Abstract
Background
Post-transplant survival in heart transplant recipients has progressively improved during the last 2 decades. It is unknown however whether the major racial groups in the United States have benefited equally.
Methods and Results
We analyzed all primary heart transplant recipients ≥18 years old in the United States during 1987-2008. We compared post-transplant survival in white, black and Hispanic recipients in 5 successive eras (1987-1992, 1993-1996, 1997-2000, 2001-2004, 2005-2008). Early survival was defined as freedom from death or re-transplantation during the first 6-months post-transplant. Longer-term, conditional survival was assessed in patients who survived the first 6 months. There were 29,986 white (81.6%), 4,745 black (12.9%) and 2,017 Hispanic (5.5%) patients in the study cohort. Black patients were at increased risk of early death or re-transplant (hazard ratio [HR] 1.15, 95% confidence interval [CI] 1.05, 1.26) in adjusted analysis. Early post-transplant survival improved (HR 0.83, CI 0.80, 0.87 for successive eras) equally in all three groups (P=0.94 for black-era, 0.40 for Hispanic-era interaction). Longer-term survival improved in white (HR 0.95, CI 0.92, 0.97 for successive eras) but not in black (HR 1.04, 95% CI 0.99, 1.09) or Hispanic (HR 1.02, CI 0.95, 1.09) recipients, resulting in increased disparities in longer-term survival with time.
Conclusions
Early post-transplant survival has improved equally in white, black and Hispanic heart transplant recipients. Longer-term survival has improved in white but not in black or Hispanic recipients resulting in a more marked disparity in outcomes in the current era. These disparities warrant further investigation and targeted interventions.
Keywords: transplantation, racial disparities, risk factors, outcomes, heart failure
Introduction
Post-transplant survival in heart transplant recipients has progressively improved since heart transplants were first performed, an observation often referred to as the “era effect” in heart transplantation.1-5 Although much of this improvement is due to improved survival in the early post-transplant period, recent multi-center registry reports have also observed improvement in longer-term survival.1, 2 Because post-transplant outcomes have been poorer historically in black (or non-white) recipients,6-11 it is important to know whether the era effect in post-transplant survival is due to improved survival in all or only some of the racial groups.
Because the post-transplant care of heart transplant recipients is protocol-driven at most centers and is expected to be the same irrespective of patient race, we hypothesized that the improvement in post-transplant survival in heart transplant recipients has benefited the major racial groups in the United States (US) equally. The objective of this study was to compare the era effect for early (first 6 months post-transplant) and longer-term post-transplant survival in white, black and Hispanic heart transplant recipients in the US.
Methods
Study Population
All white, black and Hispanic patients ≥ 18 years of age who underwent their first heart transplant in the US between January 1, 1987 and March 31, 2008 were identified in the Organ Procurement and Transplantation Network (OPTN) database. The OPTN database includes data on all transplant recipients in the US submitted by their transplant centers. The Health Resources and Services Administration, U.S. Department of Health and Human Services, provides oversight to the activities of the OPTN contractor, the United Network of Organ Sharing (UNOS).
We excluded patients who received a heart re-transplantation or multi-organ transplantation. All subjects were followed from the time of heart transplant until death, re-transplant or the day of last observation on March 31, 2009.
Study Design
The primary study hypothesis was that black and Hispanic heart transplant recipients in the US have experienced an improvement in early and longer-term post-transplant survival similar to that observed in white heart transplant recipients during the last 2 decades. We compared baseline characteristics and trends in post-transplant survival among white, black and Hispanic heart transplant recipients in 5 successive eras (transplanted during years 1987-1992, 1993-1996, 1997-2000, 2001-2004 and 2005-2008) in the OPTN database. We analyzed two time-to-event end-points, early graft loss within 6 months post-transplant and longer-term graft loss. Graft loss was defined as a composite of death (all-cause mortality) and re-transplantation. Longer-term, conditional survival was assessed in patients who survived the first 6 months post-transplant.
Demographic and clinical variables were defined at the time of transplant. Patient race/ethnicity, a mandatory variable, was reported by the transplant centers as one of the following: white, black, Hispanic/Latino, Asian, American Indian/Alaska Native, Native Hawaiian/Pacific Islander, Multiracial, and Other. Due to the small sample size for transplant recipients in minorities other than blacks and Hispanics (2.5% of all heart transplant recipients), these patients were not analyzed.
None of the subjects had any missing data for the variables age, gender, race/ethnicity, cardiac diagnosis, ventilator, extra-corporeal membrane oxygenation (ECMO), ventricular assist device (VAD), medical insurance (Medicaid), UNOS listing status, intra-aortic balloon pump, inotrope support, dialysis and the date of transplant. For patients with missing data on other variables, we created indicator variables “variable not reported” for each such variable to allow these subjects to contribute their other risk factors in the multivariable models.
Statistical Analysis
Summary data are presented as median (25th, 75th percentile) or number (percent). Baseline characteristics among groups were compared using the chi-square test for categorical and the Kruskal-Wallis test for continuous variables. Un-adjusted survival rates were assessed using the Kaplan-Meier method. We developed a multivariable Cox proportional hazards model for early post-transplant survival using a forward selection procedure retaining variables significant at the 0.10 level based on a likelihood ratio test and then added the race and era variables to the model. A second multivariable Cox model was developed for longer-term, conditional survival with a similar approach, limiting analysis to recipients who survived the first 6 months post-transplant. The effect of era was modeled in two ways, as a continuous variable coded 1 to 5 from the earliest to the most recent time period and, using 1987-1992 as the reference group with binary, indicator covariates for each subsequent period. For both early and longer-term survival, race-era interaction terms, with era as a continuous variable, were added to the main-effects models to assess whether the improvement in post-transplant survival over time was modified by race. Stratified multivariable models were developed to confirm significant race-era interactions in the overall model. To assess whether racial differences in improvement in longer-term survival were related to transplant center experience, we performed multivariable analyses for recipients stratified by the total number of recipients in each center over 20 years (<250, 250-499, ≥500 recipients during the study duration to define low, mid, and high-volume centers). In all models, we fitted continuous variables with a restricted cubic spline to allow for the most flexible relationship between the variable and the outcome.
