Abstract
Chronic stress is an established risk factor in the development of addiction. Addiction is characterized by a progressive transition from casual drug use to habitual and compulsive drug use. The ability of chronic stress to facilitate the transition to addiction may be mediated by increased engagement of the neurocircuitries underlying habitual behavior and addiction. In the present study, striatal morphology was evaluated after two weeks of chronic variable stress in male Sprague-Dawley rats. Dendritic complexity of medium spiny neurons was visualized and quantified with Golgi staining in the dorsolateral and dorsomedial striatum, as well as in the nucleus accumbens core and shell. In separate cohorts, the effects of chronic stress on habitual behavior and the acute locomotor response to methamphetamine were also assessed. Chronic stress resulted in increased dendritic complexity in the dorsolateral striatum and nucleus accumbens core, regions implicated in habitual behavior and addiction, while decreased complexity was found in the nucleus accumbens shell, a region critical for the initial rewarding effects of drugs of abuse. Chronic stress did not affect dendritic complexity in the dorsomedial striatum. A parallel shift toward habitual learning strategies following chronic stress was also identified. There was an initial reduction in acute locomotor response to methamphetamine, but no lasting effect as a result of chronic stress exposure. These findings suggest that chronic stress may facilitate the recruitment of habit- and addiction-related neurocircuitries through neuronal restructuring in the striatum.
Lifetime exposure to stressors is an established risk factor for the development of addiction (i.e., substance abuse disorders; Turner and Lloyd, 2003, Lloyd and Turner, 2008, Sinha, 2008). In addition, stress-related psychiatric disorders, such as depression and anxiety, have a high co-morbidity with drug abuse (Brady and Sinha, 2005). In rodent studies, exposure to acute or chronic stressors can lead to enhanced self-administration of various drugs, including psychostimulants such as cocaine and amphetamine (Piazza and Le Moal, 1998, Sinha, 2001, Lu et al., 2003). Despite evidence suggesting that stress creates a vulnerability to addiction in both humans and rodents, the specific mechanisms underlying the development of this vulnerability are not clearly established. The majority of research suggests that stress contributes to addiction through adaptive changes in both the reward and stress systems or through exacerbating the negative affect associated with withdrawal (Sinha, 2008, Koob, 2013). An alternative, but not mutually exclusive, mechanism is that stress potentiates maladaptive behaviors resulting in impaired behavioral control and addiction vulnerability (Sinha, 2008).
Neurocircuitries and behaviors extending beyond the canonical brain reward pathway also play a role in vulnerability to addiction (Everitt and Robbins, 2005, Baler and Volkow, 2006, Li and Sinha, 2008). A recent focus has been the role of maladaptive behavioral patterns, such as impulsivity, compulsivity, and anxiety-related behaviors in drug abuse (Aujla et al., 2008, Belin et al., 2008, Broos et al., 2012, Dilleen et al., 2012, Murphy et al., 2012, Bahi, 2013). A hypothesis that encompasses many of these maladaptive behaviors is that the development of drug addiction involves a transition from initial, voluntary and goal-directed drug use to habitual and compulsive drug use (Everitt et al., 2008, Everitt and Robbins, 2013). Interestingly, both chronic and acute stress facilitate habitual learning and behaviors (i.e., behaviors that are inflexible or insensitive to a reduction in reward value), at the expense of goal-directed or spatial learning and memory (Schwabe et al., 2008, Dias-Ferreira et al., 2009, Sadowski et al., 2009). Thus, the ability of chronic stress to facilitate both the recruitment of habitual processes and the transition to addiction may be mediated by increased engagement of the neurocircuitries underlying habitual behavior and addiction (Everitt et al., 2008, Packard, 2009, Schwabe et al., 2011).
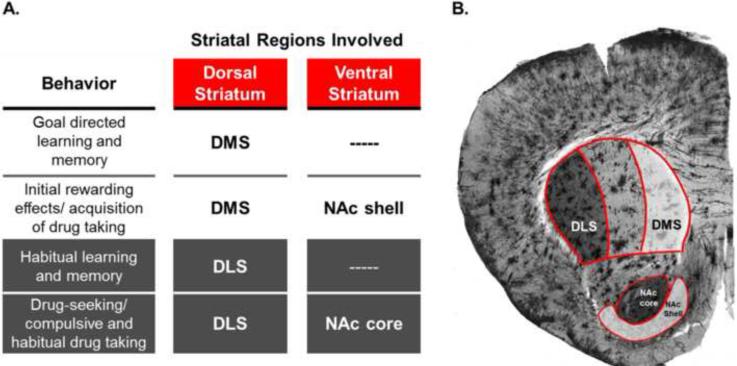
Goal-directed and habitual behaviors share some common striatal neurocircuitries that are involved in the transition from casual drug use to addiction (Figure 1A). Goal-directed learning processes are supported by the dorsomedial striatum (DMS; Figure 1B; Yin et al., 2005, Shiflett et al., 2010), while habitual learning processes are supported by the dorsolateral striatum (DLS; Figure 1B; Yin et al., 2004, Yin et al., 2005). Similarly, the transition from casual drug use to addiction appears to be mediated by a shift in relative engagement of goal-directed neurocircuitries to more habitual neurocircuitries. Evidence suggests a progressive shift in dopaminergic signaling from more ventral and medial regions of the striatum to more dorsal and lateral regions (Everitt et al., 2008). The DMS and nucleus accumbens shell (NAc shell; Figure 1B) are associated with the initial rewarding effects and acquisition of drug taking, whereas the nucleus accumbens core (NAc core; Figure 1B) and DLS are associated with the development of drug seeking behavior and ultimately compulsive and habitual drug seeking (Figure 1; Everitt et al., 2008, Packard, 2009, Schwabe et al., 2011, Murray et al., 2012, Everitt and Robbins, 2013).
Figure 1. Roles and Locations of Striatal Subregions.
A. The role of striatal subregions in habit- and addiction-related behaviors (see text for references). B. The dorsal striatum consists of the dorsolateral striatum (DLS) and dorsomedial striatum (DMS). The ventral striatum includes the nucleus accumbens core (NAc core) and nucleus accumbens shell (NAc shell).
The mechanism by which chronic stress facilitates the recruitment of habitual processes and the transition to drug abuse may involve differential neuroplastic changes in the aforementioned neurocircuitries. One frequently examined outcome following adult chronic stress exposure is changes in dendritic morphology, specifically increases or decreases in dendritic arborization (Conrad, 2006, Leuner and Shors, 2012). The majority of these morphological studies have focused on the hippocampus, prefrontal cortex, and amygdala (Conrad, 2006, McLaughlin et al., 2009, Wellman, 2011, Leuner and Shors, 2012), and some studies have also examined striatal subregions (Dias-Ferreira et al., 2009, Morales-Medina et al., 2009, Bessa et al., 2013). For the hippocampus, medial prefrontal cortex, and DMS, chronic stress typically results in dendritic retraction and impairments in corresponding spatial and goal-directed behaviors, respectively (Watanabe et al., 1992, Vyas et al., 2002, Cook and Wellman, 2004, Radley et al., 2004, Liston et al., 2006, McLaughlin et al., 2007, Dias-Ferreira et al., 2009, Hoffman et al., 2011, Hutchinson et al., 2012). On the other hand, chronic stress leads to increased dendritic complexity in the DLS, the orbitofrontal cortex, the basolateral nucleus of the amygdala, and the bed nucleus of the stria terminalis (BNST), while the neurons of the central amygdala remain unchanged (Vyas et al., 2002, Vyas et al., 2003, Dias-Ferreira et al., 2009).
