Szefler SJ, Miller DP, Chipps BE, Simons FE, Weiss ST, Wenzel SE, Borish L, Bleecker ER, TENOR Study Group Consistently very poorly controlled asthma, as defined by the impairment domain of the Expert Panel Report 3 guidelines, increases risk for future severe asthma exacerbations in The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) study. J Allergy Clin Immunol. 2009;124(5):895–902. doi: 10.1016/j.jaci.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 3.Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention.2014,.http://www.ginasthma.org/. Date last updated: May 2014. Date last accessed: 09 July 2014.
- 4.National Institutes of Health. Guidelines for the Diagnosis and Management of Asthma (EPR-3) 2007. In.: NHLBI; 2007. NIH publication no. 08–4051.
- 5.British Thoracic Society and Scottish Intercollegiate Guidelines Network. British Guideline on the Management of Asthma: a national clinical guideline.,. https://www.brit-thoracic.org.uk/document-library/clinical-information/asthma/btssign-guideline-on-the-management-of-asthma/. Date last updated: January 2012. Date last accessed: 09 July 2014.
- 6.Price D, Robertson A, Bullen K, Rand C, Horne R, Staudinger H. Improved adherence with once-daily versus twice-daily dosing of mometasone furoate administered via a dry powder inhaler: a randomized open-label study. BMC Pulm Med. 2010;10:1. doi: 10.1186/1471-2466-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bateman ED, Bleecker ER, Lötvall J, Woodcock A, Forth R, Medley H, Davis AM, Jacques L, Haumann B, Busse WW. Dose effect of once-daily fluticasone furoate in persistent asthma: a randomized trial. Respir Med. 2012;106(5):642–650. doi: 10.1016/j.rmed.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Bleecker ER, Lötvall J, O’Byrne PM, Woodcock A, Busse WW, Kerwin EM, Forth R, Medley HV, Nunn C, Jacques L, Bateman ED: Fluticasone furoate/vilanterol 100/25mcg compared with fluticasone furoate 100mcg in asthma: a randomized trial.J Allergy Clin Immunol In Pract 2014,. ePub ahead of print. [DOI] [PubMed]
- 9.Busse WW, Bleecker ER, Bateman ED, Lötvall J, Forth R, Davis AM, Jacques L, Haumann B, Woodcock A. Fluticasone furoate demonstrates efficacy in patients with asthma symptomatic on medium doses of inhaled corticosteroid therapy: an 8-week, randomised, placebo-controlled trial. Thorax. 2012;67(1):35–41. doi: 10.1136/thoraxjnl-2011-200308. [DOI] [PubMed] [Google Scholar]
- 10.Woodcock A, Bleecker ER, Busse WW, Lötvall J, Snowise NG, Frith L, Jacques L, Haumann B, Bateman ED. Fluticasone furoate: once-daily evening treatment versus twice-daily treatment in moderate asthma. Respir Res. 2011;12:160. doi: 10.1186/1465-9921-12-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodcock A, Bateman ED, Busse WW, Lötvall J, Snowise NG, Forth R, Jacques L, Haumann B, Bleecker ER. Efficacy in asthma of once-daily treatment with fluticasone furoate: a randomized, placebo-controlled trial. Respir Res. 2011;12:132. doi: 10.1186/1465-9921-12-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boscia JA, Pudi KK, Zvarich MT, Sanford L, Siederer SK, Crim C. Effect of once-daily fluticasone furoate/vilanterol on 24-hour pulmonary function in patients with chronic obstructive pulmonary disease: a randomized, three-way, incomplete block, crossover study. Clin Ther. 2012;34(8):1655–1666. doi: 10.1016/j.clinthera.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Lötvall J, Bakke PS, Bjermer L, Steinshamn S, Scott-Wilson C, Crim C, Sanford L, Haumann B. Efficacy and safety of 4 weeks’ treatment with combined fluticasone furoate/vilanterol in a single inhaler given once daily in COPD: a placebo-controlled randomised trial. BMJ Open. 2012;2(1):e000370. doi: 10.1136/bmjopen-2011-000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Busse WW, Bateman ED, O’Byrne PM, Lötvall J, Woodcock A, Medley H, Forth R, Jacques L: Once-daily fluticasone furoate 50mcg in mild to moderate asthma: a 24-week placebo-controlled randomized trial.Allergy 2014, ᅟ:ᅟ [in press]. [DOI] [PMC free article] [PubMed]
- 15.Rossios C, To Y, To M, Ito M, Barnes PJ, Adcock IM, Johnson M, Ito K. Long-acting fluticasone furoate has a superior pharmacological profile to fluticasone propionate in human respiratory cells. Eur J Pharmacol. 2011;670(1):244–251. doi: 10.1016/j.ejphar.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 16.Allen A, Bareille PJ, Rousell VM. Fluticasone furoate, a novel inhaled corticosteroid, demonstrates prolonged lung absorption kinetics in man compared with inhaled fluticasone propionate. Clin Pharmacokinet. 2013;52(1):37–42. doi: 10.1007/s40262-012-0021-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Byrne PM, Woodcock A, Bleecker ER, Bateman ED, Lotvall J, Forth R, Medley H, Jacques L, Busse WW. Efficacy and safety of once-daily fluticasone furoate (FF) 50mcg in adults with persistent asthma. Am J Respir Crit Care Med. 2013;187:A2610. [Google Scholar]
- 18.Kempsford RD, Oliver A, Bal J, Tombs L, Quinn D. The efficacy of once-daily fluticasone furoate/vilanterol in asthma is comparable with morning or evening dosing. Respir Med. 2013;107(12):1873–1880. doi: 10.1016/j.rmed.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Medarov BI, Pavlov VA, Rossoff L. Diurnal variations in human pulmonary function. Int J Clin Exp Med. 2008;1(3):267–273. [PMC free article] [PubMed] [Google Scholar]
- 20.Allen A. The relationship between fluticasone furoate systemic exposure and cortisol suppression. Clin Pharmacokinet. 2013;52(10):885–896. doi: 10.1007/s40262-013-0078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