To assess whether racial differences in era effect could be attributed to differences in use of newer immune-suppression medications in these groups, we assessed racial trends in use of maintenance tacrolimus and mycophenolate mofetil at the time of hospital discharge following transplant and in first-year rejection episodes. Finally, we compared the groups for freedom from coronary artery disease diagnosis using OPTN annual follow-up data and Kaplan Meier survival curves censoring patients at death. We evaluated racial differences in time to coronary artery diagnosis and era effect for coronary artery hazard in each group using a Cox proportional hazard model.
The data were analyzed using SAS statistical software version 9.1 (SAS Institute Inc, Cary, NC) and STATA software version 10.0 (StataCorp LP, College Station, TX). All statistical tests were two-sided and a P value of less than 0.05 was used to define statistical significance. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Study Population
During the study period, 37,682 patients ≥ 18 years of age underwent their first heart transplant in the US. Of these, 934 patients were from racial/ethnic groups other than those in the study and they were excluded from further analysis. The remaining 36748 patients formed the study cohort. Of these, 29,986 (81.6%) were white, 4,745 (12.9%) were black and 2,017 (5.5%) were Hispanic. In the OPTN database, race and ethnicity are reported as two distinct variables; however for all white, black and Hispanic patients in the study cohort, race and ethnicity variables were reported to be concordant (identical).
Table 1 summarizes baseline demographic and clinical characteristics of heart transplant recipients in the three racial groups. Compared to black and Hispanic patients, white patients were likely to be older and more likely to have ischemic cardiomyopathy as their cardiac diagnosis (P<0.001). Black patients were more likely to be female, have dilated cardiomyopathy and have a history of drug-treated hypertension compared to other groups. They were also more likely to be supported by a ventricular assist device, listed as status 1 or 1A at transplant and have a serum creatinine higher than 1.5 mg/dl at the time of their transplant. Hispanic patients had the highest prevalence of diabetes. White patients comprised a lower, and black and Hispanic patients a higher proportion of transplant recipients in successive eras (Table 1, P<0.001 for distribution by era, see online appendix for figure and for supplemental table A for comparison of additional characteristics among groups).
Table 1. Baseline Characteristics of Heart Transplant Patients, by Racial Group.
| White (n=29,986) | Black (n=4,745) | Hispanic (n=2,017) | Total (n=36,748) | P Value | |
|---|---|---|---|---|---|
| Age at Transplant, yr | 55 (47 to 60) | 48 (38 to 56) | 52 (42 to 58) | 54 (46 to 60) | <0.001 |
| Age at Transplant | <0.001 | ||||
| 18-39 | 3699 (12) | 1328 (28) | 419 (21) | 5446 (15) | |
| 40-60 | 19045 (64) | 2862 (60) | 1257 (62) | 23164 (63) | |
| >60 | 7242 (24) | 555 (12) | 341 (17) | 8138 (22) | |
| Recipient Height (n=36112) | 175 (168 to 180) | 173 (165 to 180) | 170 (163 to 175) | 175 (168 to 180) | <0.001 |
| Sex, Female | 5938 (20) | 1654 (35) | 488 (24) | 8080 (22) | <0.001 |
| Donor Age, yr (n=36746) | 28 (20 to 40) | 28 (20 to 40) | 27 (20 to 39) | 28 (20 to 40) | 0.14 |
| Diagnosis | <0.001 | ||||
| Ischemic CM | 16335 (54) | 1136 (24) | 801 (40) | 18272 (50) | |
| Dilated CM | 10981 (37) | 3294 (69) | 1024 (51) | 15299 (42) | |
| Hypertrophic CM | 382 (1) | 30 (1) | 20 (1) | 432 (1) | |
| Restrictive CM | 373 (1) | 59 (1) | 22 (1) | 454 (1) | |
| Valvular CM | 874 (3) | 114 (2) | 79 (4) | 1067 (3) | |
| Congenital Heart Disease | 620 (2) | 51 (1) | 47 (2) | 718 (2) | |
| Other | 421 (1) | 61 (1) | 24 (1) | 506 (1) | |
| Drug Treated Hypertension | <0.001 | ||||
| Yes | 6425 (21) | 1310 (28) | 467 (23) | 8202 (22) | |
| No | 10883 (36) | 1727 (36) | 839 (42) | 13449 (37) | |
| Unknown | 12678 (42) | 1708 (36) | 711 (35) | 15097 (41) | |
| Diabetes | 3804 (13) | 696 (15) | 387 (19) | 4887 (13) | <0.001 |
| Mean PAP > 30 mm Hg | 7673 (26) | 1683 (35) | 641 (32) | 9997 (27) | <0.001 |
| PVR | |||||
| <3 | 13449 (45) | 2052 (43) | 906 (45) | 16407 (45) | <0.001 |