Dendritic complexity in brain regions more directly implicated in the development of addiction, namely the ventral striatum, which includes the nucleus accumbens (NAc), has only recently been examined. One study found that prenatal stress leads to an increase in dendritic arborization in the NAc (combined core and shell; Muhammad et al., 2012) while another study found no effect of prenatal stress on dendritic length in the NAc (shell only) of either prepubertal or adult rats (Martinez-Tellez et al., 2009). In addition, postweaning isolation and neonatal maternal separation resulted in reduced dendritic intersections and reduced dendritic length in the NAc respectively (combined core and shell; Monroy et al., 2010, Wang et al., 2012). An additional study examined the effects of 3 weeks of corticosterone (i.e., a primary stress hormone in the rat) administration during periadolescence (post natal day 45) on adult NAc (shell only) dendritic morphology (Morales-Medina et al., 2009). This study found an increase in dendritic complexity near the soma but decreased complexity in the distal regions, resulting in an overall decrease in dendritic length (Morales-Medina et al., 2009). Most recently, Bessa and colleagues (2013) examined the effects of adult chronic unpredictable stress on the adult NAc and found increased dendritic complexity in both the NAc core and shell (Bessa et al., 2013). The aforementioned studies represent initial and sometimes conflicting examinations of the effects of chronic stress during different developmental stages on neuronal morphology in regions related to addiction and habitual behavior. However, a comprehensive examination of all striatal subregions in the same experimental subjects following chronic stress in adulthood is lacking.
The experiments described here tested the hypothesis that chronic stress facilitates the recruitment of habit- and addiction-related neurocircuitries, namely subregions of the dorsal and ventral striatum. Based on this hypothesis and the most similar previous studies in adult rats (Dias-Ferreira et al., 2009, Bessa et al., 2013), it was predicted that two weeks of chronic variable stress (CVS) would increase dendritic complexity in the DLS and NAc core, regions implicated in initial and habitual or compulsive drug-seeking. Conversely, it was predicted that CVS would not increase dendritic complexity in the DMS, a region involved in the acquisition of drug taking. Finally, the effect of CVS on dendritic morphology in the NAc shell was predicted to change in either direction, based on past studies mentioned above and its role in the initial rewarding effects of drugs, but not the expression of addiction-like behaviors. It was further predicted that in separate cohorts of animals, CVS exposure would increase the proportion of animals that use habitual learning strategies on a dual solution T-maze and increase the locomotor stimulant effects of methamphetamine.
2. Experimental procedures
2.1 Animals
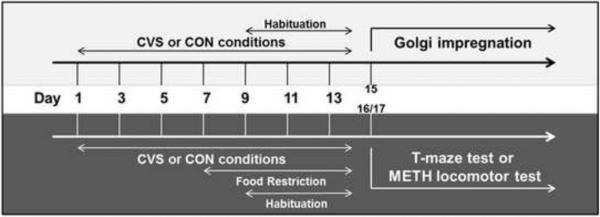
Male Sprague-Dawley rats were purchased from Charles River Laboratories (Wilmington, MA) and were pair housed in a temperature and humidity controlled vivarium with a reversed light-dark cycle (12:12; lights off at 06:00 h) at Arizona State University. Three cohorts of rats were used for either histological or behavioral assessment (Figure 2). Upon arrival, rats were given one week to acclimate before any procedures were performed. Food and water were available ad libitum with the exception of the last week of the stress procedures in the behavioral cohorts, when rats were food restricted (Figure 2). Rats were weighed once a week throughout stressor administration and daily during food restriction. Behavioral testing was conducted during the dark phase of the light-dark cycle. All procedures were conducted according to federal guidelines outlined in the Guide for Care and Use of Laboratory Rats (Institute of Laboratory Animal Resources on Life Science, National Research Council) and were approved by the Institutional Animal Care and Use Committee at Arizona State University.
Figure 2.
Experimental Timeline
2.2 Chronic Variable Stress (CVS)
The CVS paradigm used is based on established chronic variable stress paradigms (Bondi et al., 2008, McGuire et al., 2010, Taylor et al., 2013b). Rats were exposed to two variable stressors/day for 14 consecutive days; one stressor exposure occurred in the morning, and one in the afternoon (Table 1). Briefly, the stressors used in this paradigm were: (1) restraint for 1 h with a wire mesh restrainer or standard, well-ventilated clear plastic restrainers, (2) restraint for 30 min while shaking on an orbital shaker (Roto Mix, Type 50800) at ~120 rpm, (3) social crowding (6-8/cage) for 1 h while shaking on an orbital shaker at ~120 rpm, (4) forced swim in warm (30°C; 20 min) or cold (18°C; 10 min) water, (5) tail pinch during restraint for 10 min, wherein rats were placed in wire restrainers with a clothespin attached 1 cm from the base of the tail, (6) footshock for 15 min (Coulbourn Instruments, E10-18TC, Coulbourn Animal Shock Generator, H13-H15 0.5 mA; 5 cycles of: 5 s on/5 s off, repeated three times, then 155 s off) (7) overnight (active period) social crowding (6-8/cage), (8) overnight dirty bedding, wherein rats were exposed to an indirect social stressor by being placed into a dirty cage previously used by other male rats (Cano et al., 2008). Non-stressed control rats were left undisturbed in the vivarium during stress procedures. On days in which rats were handled in the vivarium or testing room, handling was conducted no less than 1 hour before or after stressor administration.
Table 1.
Chronic Variable Stress Schedule
| Time of day |
||||
|---|---|---|---|---|
| Day |
AM (~9-11) |
AM |
PM (~12-2) |
PM (~3-5/overnight) |
| 1 |
restraint |
overnight crowding |
||
| 2 |
tail pinch |
forced swim |
||
| 3 |
footshock |
shake + crowd |
||
| 4 |
restraint + shake |
footshock |
||
| 5 |
tail pinch |
overnight dirty bedding |
||
| 6 |
forced swim warm |
footshock |
||
| 7 |
restraint |
shake + crowd |
||
| 8 |
shake + crowd |
tail pinch |
||
| 9 |
forced swim cold |
overnight crowding |
||
| 10 |
footshock |
shake + crowd |
||
| 11 |
restraint |
restraint + shake |
||
| 12 |
tail pinch |
forced swim warm |
||
| 13 |
footshock |
overnight dirty bedding |
||
| 14 |
restraint + shake |
shake + crowd |
||
2.3 Dendritic Complexity
During the last five days of CVS procedures, all rats were handled in the vivarium (two days) and necropsy room (three days) to habituate them to the environment (Figure 2). Approximately 24 h after cessation of CVS, all rats (n=10 per group) were anesthetized with isoflurane and rapidly decapitated. Brains and adrenal glands were then extracted. All brains were stained with FD Rapid Golgistain™ Kits (FD NeuroTechnologies, Baltimore MD) according to the manufacturer's instructions and previous publications (Hoffman et al., 2011, Ortiz et al., 2013). Briefly, freshly extracted brains were rapidly rinsed with distilled water and then placed in an impregnation solution. The solution was replaced twice within the first 36 h and brains were stored in the dark at room temperature for a total of 2 weeks. After impregnation, brains were transferred to fixing solution for a minimum of 48 h. Brains were then rapidly frozen in 2-methylbutane and later cut into 200 μm coronal sections (Microm Cryostat, −22°C). Sections were mounted onto 2% gelatin-coated slides with a small volume of fixing solution. After removing excess solution, slides were dried flat in the dark at room temperature for a maximum of 48 h. Next, slides were rinsed in distilled water, incubated in developing solution, dehydrated in ascending series of ethanol solutions, cleared with xylene, and cover slipped with Permount™ Mounting Medium (Thermo Fisher Scientific, Waltham, MA). All slides were dried flat in the dark for a minimum of 1 week.