| >3 | 4893 (16) | 1337 (28) | 498 (25) | 6728 (18) | |
| Unknown | 11644 (39) | 1356 (29) | 613 (30) | 13613 (37) | |
| Intravenous Inotropes | 11121 (37) | 2158 (45) | 894 (44) | 14173 (39) | <0.001 |
| Mechanical Ventilation | 832 (3) | 101 (2) | 66 (3) | 999 (3) | 0.01 |
| Intra-aortic Balloon Pump | 1769 (6) | 251 (5) | 110 (5) | 2130 (6) | 0.20 |
| Ventricular Assist Device | 3748 (13) | 771 (16) | 256 (13) | 4775 (13) | <0.001 |
| ECMO | 63 (<1) | 9 (<1) | 3 (<1) | 75 (<1) | 0.82 |
| ICD | 8004 (27) | 1533 (32) | 637 (32) | 10174 (28) | <0.001 |
| Listing Status | <0.001 | ||||
| 1/1A | 13514 (45) | 2362 (50) | 929 (46) | 16805 (46) | |
| 1B | 4903 (16) | 1138 (24) | 459 (23) | 6500 (18) | |
| 2 | 10661 (36) | 1146 (24) | 610 (30) | 12417 (34) | |
| Other: Inactive/unknown | 908 (3) | 99 (2) | 19 (1) | 1026 (3) | |
| Ischemic Time, hr (n=34606) | 2.9 (2.2 to 3.6) | 2.9 (2.2 to 3.6) | 2.9 (2.2 to 3.6) | 2.9 (2.2 to 3.6) | 0.07 |
| PRA > 10% (n=26872) | 3319 (11) | 716 (15) | 218 (11) | 4253 (12) | <0.001 |
| Dialysis at Transplant | 370 (1) | 88 (2) | 37 (2) | 495 (1) | <0.001 |
| Creatinine at Transplant (n=26137) | 1.2 (1.0 to 1.5) | 1.2 (1.0 to 1.6) | 1.1 (0.9 to 1.4) | 1.2 (1.0 to 1.5) | <0.001 |
| Creatinine at Transplant >1.5 (n=26137) | 4686 (16) | 985 (21) | 293 (15) | 5964 (16) | <0.001 |
| Bilirubin at Transplant (n=24087) | 0.8 (0.5 to 1.3) | 0.9 (0.5 to 1.4) | 0.9 (0.6 to 1.5) | 0.8 (0.6 to 1.3) | <0.001 |
| IV Antibiotics <2 Weeks | 1967 (7) | 473 (10) | 155 (8) | 2595 (7) | <0.001 |
| Before Transplant | |||||
| Medicaid Insurance | 1895 (6) | 908 (19) | 425 (21) | 3228 (9) | <0.001 |
| Non ABO-Identical Transplant | 4185 (14) | 789 (17) | 266 (13) | 5240 (14) | <0.001 |
| Era of Transplant | <0.001 | ||||
| 1987-1992 | 7571 (25) | 754 (16) | 317 (16) | 8642 (24) | |
| 1993-1996 | 6511 (22) | 882 (19) | 337 (17) | 7730 (21) | |
| 1997-2000 | 6075 (20) | 916 (19) | 386 (19) | 7377 (20) | |
| 2001-2004 | 5269 (18) | 1015 (21) | 461 (23) | 6745 (18) | |
| 2005-2008 | 4560 (15) | 1178 (25) | 516 (26) | 6254 (17) |
Values are summarized as number (percent) or median (25th to 75th percentile). yr (year), CM (Cardiomyopathy), PAP (Pulmonary Artery Pressure), PVR (Pulmonary Vascular Resistance), ECMO (Extra-corporeal Membrane Oxygenation), ICD (Implantable Cardioverter Defibrillator), VAD (Ventricular Assist Device), hr (hour), IV (intravenous), PRA (Panel Reactive Antibodies)
Early (6-month) Post-transplant Survival
Overall, death or re-transplantation occurred in 17,000 transplant recipients during the study period (16226 deaths and 774 re-transplants). Early graft loss occurred in 4349 (11.8%) transplant recipients (4161 deaths, 188 re-transplants). Unadjusted 6-month post-transplant survival improved from 86.3% in the earliest era (1987-1992) to 90.8% in the most recent era (2005-2008). Early post-transplant survival improved with time in all racial groups (Figure 1, panels A-C).
Figure 1.
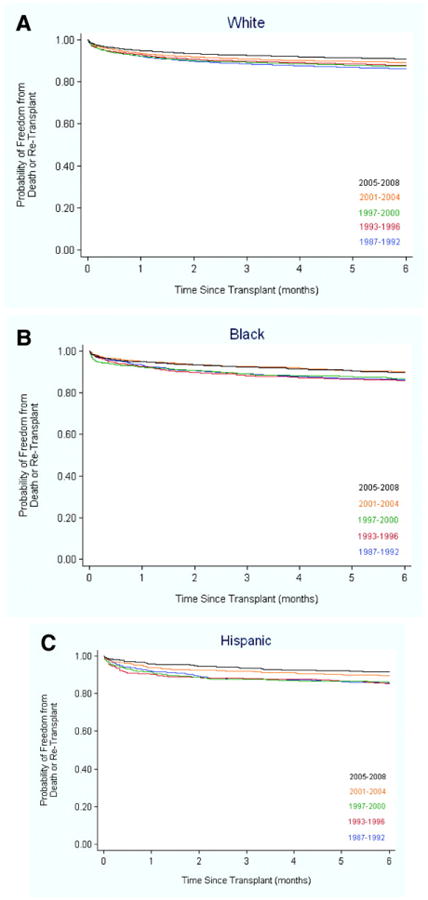
Early (first 6 months) post-transplant survival in white (A), black (B), and Hispanic (C) heart transplant recipients during the five eras. The improvement in survival in the three groups, adjusted for baseline risk factors, was similar (P=0.94 for black-era and 0.40 for Hispanic-era interaction).
In a multivariable model adjusted for patient factors and era of transplant, the risk of death or re-transplant within 6 months post-transplant was significantly higher in black transplant recipients compared to white recipients (hazard ratio [HR] 1.15, 95% confidence interval [CI] 1.05, 1.26, P=0.004, Table 2). Early post-transplant survival improved significantly in successive eras (HR 0.83, 95% CI 0.80, 0.87, P<0.001, Table 2). Furthermore, when the transplant eras were modeled as binary, indicator variables with recipients during 1987-1992 as the reference group, the risk of death or re-transplantation within the first 6 months post-transplant was 49% lower for transplant recipients in 2005-2008 compared to the reference group (HR 0.51, 95% CI 0.43, 0.60, P<0.001). When race-era interaction terms were added to the main-effects model in Table 2, they were not statistically significant (P=0.94 for black-era interaction, P=0.40 for Hispanic-era interaction).