Medium spiny neurons (MSNs) were identified in the dorsal striatum (between 1.08 to 2.16 mm anterior to bregma) and the nucleus accumbens (1.20 to 2.28 mm anterior to bregma). Subregion boundaries (Figure 1B) within the dorsal striatum (DLS and DMS) and nucleus accumbens (NAc core and NAc shell) were identified using a rat brain atlas (Paxinos and Watson, 2005) according to the boundaries used by Dias-Ferrerira and colleagues (Dias-Ferreira et al., 2009). Neurons were selected randomly with an equal distribution from both hemispheres for reconstruction if the following criteria were met: (1) the cell body and dendrites were fully impregnated, (2) the cell was relatively isolated from surrounding neurons, and (3) the cell was located in the region of interest.
Neurons were manually reconstructed at 320x magnification, using an Olympus BX51 microscope fitted with a camera lucida attachment. Upon reconstruction, the inclusion criteria above were cross-checked by an additional experimenter, and then quantified via Sholl analysis (Uylings and van Pelt, 2002). Dendritic intersections were measured at equidistant concentric spheres in increments of 20 μm from the cell soma. For an animal to be included in the analysis of a given brain region, a minimum of five neurons were required per region. All experimenters were blind to experimental group throughout morphological analysis.
2.4 Food Restriction
In the following behavioral experiments, all rats were progressively introduced to reduced food access over three days, beginning with access for 5 h (day 1), 3 h (day 2), and finally 1 h (day 3). Rats were then given 1 h food access for a maximum of six additional days to reduce body weight. The target weight was 85% of ad libitum weight (Korol et al., 2004, Sadowski et al., 2009) with a minimum of 75% ad libitum weight. Rats that weighed less than 85% of their ad libitum weight for five consecutive days were given an additional 1 h of feeding until weight increased to above 85%. No animals in the present studies demonstrated signs of malnourishment (lethargy, unusual huddling, ruffled coat, watery/closed eyes). Rats were weighed daily and monitored for health during this period.
2.5 Dual Solution T- Maze
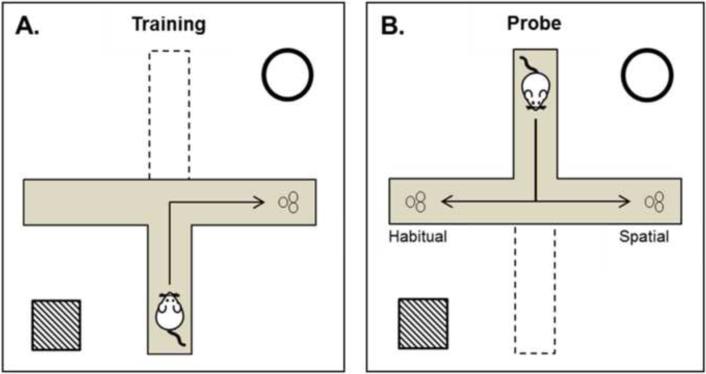
Two days after termination of CVS (i.e., on day 16), a dual solution T-maze (DST-maze; i.e., Tollman maze; Figure 3) task was employed to assess the learning strategy used to find a food reward by a separate cohort of CVS and CON rats. This task can test for spatial (i.e., place) or habitual (i.e., response) learning (Korol et al., 2004, Yin and Knowlton, 2006). Spatial learning is sensitive to manipulations of the hippocampus, while habitual learning is sensitive to manipulations of the dorsal striatum (Packard and McGaugh, 1996). Prior to training, rats (n=14/group) were food restricted for seven days (during CVS procedures) to 85% of ad libitum weight. Animals were trained in a black Plexiglas® plus-shaped maze with four arms (46 cm long and 13 cm wide, 8 cm high walls). The maze was placed on a table 71 cm above the floor and the T-maze was formed by placing a removable barrier on the arm opposite the start arm. Animals were first handled for two days in the vivarium, and then for three days in the testing room. To minimize neophobia to the apparatus and food reward, rats were then habituated to the maze with the food reward present for an additional two days prior to training.
Figure 3. Dual Solution T-Maze.
A. In training trials, rats began in a start arm held in a constant position and were allowed to turn left or right to find a food reward. Extra maze spatial cues are represented in the corners. Rats were able to use either spatial strategies (reward location relative to spatial cues) or habitual/response strategies (reward location relative to body turn) at the choice point. B. In the single probe trial, the start arm was rotated 180 degrees from its location during the probe trial. A spatial strategy was scored if a rat went to the previously rewarded location. A habitual strategy was scored if a rat made the same body turn as during training, but went to the previously unrewarded location.
During training, 1/3 of a Frosted Cheerio® (General Mills) was place in the reward arm, while the non-reward arm contained a decoy consisting of an equal sized piece of corncob bedding scented with a fine powder from crushed Frosted Cheerios®. To further minimize use of odor cues, the end of each arm was scented with crushed Frosted Cheerios® and odor-neutralizing disinfectant (Quatricide®, Pharmacal, Naugatuck, CT) was used to clean the maze between each trial. To minimize the use of intra-maze cues, the entire maze itself was rotated 90° before each training trial. The location of the start arm was kept constant with respect to spatial cues around the room. During each training trial, rats were placed in the start arm of the maze and given a maximum of two min to move to the intersection of the arms and make a single right or left turn into one of the arms, with an intertrial interval of ~ 30 sec. Thus, rats were able to use either the spatial cues (dark shapes painted on white walls) or a habitual/response strategy (body turns at choice point) in order to locate the food reward trial after trial (Figure 3A). Rats were allowed a maximum of 75 trials to meet a criterion of 90% correct choices. Immediately after criterion was reached, a single probe trial was given. The start arm was rotated 180° from the original position used during training and a food reward was placed in both arms (Figure 3B). In this probe trial, a habitual strategy was scored if the rat turned in the same direction as in training and went to the previously unrewarded location. A spatial strategy was scored if the rat turned in the opposite direction as in training and went to the previously rewarded location (Figure 3B). Non-strategy measures were also recorded, including trials to criterion, errors, and latency to choice.
2.6 METH-Induced Locomotor Activity
During the last seven days of exposure to either CVS or control conditions, a separate cohort of rats (n=4/group) were food restricted as previously described. Food restriction was implemented in this experiment to maintain consistency between behavioral experiments. Two different types of arenas were used, but the first (RotoRat bowl-shaped and square open field arenas; Med Associates, St. Albans, VT) was determined to be too sensitive to motor movements to use under these conditions and was discontinued. In a protocol similar to that used by Cruz et. al., (2011), three days after termination of CVS procedures (i.e., on day 17), animals were placed into a square open field arena Photobeam Activity System (PAS)-Open Field stations (41 cm wide × 41 cm deep × 38 cm high; San Diego Instruments, San Diego, CA). Rats were injected with METH (1 mg/kg) or saline (1ml/kg) via the intraperitoneal route and then immediately placed into the arenas for 60 min. Locomotor activity was measured as beam breaks (2.5 cm apart) and then transformed by PAS software into distance traveled and number of rearings.
2.7 Statistical Methods
Statistical analyses were conducted with SPSS (version 22) and GraphPad (version 4) software. Repeated measures Analysis of Variance (rmANOVAs) were used to analyze locomotor activity and dendritic complexity. Multivariate ANOVAs were used for post hoc analysis of significant interactions. Non-strategy measures on the DST-maze were analyzed with two-way ANOVAs. Fisher's exact test was used to analyze the 2x2 contingency table of DST-maze strategy (GraphPad Software online Quick Calcs). Student's T-tests were used to analyze percent weight gained and adrenal weights. For all statistical analyses, p<0.05 was considered significant.
3. Results
3.1 Physiological Outcomes
To confirm stressor effectiveness, weight gained during CVS exposure was compared between CVS and CON conditions and is summarized in Table 2. In the dendritic morphology cohort, CVS rats exhibited attenuated weight gain compared to CON rats (t18 =6.26, p<0.001; Table 2). In addition, relative adrenal weights (mg/100 g body weight) were significantly increased in CVS rats compared to CON rats (t14 =2.88, p<0.05; Table 2). In the two behavioral cohorts, all rats were food restricted during the last week of CVS exposure or CON conditions. CVS rats gained significantly less weight than CON rats in both experiments (DST-maze: t20 =6.24, p<0.001; Locomotor: t14 =5.8, Table 2). Adrenal weights were not analyzed in these cohorts due to possible confounds introduced by behavioral testing after cessation of CVS procedures.
Table 2.
Physiological Outcomes
| Body Weight (g) Gained During CVS (14 days) |
Relative Adrenal Weights (mg/100g) |
|||
|---|---|---|---|---|
| Cohort 1 (Golgi) |
Cohort 2# (DST-Maze) |
Cohort 3# (Locomotor) |
Cohort 1 (Golgi) |
|
| CON | 75.4 ± 4.6 | 10.6 ± 3 | 16 ± 3 | 1.39 ± 0.05 |
| CVS | 35.4 ± 4.5* | −17.3 ± 3.3* | −9.3 ± 3.1* | 1.65 ± 0.08* |
p<0.05 compared to CON within the same cohort
Food restricted
3.2 Dendritic Complexity
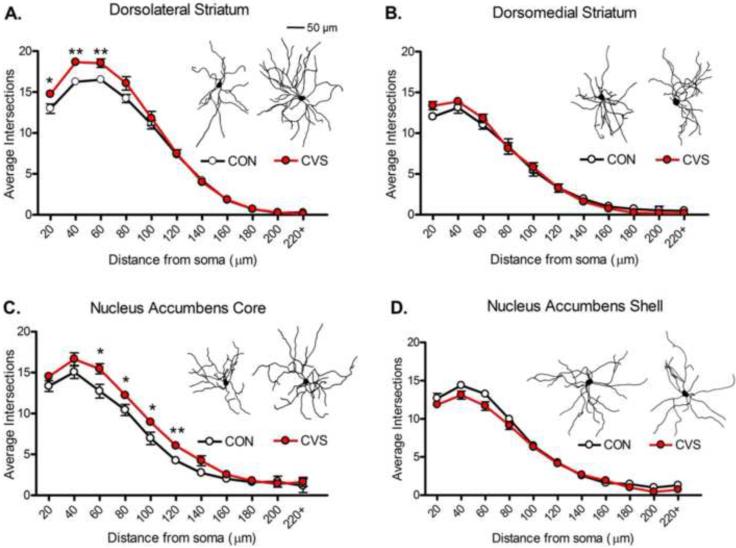
Exposure to CVS resulted in region-specific dendritic reorganization. In the DLS (Figure 4A), rmANOVAs revealed a main effect of stress (F1,15 =4.68, p<0.05) such that MSNs in CVS rats were more complex than those of CON rats. A significant interaction between ring and stress (F10,150 =3.87, p<0.001) was also found. Post hoc analyses revealed significant differences between CVS and CON groups at the proximal dendrites (20-60 μm rings; Figure 4A). In the DMS (Figure 4B), no main effect of stress (F1,15 =0.04, p=0.83) or significant interaction between stress and ring (F10,150 =1.79, p=0.07) were identified. In the NAc core (Figure 4C), a main effect of stress was found (F1,12 =6.57, p<0.05) such that MSNs in CVS rats were more complex than those of CON rats. A significant interaction between ring and stress (F10,120 =1.95, p<0.05) was also identified. Post hoc analyses revealed significant differences between CVS and CON groups at the rings 60-120 μm from the soma (Figure 4C). Conversely, in the NAc shell (Figure 4D), rmANOVAs revealed a main effect of stress (F1,11 =12.01, p<0.001) such that MSNs in CVS rats were less complex than those of CON rats. No significant interaction between ring and stress (F10,110 =0.86, p=0.58) was identified. The final sample sizes were as follows: DLS: n=8-9/group, 5-10 neurons/rat; DMS: n=8-9/group, 5-9 neurons/rat; NAc core: n= 6-8/group, 5-7 neurons/rat; NAc shell: n= 6-7/group, 5-9 neurons/rat.
Figure 4. Dendritic Complexity of Striatal Medium Spiny Neurons.
A. Increased dendritic complexity was found in the dorsolateral striatum of chronic variable stress (CVS) exposed rats. B. CVS exposure had no effect on dendritic complexity in the dorsomedial striatum. C. CVS exposure resulted in increased dendritic complexity in the nucleus accumbens core. D. CVS exposure decreased dendritic complexity in the nucleus accumbens shell. *p<0.05, **p<0.01. n=5-10 neurons/rat, 6-9 rats/group.
3.3 Dual Solution T-Maze Task
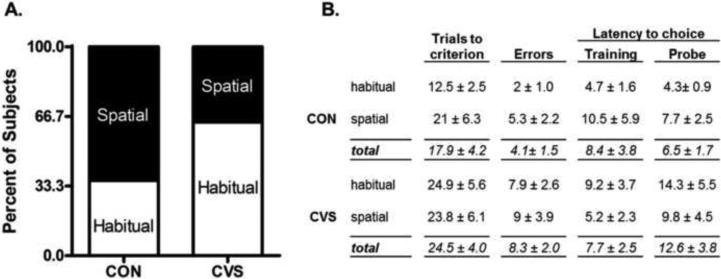
The strategies used by CON and CVS rats, while not statistically significant (p=0.4, Fishers Exact Test) suggested a pattern that was proportionally opposite. A habitual strategy was used by 67% of CVS rats (n=7) and 33% of CON rats (n=4), while a spatial strategy was used by 67% of CON rats (n=7) and 33% of CVS rats (n=4; Figure 5A). In addition, two way ANOVAs revealed no significant effect of stress or choice on trials to criterion, errors during training, latency during training, or latency during the probe trial (Figure 5B). However, there was a tendency for CVS rats to commit more errors during training (F1,18 =3.14, p=0.09, Figure 5B). The final sample size for each group was n=11 because three rats in each group did not properly acquire performance in the task and were excluded from all analyses.