Table 2. Multivariable Model of Predictors of Early (6-month) Death or Re-transplant in Heart Transplant Recipients.
| Predictor | Hazard Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Age at Transplant | ** | ** | <0.001 |
| Recipient Height | ** | ** | <0.001 |
| Donor Age | ** | ** | <0.001 |
| Diagnosis (vs. Dilated CM) | |||
| Ischemic CM | 1.22 | (1.14, 1.31) | <0.001 |
| Hypertrophic CM | 1.36 | (1.02, 1.81) | 0.04 |
| Congenital Heart Disease | 2.69 | (2.25, 3.22) | <0.001 |
| Other | 1.29 | (1.13, 1.47) | <0.001 |
| Drug Treated | 1.14 | (1.06, 1.23) | 0.001 |
| Hypertension | |||
| Mean PAP > 30 mmHg | 1.08 | (1.00, 1.16) | 0.06 |
| PVR < 3 | 0.89 | (0.82, 0.96) | 0.002 |
| Mechanical Ventilation | 2.41 | (2.12, 2.73) | <0.001 |
| Intra-aortic Balloon Pump | 1.21 | (1.08, 1.36) | 0.001 |
| VAD | 1.57 | (1.44, 1.72) | <0.001 |
| ECMO | 2.10 | (1.44, 3.08) | <0.001 |
| ICD | 1.09 | (1.00, 1.19) | 0.06 |
| Ischemic Time | ** | ** | 0.001 |
| PRA | |||
| > 10% | 1.22 | (1.11, 1.33) | <0.001 |
| Missing | 1.01 | (0.94, 1.09) | 0.81 |
| Bilirubin at Transplant | ** | ** | 0.001 |
| IV Antibiotic <2 Weeks Before Transplant Gender Match* | 1.24 | (1.12, 1.38) | <0.001 |
| M recipient / F donor | 1.20 | (1.11, 1.30) | <0.001 |
| F recipient / M donor | 1.11 | (0.99, 1.25) | 0.06 |
| F recipient / F donor | 1.07 | (0.96, 1.20) | 0.24 |
| Medicaid Insurance | 1.12 | (1.00, 1.25) | 0.06 |
| Era (treated as linear 1-5) | 0.83 | (0.80, 0.87) | <0.001 |
| Race (vs. White) | |||
| Black | 1.15 | (1.05, 1.26) | 0.004 |
| Hispanic | 1.03 | (0.90, 1.18) | 0.66 |
Restricted cubic splines, with a separate term to identify missing values if required. CM (Cardiomyopathy), PAP (Pulmonary Artery Pressure), PVR (Pulmonary Vascular Resistance), ECMO (Extra-corporeal Membrane Oxygenation), ICD (Implantable Cardioverter Defibrillator), VAD (Ventricular Assist Device), hr (hour), IV (intravenous), PRA (Panel Reactive Antibodies),
The reference category is M recipient-M donor. The donor-recipient BMI ratio or donor LV ejection fraction was not significant.
Longer-Term, Conditional Survival
Overall, the annual rate of death or re-transplantation in 6-month survivors was 4.3% in white, 5.5% in black and 4.3% in Hispanic transplant recipients. In multivariable analysis, a significant race-era interaction was identified for longer-term survival (P<0.001 for black-era interaction, P=0.06 for Hispanic-era interaction). In a model adjusted for baseline risk factors, longer-term survival improved in successive eras in white (HR 0.95, 95% CI 0.92, 0.97, P<0.001) but not in black or Hispanic transplant recipients (Table 3). As a result, the risk of death or re-transplantation in black and Hispanic recipients (vs. white recipients) increased progressively during the five eras (Figure 2). Other independent predictors of late death or re-transplantation included ischemic etiology, diabetes, renal dysfunction and Medicaid insurance.
Table 3. Multivariable Model of Predictors for Time to Graft Loss in 6-Month Survivors of Heart Transplantation.
| Predictor | Hazard Ratio | 95% CI | P Value |
|---|---|---|---|
| Age at Transplant | ** | ** | <0.001 |
| Donor Age | ** | ** | <0.001 |
| Diagnosis (vs. Dilated CM) | |||
| Ischemic CM | 1.22 | (1.17, 1.27) | <0.001 |
| Hypertrophic CM | 0.75 | (0.59, 0.94) | 0.01 |
| Congenital Heart Disease | 0.88 | (0.75, 1.03) | 0.11 |
| Other | 1.03 | (0.95, 1.12) | 0.52 |
| Drug Treated Hypertension | 1.04 | (0.98, 1.09) | 0.20 |
| Diabetes | |||
| Yes | 1.19 | (1.12, 1.27) | <0.001 |
| Not reported | 1.16 | (1.09, 1.24) | <0.001 |
| VAD | 0.95 | (0.88, 1.02) | 0.16 |
| ICD | 0.90 | (0.85, 0.96) | 0.001 |
| Creatinine at Transplant | |||
| > 1.5 | 1.20 | (1.13, 1.26) | <0.001 |
| Not reported | 1.08 | (1.02, 1.14) | 0.008 |
| IV Antibiotic <2 Weeks Before Transplant | 1.14 | (1.05, 1.24) | 0.002 |
| Medicaid Insurance | 1.43 | (1.33, 1.54) | <0.001 |
| Interaction: Era by Race† | |||
| White | 0.95 | (0.92, 0.97) | <0.001 |
| Black | 1.04 | (0.99, 1.09) | 0.07 |
| Hispanic | 1.02 | (0.95, 1.09) | 0.63 |
Restricted cubic splines, with a separate term to identify missing values if required.
Race-era interaction terms were interpreted in two ways, as era effect within racial groups (above) and as racial differences in outcomes within all eras (Figure 2). CM (Cardiomyopathy), ICD (Implantable Cardioverter Defibrillator), VAD (Ventricular Assist Device), IV (intravenous)
Figure 2.