Figure 5. Strategy Use on Dual Solution T-Maze.
A. CVS exposure resulted in a non-significant pattern suggesting the reversal of the proportion of rats that used habitual strategies. Two thirds of CVS rats used habitual strategies, compared to only one third of CON rats. n=11 rats/group. B. No statistical differences were identified between CON and CVS rats in trials to criterion, errors during training, or latency to make a choice during training or the probe trial. All rats reached criterion in less than 75 trials.
3.4 METH-Induced Locomotor Activity
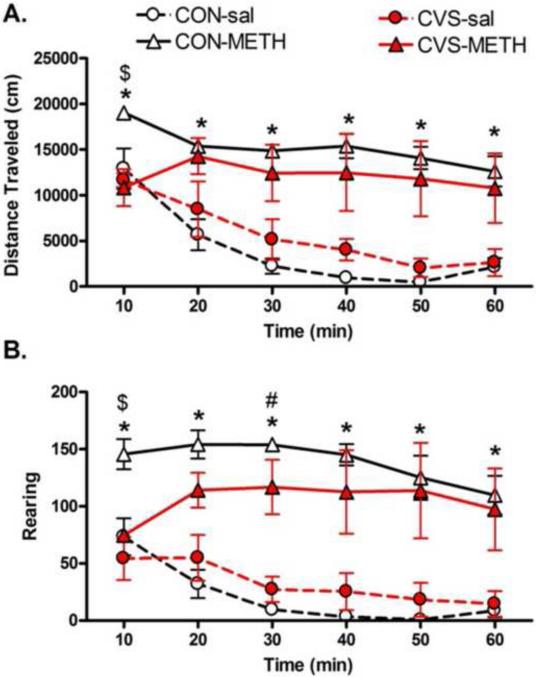
CVS resulted in an initial reduction in METH-induced locomotor activity. A two way rmANOVA (Stress (CON vs. CVS) and Drug (sal vs. METH)) across six 10 min time bins was conducted on distance traveled and rearing. This analysis revealed a significant between subjects effect of drug for both distance traveled (F1,12 =40.0, p<0.001) and rearing (F1,12 =46.4, p<0.001) such that METH administration resulted in increased distance traveled and rearing compared to saline treated animals (Figure 6). Additional rmANOVA analyses revealed a significant within subjects interaction between time bin and group (CON-sal, CON-METH, CVS-sal, CVS-METH) on both distance traveled (F15,60 =1.9, p<0.05) and rearing (F15,60 =2, p<0.05). Follow up multivariate ANOVAs of individual time bins revealed that initially only the CON rats exhibited increased locomotor activity and rearing as a result of METH administration (p<0.05 compared to all other groups at 10 min bin, Figure 6). Furthermore, CON-METH rats maintained increased locomotor activity and rearing (p<0.05) throughout the session compared to CON-sal and CVS-sal rats (Figure 6). However, CVS-METH rats did not exhibit significantly more activity or rearing than CVS-sal rats, with the exception of increased rearing at the 30 min time bin (Figure 6).
Figure 6. METH Induced Locomotor Activity.
A. Distance Traveled. B. Rearings. During the first ten minutes after administration of drug, CON-METH rats exhibited increased distance traveled (A) and rearing (B) compared to CVS-METH ($p<0.01) and both CON-sal and CVS-sal groups (*p<0.05). Throughout testing, CON-METH rats were significantly more active compared to CON-sal and CVS-sal rats (*p<0.05). After the initial reduction in activity at the 10 minute time point, CVS-METH rats demonstrated activity equivalent to CON-METH rats. However, CVS-METH rats did not exhibit significantly more activity than CVS-sal rats, with the exception of increased rears at the 30 minute time point. n= 4 rats/group.
4. Discussion
The present findings support the hypothesis that chronic stress may facilitate the recruitment of habit- and addiction-related neurocircuitries through neuronal restructuring in the striatum. The effectiveness of the CVS paradigm was confirmed with significantly attenuated weight gain and adrenal hypertrophy in CVS exposed rats compared to CON rats, as reported by others using similar paradigms (Herman et al., 1995, Bondi et al., 2008, Dias-Ferreira et al., 2009, Taylor et al., 2013b). Two weeks of CVS exposure resulted in differential changes in striatal dendritic complexity. As predicted, dendritic complexity in striatal regions associated with habitual learning and drug seeking (DLS and NAc core) was significantly increased following CVS exposure. Conversely, dendritic complexity in the striatal region associated with goal-directed learning and acquisition of drug taking (DMS) was unaltered and complexity in the region associated with the initial rewarding effects of psychostimulants (NAc shell) was significantly reduced. In addition, rats exposed to CVS exhibit a pattern of increased use of habitual (stimulus-response) strategies to locate a food reward. However, rats exposed to CVS did not demonstrate an increase in the locomotor stimulating effects of METH, but rather demonstrated an initial reduction in METH-induced locomotor activity.
4.1 Dendritic Complexity
Two weeks of CVS exposure differentially altered the dendritic complexity of striatal subregions. Rats exposed to CVS exhibited increased dendritic complexity in striatal regions that are implicated in habitual behavior and the transition to addiction, namely the DLS and NAc core. These findings are consistent with the work of others, suggesting chronic stress has a robust and replicable effect on dendritic complexity in the NAc core and DLS. Dias-Ferreira and colleagues (2009) were the first to demonstrate a similar increase in dendritic complexity in the DLS. These effects are consistent despite differences in rat strain (Long Evans vs. Sprague-Dawley used here) and various aspects of chronic stress paradigms. For the stress paradigm, Dias-Ferreira and colleagues (2009) utilized chronic unpredictable stress over three weeks, administering one of three stressors randomly each day. The present study used chronic variable stress over two weeks, administering two of eight stressors pseudo-randomly each day. Both stress paradigms utilized stressors with psychological and/or physical components. One notable difference between the findings in DLS dendritic complexity is that in the present study, the increased dendritic complexity was confined primarily to the proximal dendrites. Proximal dendrites of medium spiny neurons typically receive neuronal communication from interneurons, such as local cholinergic or parvalbumin GABAergic interneurons (Steiner and Tseng, 2010). The specificity of increased proximal dendritic complexity in the DLS may reflect initial changes as a result of chronic stress, with increased complexity in distal regions occurring with longer exposure to stressors. Similarly, the reduction of dendritic complexity in the DMS, which was found by Dias-Ferreira and colleagues (2009), may occur at a later stage when chronic stress persists for three weeks. This would be consistent with the lack of effect of two weeks of CVS on dendritic complexity in the DMS in the present study.
As with the DLS, the present study revealed changes in NAc core dendritic complexity that closely align with the work of others. Bessa and colleagues (2013) identified a similar chronic stress-induced increase in dendritic complexity (quantified as length) in the the adult NAc core. Once again, the comparable findings in the present study confirmed a robust and consistent effect of chronic stress on NAc core dendritic complexity, despite strain (Wistar vs. Sprague-Dawely used here) and stress paradigm differences. Bessa and colleagues (2013) used a modified unpredictable chronic mild stress paradigm, consisting of seven stressors administered over six weeks, though the frequency of stressor exposure was not specified. Conversely, the effects of the two chronic stress paradigms on NAc shell dendritic complexity were opposite. Bessa and colleagues (2013) found an increase in NAc shell dendritic complexity, whereas the present study demonstrated a reduction in NAc shell dendritic complexity. While strain and duration of stress paradigm could easily account for these differences, it was not necessarily expected that the dendritic complexity of the NAc core and shell would change in the same direction. Substantial evidence suggests that the subregions of the NAc serve different roles in the behavioral and neurochemical response to drugs of abuse (Di Chiara, 2002, Ito et al., 2004, Belin and Everitt, 2008, Everitt and Robbins, 2013). The NAc shell appears to mediate the locomotor-stimulating effects of psychostimulants, as well as the primary reinforcing effects of amphetamines (Parkinson et al., 1999, Ikemoto et al., 2005). The NAc core plays a more critical role in the response to reward (both natural and drug) associated stimuli (Parkinson et al., 1999, Di Ciano and Everitt, 2001, Fuchs et al., 2004). Consequently, the NAc core is critical for the acquisition of drug seeking (Ito et al., 2004). Thus, the findings of the present study are more in line with the hypothesis that chronic stress may prime striatal neurocircuitry to allow for a less resistant or more rapid transition to more advanced stages of addiction. An additional note is that, as mentioned in the introduction and consistent with many outcomes following chronic stress, there remains a great deal of variability in the impact of chronic stress on striatal morphology. Some of the variables that might impact the outcomes include age during stress manipulation, post stress delay period and age at brain collection, type of stress paradigm, as well as strain of rat used.
A limitation of analyzing structural plasticity, such as dendritic complexity, is the assumption that these structural changes have functional relevance. While this assumption is routinely made, and generally supported by behavioral and cognitive endpoints, other intermediaries (e.g., altered receptor expression) also likely contribute to the final functional outcome (Conrad, 2006, McLaughlin et al., 2009). In the case of the current findings, functional outcomes of the increased dendritic complexity in the DLS ultimately depend on much more complex intra-striatal and cortico-striatal neurocircuitries. In addition, functional outcomes of altered dendritic complexity in the NAc core and shell will be highly dependent on inputs from the VTA and inhibitory feedback loops from the NAc back to the VTA, in addition to connections with the dorsal striatum. Consequently, our findings add to the mounting evidence of the interaction between chronic stress and striatal subregions with relevance to the progressive development of addiction.
4.2 Dual Solution T-maze
The Dual Solution T-maze (or Tollman maze) uses appetitive motivation to probe an experimental subject's presumed preferred strategy to locate a food reward. The present study identified a pattern suggesting that a larger proportion of rats exposed to CVS used habitual (i.e., stimulus-response) strategies, whereas a larger proportion of non-stressed rats used spatial strategies. These findings, while not statistically significant, demonstrate a similar pattern as those found by others (Sadowski et al., 2009). Sadowski and colleagues (2009) tested rats on a modified version of the Dual Solution T-maze, wherein the number of trials to learn either the spatial (place) version or the habitual (response) version of the task were measured. These authors found a less robust but similar pattern, in that rats exposed to chronic restraint stress (6h/d/21d) required a greater number of trials to learn the spatial task as compared to non-stressed control rats (Sadowski et al., 2009). Furthermore, other studies, utilizing more complex or operant tasks, have identified that chronic stress exposure results in increased use of habitual systems (i.e., striatal), while decreasing the use of spatial systems (Schwabe et al., 2008, Dias-Ferreira et al., 2009). In addition, the present findings are consistent with the morphological findings in the DLS in these studies. After two weeks of CVS exposure, only the proximal dendrites of the DLS became more complex and had the duration of CVS lengthened, the proportion of rats using habitual strategies would be predicted to increase.
It is important to note that increased dendritic complexity in the DLS suggests enhanced propensity toward habitual (striatal-based) learning strategies likely work in tandem with changes in the hippocampus. Chronic stress is well-established to produce robust dendritic retraction in hippocampal subregions, which corresponds with spatial learning and memory deficits (Conrad, 2006, Conrad, 2010). These complimentary changes in the hippocampus and striatum support the notion that chronic stress modifies multiple memory systems (Packard, 2009). Moreover, the hippocampal and striatal memory systems are further modified by amygdalar subregions, such as the basolateral amygdala (Packard, 2009). In fact, administration of pretraining stress results in greater use of habit/striatal memory systems compared to spatial/hippocampal memory systems on probe trials in a water maze version of the DST-Maze (Kim et al., 2001). Furthermore, increasing arousal with anxiogenic drugs (e.g., α2 adrenergic receptor antagonists) prior to training or the probe trial on a modification of the water DST-maze leads to a robust use of habit memory systems (Packard and Wingard, 2004, Elliott and Packard, 2008). These observations, coupled with the findings from the present study suggest that other maladaptive behaviors caused by chronic stress may play a role in producing biases towards use of striatal based memory system. Interestingly, we have also identified that rats exposed to our CVS paradigm also exhibit increased anxiety-like behavior in the elevated plus maze compared to unstressed rats (unpublished observations).
4.3 METH-Induced Locomotor Activity
It was predicted that, similar to previous studies with amphetamine, chronic stress would result in increased METH-induced locomotor activity (Antelman et al., 1980, Deroche et al., 1992, Cruz et al., 2011). Instead, rats exposed to CVS exhibited an initial reduction (i.e., first 10 minutes following administration) of the locomotor stimulating effects of METH (Figure 5). This effect was not evident at the 20 minute time point and there was no difference between the activity CON and CVS rats for the reminder of the test. In addition, CVS-METH rats did not demonstrate significantly increased locomotor activity compared to CVS-sal rats. The finding that CVS exposure reduces the locomotor stimulating effects of METH was opposite of the predicted outcome. Interestingly, however, these findings are in line with the fact that the current study also identified a reduction in NAc shell dendritic complexity. Altogether, these findings corroborate evidence suggesting that the NAc shell mediates the locomotor-stimulating effects of psychostimulants (Parkinson et al., 1999). However, due to the small sample size and the correlational nature of our observations, these findings should be cautiously interpreted.
A potential explanation and limitation for the lack CVS-induced increases in locomotor effects may be the fact that METH, as opposed to amphetamine, was used in the current study. It is known that METH produces greater increases in extracellular DA in the NAc compared to amphetamine, though no differences in the magnitude of DA release are observed in the dorsal striatum (Melega et al., 1995, Goodwin et al., 2009, Taylor et al., 2013a). Other differences in the pharmacodynamic properties of METH and amphetamine may also have accounted for the observed lack of increased locomotor response to METH in CVS rats. In addition, the current study implemented food restriction to maintain consistency with the DST-maze behavioral task, and it can not be ruled out that this could have impacted the locomotor stimulating properties of METH. However, at the time of testing, rats had received several days of free feeding, and food restriction is routinely used in combination with chronic stress procedures (Bondi et al., 2008, Sadowski et al., 2009). Finally, the small sample size utilized in the present study limits the generalizability of these findings. Future studies examining CVS induced locomotor response to METH and more complex addiction-relevant behaviors are warranted.