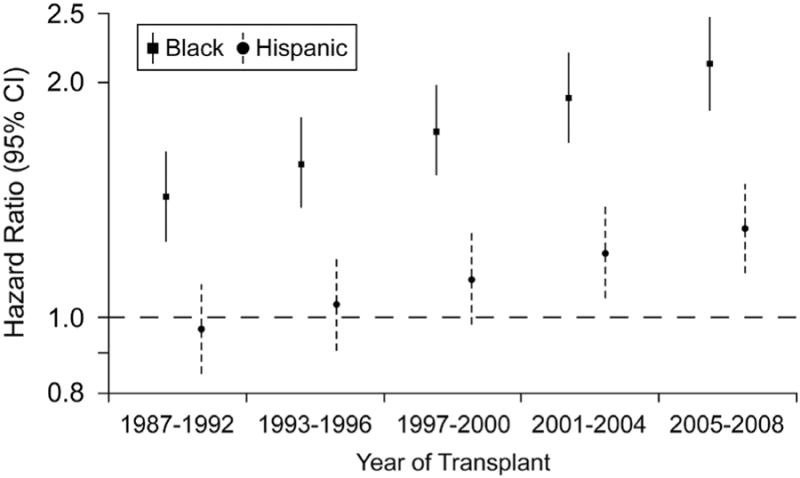
Widening racial disparities for longer-term survival, conditional upon surviving the first 6-month after heart transplant. The reference group is white heart transplant recipients. The hazard ratios and 95% confidence intervals are adjusted for baseline risk factors (see Table 3).
In multivariable models stratified by race, race-era interaction findings demonstrated in Table 3 and Figure 2 were confirmed. Thus, longer-term survival improved in white (HR 0.94, 95% CI 0.91, 0.97, P<0.001) but not in black or Hispanic transplant recipients (see online appendix). In multivariable models stratified by era, black recipients were at a higher risk of longer-term graft loss (vs. white recipients) in all eras with an increase in relative risk from the earliest (HR 1.59, 95% CI 1.45, 1.75, P<0.001) to the most recent (HR 2.37, 95% CI 1.86, 3.02, P<0.001) era. The risk of longer-term graft loss in Hispanic recipients was similar to white recipients during the first 3 eras but was higher during 2001-2004 (HR 1.25, 95% CI 1.01, 1.55, P=0.04) and 2005-08 (HR 1.55, 95% CI 1.08, 2.22, P=0.02) (see online appendix).
There was no improvement in longer-term survival in any racial group in recipients from low-volume centers (<250 total recipients). The improvement in survival in white recipients from mid-volume centers (250-499 total recipients) was of borderline statistical significance (HR 0.95 for successive eras, P=0.06) and was highly significant in white recipients from high-volume centers (HR 0.88, P<0.001). Longer-term survival did not improve in black or Hispanic recipients in either mid or high-volume centers.
Racial Trends in Immune Suppression, Rejection and Coronary Artery Disease
The percent transplant recipients on tacrolimus immune suppression and those on mycophenolate immune suppression at hospital discharge increased in successive eras in all racial groups. The proportion of white, black and Hispanic recipients on myocophenolate were similar in all eras, however a higher proportion of black recipients (vs. white recipients) appeared to be on tacrolimus in successive eras (Figure 3, Panels A-B).
Figure 3.
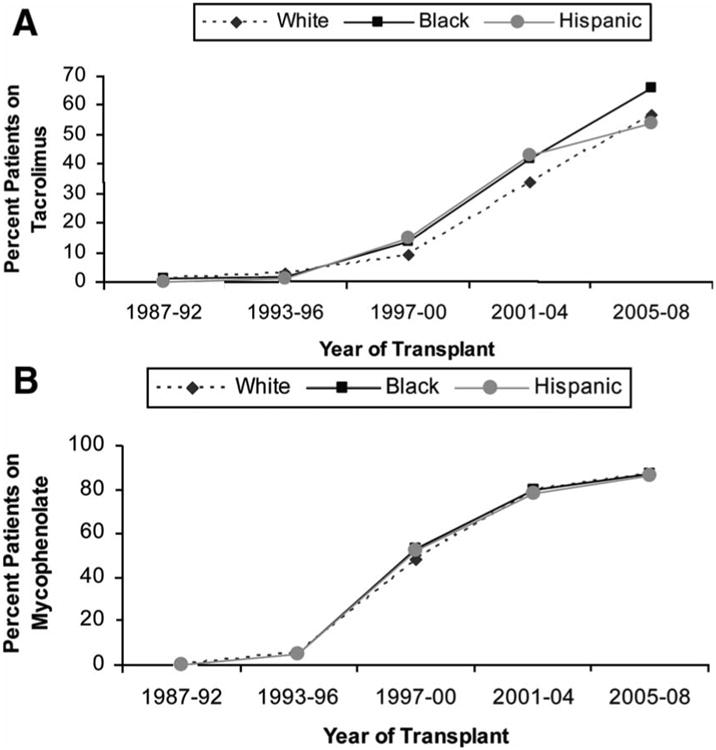
Racial trends in use of tacrolimus (A) and mycophenolate mofetil (B) as maintenance immune suppression at hospital discharge.
First-year rejection data were not available for transplant recipients in the first and the majority of the second era. The percentage of recipients who were reported to have a rejection episode during the first post-transplant year declined between the 3rd and the 5th era in white (56%, 40% and 24% during 1997-2000, 2001-2004 and 2005-2008, respectively), black (62%, 43% and 33%, respectively) and Hispanic (60%, 36% and 26%, respectively) recipients.