4.4 Conclusions
The findings reported here demonstrate that chronic stress increases dendritic complexity in the NAc core and DLS, in parallel with shifts toward habitual behaviors. However, a subtle change in the ability of acute administration of METH to alter locomotor activity was observed, such that CVS treated rats exhibited an initially reduced locomotor response to METH. Thus, the extent to which these morphological changes contribute to the locomotor stimulant effects of METH is currently unknown. Future studies are needed to determine the interactions between chronic stress, striatal morphology, chronic drug exposure, and vulnerability towards drug self-administration. Such studies will clarify the role of structural plasticity in the development of addiction-like behaviors. In addition, determining the predictive ability of increased reliance on habitual (striatal-based) learning and memory, or reduced locomotor stimulating effects of psychostimulants on drug self-administration may help identify novel preventative measures for psychostimulant addiction.
Research Highlights.
- Chronic stress differentially alters striatal dendritic complexity
- Chronic stress may promote habitual learning strategies
- Methamphetamine-induced locomotor activity is minimally affected by chronic stress
Acknowledgements
The authors would like to thank Amanda Krigbaum, Tiffany Tang, Franchezka Lapitan, and J. Bryce Ortiz for their additional technical support. This research was funded by the College of Liberal Arts and Sciences (CDC) and the School of Life Sciences Undergraduate Enrichment Program (PRP) at Arizona State University and NIDA Grant DA025606 (MFO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antelman SM, Eichler AJ, Black CA, Kocan D. Interchangeability of stress and amphetamine in sensitization. Science. 1980;207:329–331. doi: 10.1126/science.7188649. [DOI] [PubMed] [Google Scholar]
- Aujla H, Martin-Fardon R, Weiss F. Rats with extended access to cocaine exhibit increased stress reactivity and sensitivity to the anxiolytic-like effects of the mGluR 2/3 agonist LY379268 during abstinence. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:1818–1826. doi: 10.1038/sj.npp.1301588. [DOI] [PubMed] [Google Scholar]
- Bahi A. Individual differences in elevated plus-maze exploration predicted higher ethanol consumption and preference in outbred mice. Pharmacology, biochemistry, and behavior. 2013;105:83–88. doi: 10.1016/j.pbb.2013.01.023. [DOI] [PubMed] [Google Scholar]
- Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med. 2006;12:559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessa JM, Morais M, Marques F, Pinto L, Palha JA, Almeida OF, Sousa N. Stress-induced anhedonia is associated with hypertrophy of medium spiny neurons of the nucleus accumbens. Translational psychiatry. 2013;3:e266. doi: 10.1038/tp.2013.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:320–331. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- Brady KT, Sinha R. Co-occurring mental and substance use disorders: the neurobiological effects of chronic stress. The American journal of psychiatry. 2005;162:1483–1493. doi: 10.1176/appi.ajp.162.8.1483. [DOI] [PubMed] [Google Scholar]
- Broos N, Diergaarde L, Schoffelmeer AN, Pattij T, De Vries TJ. Trait impulsive choice predicts resistance to extinction and propensity to relapse to cocaine seeking: a bidirectional investigation. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:1377–1386. doi: 10.1038/npp.2011.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano G, Mochizuki T, Saper CB. Neural circuitry of stress-induced insomnia in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:10167–10184. doi: 10.1523/JNEUROSCI.1809-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD. What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus? Behavioral and cognitive neuroscience reviews. 2006;5:41–60. doi: 10.1177/1534582306289043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD. A critical review of chronic stress effects on spatial learning and memory. Prog Neuropsychopharmacol Bol Psychiatry. 2010;34:742–755. doi: 10.1016/j.pnpbp.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Cruz FC, Marin MT, Leao RM, Planeta CS. Stress-induced cross-sensitization to amphetamine is related to changes in the dopaminergic system. J Neural Transm. 2011 doi: 10.1007/s00702-011-0720-8. [DOI] [PubMed] [Google Scholar]
- Deroche V, Piazza PV, Casolini P, Maccari S, Le Moal M, Simon H. Stress-induced sensitization to amphetamine and morphine psychomotor effects depend on stress-induced corticosterone secretion. Brain research. 1992;598:343–348. doi: 10.1016/0006-8993(92)90205-n. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behavioural brain research. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2001;25:341–360. doi: 10.1016/S0893-133X(01)00235-4. [DOI] [PubMed] [Google Scholar]
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, Sousa N. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- Dilleen R, Pelloux Y, Mar AC, Molander A, Robbins TW, Everitt BJ, Dalley JW, Belin D. High anxiety is a predisposing endophenotype for loss of control over cocaine, but not heroin, self-administration in rats. Psychopharmacology (Berl) 2012;222:89–97. doi: 10.1007/s00213-011-2626-4. [DOI] [PubMed] [Google Scholar]
- Elliott AE, Packard MG. Intra-amygdala anxiogenic drug infusion prior to retrieval biases rats towards the use of habit memory. Neurobiology of learning and memory. 2008;90:616–623. doi: 10.1016/j.nlm.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. From the ventral to the dorsal striatum: Devolving views of their roles in drug addiction. Neuroscience and biobehavioral reviews. 2013 doi: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2004;176:459–465. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Goodwin JS, Larson GA, Swant J, Sen N, Javitch JA, Zahniser NR, De Felice LJ, Khoshbouei H. Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. The Journal of biological chemistry. 2009;284:2978–2989. doi: 10.1074/jbc.M805298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61:180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- Hoffman AN, Krigbaum A, Ortiz JB, Mika A, Hutchinson KM, Bimonte-Nelson HA, Conrad CD. Recovery after chronic stress within spatial reference and working memory domains: correspondence with hippocampal morphology. Eur J Neurosci. 2011;34:1023–1030. doi: 10.1111/j.1460-9568.2011.07820.x. [DOI] [PubMed] [Google Scholar]
- Hutchinson KM, McLaughlin KJ, Wright RL, Bryce Ortiz J, Anouti DP, Mika A, Diamond DM, Conrad CD. Environmental enrichment protects against the effects of chronic stress on cognitive and morphological measures of hippocampal integrity. Neurobiology of learning and memory. 2012;97:250–260. doi: 10.1016/j.nlm.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Qin M, Liu ZH. The functional divide for primary reinforcement of D-amphetamine lies between the medial and lateral ventral striatum: is the division of the accumbens core, shell, and olfactory tubercle valid? The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:5061–5065. doi: 10.1523/JNEUROSCI.0892-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Lee HJJ, Han JS, Packard MG. Amygdala is critical for stress-induced modulation of hippocampal long-term potentiation and learning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:5222–5228. doi: 10.1523/JNEUROSCI.21-14-05222.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Addiction is a Reward Deficit and Stress Surfeit Disorder. Frontiers in psychiatry / Frontiers Research Foundation. 2013;4 doi: 10.3389/fpsyt.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo J. Shifts in preferred learning strategy across the estrous cycle in female rats. Hormones and behavior. 2004;45:330–338. doi: 10.1016/j.yhbeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Leuner B, Shors TJ. Stress, anxiety, and dendritic spines: What are the connections? Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Li CS, Sinha R. Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neuroscience and biobehavioral reviews. 2008;32:581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd DA, Turner RJ. Cumulative lifetime adversities and alcohol dependence in adolescence and young adulthood. Drug and alcohol dependence. 2008;93:217–226. doi: 10.1016/j.drugalcdep.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Shepard JD, Hall FS, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neuroscience and biobehavioral reviews. 2003;27:457–491. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Martinez-Tellez RI, Hernandez-Torres E, Gamboa C, Flores G. Prenatal stress alters spine density and dendritic length of nucleus accumbens and hippocampus neurons in rat offspring. Synapse. 2009;63:794–804. doi: 10.1002/syn.20664. [DOI] [PubMed] [Google Scholar]