The difference among racial groups for freedom from coronary artery disease diagnosis was statistically significant (P=0.02, log rank test; see figure in online supplement) with shorter time to diagnosis for black compared to white recipients (HR 1.13, 95% CI 1.06, 1.21). There was no difference between Hispanic and white recipients for time to coronary artery diagnosis (HR 0.95, 95% CI 0.86, 1.04). There was no era effect for time to diagnosis of coronary artery disease in white (HR for successive eras 1.02, 95% CI 099, 1.05), black (HR 0.98, 95% CI 0.92, 1.05) or Hispanic recipients (HR 1.05, 95% CI 0.94, 1.17)
Discussion
In this study, we analyzed trends in post-heart transplant survival in three major US racial groups during the last 2 decades. There are three main findings of this study. First, the risk of death or re-transplantation within 6 months post-transplant, adjusted for baseline risk factors at the time of transplant, has decreased equally in white, black and Hispanic recipients during the last 2 decades. Second, among patients who survived the first 6 months post-transplant, longer-term survival has progressively improved in white but not in black or Hispanic recipients. As a result, disparities in longer-term post-transplant survival among racial groups have increased with time. Third, black heart transplant recipients have worse post-transplant outcomes compared to white recipients both during early post-transplant period and on longer-term follow-up. For example, the risk of death or re-transplant in black recipients within the first 6-months post-transplant is 15% higher compared to white recipients, and for longer-term follow-up is 111% higher compared to white recipients in the current era. These disparities in post-transplant outcomes warrant further investigation and may be amenable to intervention.
Risk factors for early post-transplant mortality in our analysis included the listing diagnosis, the level of cardiac support (and thus the severity of heart failure), pre-transplant anti-HLA antibodies>10%, male recipients who received a heart from a female donor, and co-morbidity at the time of transplant (Table 2). The finding that early outcomes have improved similarly among racial groups adjusted for these risk factors suggests that advances in recipient selection, in the care of patients awaiting a heart transplant, in peri-operative care of transplant recipients and in immune suppression, which have contributed to improvement in early survival after heart transplantation,3-5 have been implemented widely among centers and have benefited the racial groups equally. Several risk factors for early mortality such as VAD support, PVR>3, anti-HLA antibodies>10% and administration of intravenous antibiotics <2 weeks prior to transplant were more prevalent in black recipients and residual confounding with respect to these risk factors could have contributed to their worse early outcomes. Risk factors not captured in the OPTN database such as differences in access to care, illness severity at presentation and rate of disease progression could have also contributed to these outcome differences.
We were surprised to find that the improvement in longer-term survival has been limited to white heart transplant recipients. Although we did anticipate better conditional survival in recipients from more recent years,1, 2 we expected this finding in either all racial groups or primarily in higher-risk black recipients (because recognition of a risk factor often leads to efforts to improve outcomes associated with that risk factor). Several potential mechanisms have been invoked to explain worse longer-term outcomes in black heart transplant recipients. These include biologic factors such as a higher prevalence of pre- and post-transplant hypertension12, a higher likelihood of donor-recipient HLA mismatch13, and immunologic and metabolic differences from whites. Black recipients have a higher prevalence of genotypes associated with reduced immune suppression exposure and efficacy as well as genotypes associated with a pro-inflammatory state.14 Lower socioeconomic position and fewer years of formal education, known to be more prevalent in black population, have been previously associated with worse post-transplant outcomes.12, 15, 16 A similar association of black race with worse graft survival has also been described in renal transplantation and has been attributed to a combination of genetic, immunologic and socioeconomic factors.17-19 These biologic and socioeconomic factors may also explain the lack of improvement in longer-term survival in black recipients observed in our study. Our analysis shows that although rejection rates have decreased progressively in all groups, a modestly higher proportion of black recipients had a rejection episode during the first post-transplant year in all eras. The risk of developing graft coronary artery disease was also higher in black recipients suggesting that racial differences in longer-term survival are in part due to rejection-related mechanisms. Although black and Hispanic transplant recipients in the current study were three times as likely to have Medicaid insurance as white recipients, the reported race effects were seen after adjusting for insurance. Given that newer immune suppression agents (such as mycophenolate mofetil, tacrolimus and sirolimus) reduce rejection rates, prevent progression of cardiac allograft vasculopathy and improve graft and patient survival in heart transplant recipients,5, 20-23 our finding that a similar or higher percentage of black and Hispanic transplant recipients (vs. white recipients) received maintenance mycophenolate and tacrolimus in all eras makes it unlikely that the lack of improvement in longer-term survival in these recipients was due to a disparity in choice of immune suppression.
A few single-center studies have reported equivalent post-transplant survival in white and black heart transplant recipients and have attributed their success in black recipients to either newer, more efficacious immune suppression protocols or to specialized care, i.e. a quality improvement initiative at the center.20, 21 Equivalence of outcomes in white and black transplant recipients using newer immune suppression has also been reported recently in renal transplantation.24 These preliminary reports suggest that approaches that combine current immune suppression agents with quality control initiatives and with interventions to reduce disparities may help bridge survival differences between racial groups despite their underlying immunologic and metabolic differences and may improve overall post-transplant survival.25 For example, enhanced patient education with respect to their medical management and symptoms of rejection in those with limited formal schooling, and improved support system for patients with socioeconomic challenges that allows easy access to transplant team members may help improve longer-term outcomes in minorities.
Our results also demonstrate that the minorities represent an increasing proportion of heart transplant recipients in the US. This demographic shift is expected as the racial distribution of US population changes over time but may also be due to other factors such as an increase in referral of minority patients to transplant centers and a higher incidence of heart failure in minorities, particularly blacks.26, 27 Further improvement in survival after heart transplantation will require a concurrent use of two strategies similar to those described for preventing cardiovascular disease:28 (1) developing interventions that improve outcomes in all heart transplant recipients, and (2) identifying transplant recipients at high risk of graft loss and targeting interventions to improve their outcomes. The present analysis provides a framework for such interventions by describing the magnitude of racial disparity associated with early and longer-term post-transplant survival. Reduction and elimination of racial disparities in health care and in health outcomes are national priorities in the US.29 Because racial disparities have complex, multi-factorial origins, interventions likely to succeed in reducing disparities in post-transplant outcomes are also likely to be multi-level.30
This study has a few limitations. First, being a retrospective analysis of a national database, the quality control of these data may be variable among transplant centers. However, because the OPTN data are used by the UNOS to mediate organ allocation in the US and to evaluate and report transplant center performance, certain safeguards to data quality are to be expected. Second, race was analyzed as reported by the transplant centers and there is a possibility that some recipients were misclassified. However, a non-differential misclassification of race would likely result in a loss of statistical power which was not a major problem in this study because of the relatively large sample size. Finally, the duration of follow-up was different in transplant recipients from different eras. Although Cox models allowed us to evaluate recipients with different duration of follow-up, these models may not predict future survival accurately in transplant recipients from the more recent eras.
In conclusion, the progressive improvement in early post-transplant survival during the last 2 decades has benefited white, black and Hispanic heart transplant recipients equally. Longer-term survival has improved in white but not in black or Hispanic transplant recipients resulting in a more marked disparity in outcomes in the current era. Black heart transplant recipients are at higher risk of early and longer-term graft loss compared to other groups. Targeted interventions in high-risk transplant recipients may improve long-term and overall survival in heart transplantation.
Supplementary Material
Clinical Perspective.
Survival after heart transplantation has progressively improved since heart transplants were first performed, a finding described as the “era effect” in heart transplantation. Whether the major racial groups in the United States (white, black, and Hispanic) have benefited similarly from the medical progress in this field is unknown. This study analyzed trends in survival after heart transplant among these 3 racial groups during the past 2 decades. As expected, the minorities were found to represent an increasing proportion of heart transplant recipients with time. There are 3 main findings of this study. First, the risk of death or retransplantation within 6 months posttransplant, adjusted for baseline risk factors at the time of transplant, has decreased equally in white, black, and Hispanic recipients. Second, among patients who survived the first 6 months after transplant, longer-term survival has progressively improved in white but not in black or Hispanic recipients. Third, black heart transplant recipients have worse posttransplant outcomes than white recipients both during the early posttransplant period (15% higher risk of death or retransplant) and on longer-term follow-up (111% higher risk). We discuss the potential biological and socioeconomic mechanisms that may explain these findings and suggest that targeted interventions in high-risk transplant recipients may improve long-term and overall survival in heart transplantation.
Acknowledgments
The work was supported in part by Health Resources and Services Administration contract 234-2005-370011C. The data were supplied by the UNOS as the contractor for the OPTN. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the OPTN or the US Government. This work was supported by the National Institutes of Health under award number: T32HL007572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding Sources: This study was supported by Heart Transplant Research and Education Fund, Department of Cardiology, Children's Hospital Boston, Boston, MA.
Footnotes
Disclosures: None
References
- 1.Taylor DO, Edwards LB, Aurora P, Christie JD, Dobbels F, Kirk R, Rahmel AO, Kucheryavaya AY, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: twenty-fifth official adult heart transplant report--2008. J Heart Lung Transplant. 2008;27:943–956. doi: 10.1016/j.healun.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 2.Taylor DO, Stehlik J, Edwards LB, Aurora P, Christie JD, Dobbels F, Kirk R, Kucheryavaya AY, Rahmel AO, Hertz MI. Registry of the international society for heart and lung transplantation: twenty-sixth official adult heart transplant report-2009. J Heart Lung Transplant. 2009;28:1007–1022. doi: 10.1016/j.healun.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Marelli D, Laks H, Kobashigawa JA, Bresson J, Ardehali A, Esmailian F, Plunkett MD, Kubak B. Seventeen-year experience with 1,083 heart transplants at a single institution. Ann Thorac Surg. 2002;74:1558–1566. doi: 10.1016/s0003-4975(02)03933-4. [DOI] [PubMed] [Google Scholar]
- 4.John R, Rajasinghe HA, Chen JM, Weinberg AD, Sinha P, Mancini DM, Naka Y, Oz MC, Smith CR, Rose EA, Edwards NM. Long-term outcomes after cardiac transplantation: an experience based on different eras of immunosuppressive therapy. Ann Thorac Surg. 2001;72:440–449. doi: 10.1016/s0003-4975(01)02784-9. [DOI] [PubMed] [Google Scholar]
- 5.Kofler S, Bigdeli AK, Kaczmarek I, Kellerer D, Muller T, Schmoeckel M, Steinbeck G, Uberfuhr P, Reichart B, Meiser B. Long-term outcomes after 1000 heart transplantations in six different eras of innovation in a single center. Transpl Int. 2009;22:1140–1150. doi: 10.1111/j.1432-2277.2009.00931.x. [DOI] [PubMed] [Google Scholar]
- 6.Cohen O, De La Zerda D, Beygui RE, Hekmat D, Laks H. Ethnicity as a predictor of graft longevity and recipient mortality in heart transplantation. Transplant Proc. 2007;39:3297–3302. doi: 10.1016/j.transproceed.2007.06.086. [DOI] [PubMed] [Google Scholar]
- 7.Mills RM, Naftel DC, Kirklin JK, Van Bakel AB, Jaski BE, Massin EK, Eisen HJ, Lee FA, Fishbein DP, Bourge RC. Heart transplant rejection with hemodynamic compromise: a multiinstitutional study of the role of endomyocardial cellular infiltrate. Cardiac Transplant Research Database. J Heart Lung Transplant. 1997;16:813–821. [PubMed] [Google Scholar]
- 8.Park MH, Tolman DE, Kimball PM. Disproportionate HLA matching may contribute to racial disparity in patient survival following cardiac transplantation. Clin Transplant. 1996;10:625–628. [PubMed] [Google Scholar]
- 9.Valente JF, Hariharan S, Peddi VR, Schroeder TJ, Ogle CK, Alexander JW, First MR. Causes of renal allograft loss in black vs. white transplant recipients in the cyclosporine era. Clin Transplant. 1997;11:231–236. [PubMed] [Google Scholar]