- McGuire J, Herman JP, Horn PS, Sallee FR, Sah R. Enhanced fear recall and emotional arousal in rats recovering from chronic variable stress. Physiol Behav. 2010;101:474–482. doi: 10.1016/j.physbeh.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Conrad CD. Chronic stress-and sex-specific neuromorphological and functional changes in limbic structures. Mol Neurobiol. 2009;40:166–182. doi: 10.1007/s12035-009-8079-7. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Gomez JL, Baran SE, Conrad CD. The effects of chronic stress on hippocampal morphology and function: an evaluation of chronic restraint paradigms. Brain research. 2007;1161:56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melega WP, Williams AE, Schmitz DA, DiStefano EW, Cho AK. Pharmacokinetic and pharmacodynamic analysis of the actions of D-amphetamine and D-methamphetamine on the dopamine terminal. J Pharmacol Exp Ther. 1995;274:90–96. [PubMed] [Google Scholar]
- Monroy E, Hernandez-Torres E, Flores G. Maternal separation disrupts dendritic morphology of neurons in prefrontal cortex, hippocampus, and nucleus accumbens in male rat offspring. J Chem Neuroanat. 2010;40:93–101. doi: 10.1016/j.jchemneu.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Morales-Medina JC, Sanchez F, Flores G, Dumont Y, Quirion R. Morphological reorganization after repeated corticosterone administration in the hippocampus, nucleus accumbens and amygdala in the rat. J Chem Neuroanat. 2009;38:266–272. doi: 10.1016/j.jchemneu.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Muhammad A, Carroll C, Kolb B. Stress during development alters dendritic morphology in the nucleus accumbens and prefrontal cortex. Neuroscience. 2012;216:103–109. doi: 10.1016/j.neuroscience.2012.04.041. [DOI] [PubMed] [Google Scholar]
- Murphy A, Taylor E, Elliott R. The detrimental effects of emotional process dysregulation on decision-making in substance dependence. Front Integr Neurosci. 2012;6 doi: 10.3389/fnint.2012.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Belin D, Everitt BJ. Double Dissociation of the Dorsomedial and Dorsolateral Striatal Control Over the Acquisition and Performance of Cocaine Seeking. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:2456–2466. doi: 10.1038/npp.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz JB, McLaughlin KJ, Hamilton GF, Baran SE, Campbell AN, Conrad CD. Cholesterol and perhaps estradiol protect against corticosterone-induced hippocampal CA3 dendritic retraction in gonadectomized female and male rats. Neuroscience. 2013;246:409–421. doi: 10.1016/j.neuroscience.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG. Anxiety, cognition, and habit: a multiple memory systems perspective. Brain research. 2009;1293:121–128. doi: 10.1016/j.brainres.2009.03.029. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiology of learning and memory. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Packard MG, Wingard JC. Amygdala and “emotional” modulation of the relative use of multiple memory systems. Neurobiology of learning and memory. 2004;82:243–252. doi: 10.1016/j.nlm.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-amphetamine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:2401–2411. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press Inc.; Orlando: 2005. [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends in pharmacological sciences. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Sadowski RN, Jackson GR, Wieczorek L, Gold PE. Effects of stress, corticosterone, and epinephrine administration on learning in place and response tasks. Behavioural brain research. 2009;205:19–25. doi: 10.1016/j.bbr.2009.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Dalm S, Schachinger H, Oitzl MS. Chronic stress modulates the use of spatial and stimulus-response learning strategies in mice and man. Neurobiology of learning and memory. 2008;90:495–503. doi: 10.1016/j.nlm.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Dickinson A, Wolf OT. Stress, habits, and drug addiction: a psychoneuroendocrinological perspective. Experimental and clinical psychopharmacology. 2011;19:53–63. doi: 10.1037/a0022212. [DOI] [PubMed] [Google Scholar]
- Shiflett MW, Brown RA, Balleine BW. Acquisition and performance of goal-directed instrumental actions depends on ERK signaling in distinct regions of dorsal striatum in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:2951–2959. doi: 10.1523/JNEUROSCI.1778-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Annals of the New York Academy of Sciences 030. 2008 doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Tseng KY. Handbook of Basal Ganglia Structure and Function: A Decade of Progress. Elsevier Science; 2010. [Google Scholar]
- Taylor SB, Lewis CR, Olive MF. The neurocircuitry of illicit psychostimulant addiction: acute and chronic effects in humans. Substance Abuse and Rehabilitation. 2013a;2013;4:29–43. doi: 10.2147/SAR.S39684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SB, Taylor AR, Koenig JI. The interaction of disrupted Type II Neuregulin 1 and chronic adolescent stress on adult anxiety-and fear-related behaviors. Neuroscience. 2013b;249:31–42. doi: 10.1016/j.neuroscience.2012.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RJ, Lloyd DA. Cumulative adversity and drug dependence in young adults: racial/ethnic contrasts. Addiction. 2003;98:305–315. doi: 10.1046/j.1360-0443.2003.00312.x. [DOI] [PubMed] [Google Scholar]
- Uylings HB, van Pelt J. Measures for quantifying dendritic arborizations. Network. 2002;13:397–414. [PubMed] [Google Scholar]
- Vyas A, Bernal S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain research. 2003;965:290–294. doi: 10.1016/s0006-8993(02)04162-8. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Ho UC, Ko MC, Liao CC, Lee LJ. Differential neuronal changes in medial prefrontal cortex, basolateral amygdala and nucleus accumbens after postweaning social isolation. Brain structure & function. 2012;217:337–351. doi: 10.1007/s00429-011-0355-4. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain research. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Wellman CL. Chronic stress effects on corticolimibic morphology. In: Conrad CD, editor. The Handbook of Stress:neuropsychological effects on the brain. Wiley-Blackwell; New York: New York: 2011. pp. 201–229. [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nature reviews Neuroscience. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. The European journal of neuroscience. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Blockade of NMDA receptors in the dorsomedial striatum prevents action-outcome learning in instrumental conditioning. The European journal of neuroscience. 2005;22:505–512. doi: 10.1111/j.1460-9568.2005.04219.x. [DOI] [PubMed] [Google Scholar]