- 10.Mahle WT, Kanter KR, Vincent RN. Disparities in outcome for black patients after pediatric heart transplantation. J Pediatr. 2005;147:739–743. doi: 10.1016/j.jpeds.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 11.Costanzo MR, Naftel DC, Pritzker MR, Heilman JK, 3rd, Boehmer JP, Brozena SC, Dec GW, Ventura HO, Kirklin JK, Bourge RC, Miller LW. Heart transplant coronary artery disease detected by coronary angiography: a multiinstitutional study of preoperative donor and recipient risk factors. Cardiac Transplant Research Database. J Heart Lung Transplant. 1998;17:744–753. [PubMed] [Google Scholar]
- 12.Allen JG, Weiss ES, Arnaoutakis GJ, Russell SD, Baumgartner WA, Conte JV, Shah AS. The impact of race on survival after heart transplantation: an analysis of more than 20,000 patients. Ann Thorac Surg. 2010;89:1956–1963. doi: 10.1016/j.athoracsur.2010.02.093. [DOI] [PubMed] [Google Scholar]
- 13.Park MH, Tolman DE, Kimball PM. The impact of race and HLA matching on long-term survival following cardiac transplantation. Transplant Proc. 1997;29:1460–1463. doi: 10.1016/s0041-1345(96)00567-2. [DOI] [PubMed] [Google Scholar]
- 14.Girnita DM, Webber SA, Ferrell R, Burckart GJ, Brooks MM, McDade KK, Chinnock R, Canter C, Addonizio L, Bernstein D, Kirklin JK, Girnita AL, Zeevi A. Disparate distribution of 16 candidate single nucleotide polymorphisms among racial and ethnic groups of pediatric heart transplant patients. Transplantation. 2006;82:1774–1780. doi: 10.1097/01.tp.0000250656.33731.08. [DOI] [PubMed] [Google Scholar]
- 15.Singh TP, Givertz MM, Semigran M, Denofrio D, Costantino F, Gauvreau K. Socioeconomic position, ethnicity, and outcomes in heart transplant recipients. Am J Cardiol. 2010;105:1024–1029. doi: 10.1016/j.amjcard.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Singh TP, Gauvreau K, Bastardi HJ, Blume ED, Mayer JE. Socioeconomic position and graft failure in pediatric heart transplant recipients. Circ Heart Fail. 2009;2:160–165. doi: 10.1161/CIRCHEARTFAILURE.108.800755. [DOI] [PubMed] [Google Scholar]
- 17.Stephens MR, Evans M, Ilham MA, Marsden A, Asderakis A. The influence of socioeconomic deprivation on outcomes following renal transplantation in the United kingdom. Am J Transplant. 2010;10:1605–1612. doi: 10.1111/j.1600-6143.2010.03041.x. [DOI] [PubMed] [Google Scholar]
- 18.Hutchings A, Purcell WM, Benfield MR. Increased costimulatory responses in African-American kidney allograft recipients. Transplantation. 2001;71:692–695. doi: 10.1097/00007890-200103150-00021. [DOI] [PubMed] [Google Scholar]
- 19.Gordon EJ, Ladner DP, Caicedo JC, Franklin J. Disparities in kidney transplant outcomes: a review. Semin Nephrol. 2010;30:81–89. doi: 10.1016/j.semnephrol.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobashigawa JA, Meiser BM. Review of major clinical trials with mycophenolate mofetil in cardiac transplantation. Transplantation. 2005;80:S235–243. doi: 10.1097/01.tp.0000186383.22264.b3. [DOI] [PubMed] [Google Scholar]
- 21.Kobashigawa JA, Miller LW, Russell SD, Ewald GA, Zucker MJ, Goldberg LR, Eisen HJ, Salm K, Tolzman D, Gao J, Fitzsimmons W, First R. Tacrolimus with mycophenolate mofetil (MMF) or sirolimus vs. cyclosporine with MMF in cardiac transplant patients: 1-year report. Am J Transplant. 2006;6:1377–1386. doi: 10.1111/j.1600-6143.2006.01290.x. [DOI] [PubMed] [Google Scholar]
- 22.Mancini D, Pinney S, Burkhoff D, LaManca J, Itescu S, Burke E, Edwards N, Oz M, Marks AR. Use of rapamycin slows progression of cardiac transplantation vasculopathy. Circulation. 2003;108:48–53. doi: 10.1161/01.CIR.0000070421.38604.2B. [DOI] [PubMed] [Google Scholar]
- 23.Kobashigawa JA, Katznelson S, Laks H, Johnson JA, Yeatman L, Wang XM, Chia D, Terasaki PI, Sabad A, Cogert GA, Trosian K, Hamilton MA, Moriguchi JD, Kawata N, Hage A, Drinkwater DC, Stevenson LW. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med. 1995;333:621–627. doi: 10.1056/NEJM199509073331003. [DOI] [PubMed] [Google Scholar]
- 24.Oliver JD, 3rd, Neff RT, Leeser DB, Swanson SJ, Yuan CM, Falta EM, Elster E, Reinmuth B, Bohen EM, Jindal RM, Abbott KC. Influence of race on kidney transplantation in the Department of Defense healthcare system. Am J Nephrol. 2009;29:327–333. doi: 10.1159/000163558. [DOI] [PubMed] [Google Scholar]
- 25.Schlotthauer AE, Badler A, Cook SC, Perez DJ, Chin MH. Evaluating interventions to reduce health care disparities: an RWJF program. Health Aff (Millwood) 2008;27:568–573. doi: 10.1377/hlthaff.27.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bibbins-Domingo K, Pletcher MJ, Lin F, Vittinghoff E, Gardin JM, Arynchyn A, Lewis CE, Williams OD, Hulley SB. Racial differences in incident heart failure among young adults. N Engl J Med. 2009;360:1179–1190. doi: 10.1056/NEJMoa0807265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 28.Rose G. Strategy of prevention: lessons from cardiovascular disease. Br Med J (Clin Res Ed) 1981;282:1847–1851. doi: 10.1136/bmj.282.6279.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson AR. Unequal treatment: report of the Institute of Medicine on racial and ethnic disparities in healthcare. Ann Thorac Surg. 2003;76:S1377–1381. doi: 10.1016/s0003-4975(03)01205-0. [DOI] [PubMed] [Google Scholar]
- 30.Chin MH, Walters AE, Cook SC, Huang ES. Interventions to reduce racial and ethnic disparities in health care. Med Care Res Rev. 2007;64:7S–28S. doi: 10.1177/1077558707305413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


