Abstract
Recent research using event-related potentials (ERPs) has shown that individuals low in alcohol sensitivity show increased P3 reactivity to alcohol cues (Bartholow, Henry, & Lust, 2007). The current research sought to test the specificity of this effect by including other arousing cues in addition to alcohol, and by controlling for individual differences in trait impulsivity. Forty-seven participants varying in self-reported alcohol sensitivity completed a visual oddball task including neutral, arousing (erotic and adventure-related), and beverage-related images while ERPs were recorded. Low-sensitivity participants showed increased P3 reactivity to alcohol cues relative to their high-sensitivity peers. However, P3 amplitude elicited by all other targets did not differ as a function of alcohol sensitivity levels. Differences in impulsivity and recent alcohol consumption did not account for sensitivity group differences in alcohol cue reactivity. These results point to the specific motivational salience of alcohol cues to individuals at risk for alcohol problems because of low alcohol sensitivity and suggest that P3 reactivity to alcohol cues could be a new endophenotype for alcohol use disorder risk.
Keywords: alcohol sensitivity, cue-reactivity, P3, endophenotype, alcoholism risk
Alcohol sensitivity is defined by the amount of alcohol an individual must consume, relative to others, to experience given subjective effects that result from drinking (see Bartholow et al., 2003; O’Neill, Sher, & Bartholow, 2002; Williams, Sher, Bartholow, & Wood, 2008; see also Schuckit, Tipp, Smith, Wiesbeck, & Kalmijn, 1997). For example, low-sensitivity (LS) individuals report needing more alcohol than high-sensitivity (HS) individuals to experience the same subjective alcohol-related sensations or impairments. Considerable research has established that low sensitivity (or a low level of response) to the acute effects of alcohol is a risk factor for development of alcohol use disorders (AUDs; e.g., Heath et al., 1999; Rodriguez, Wilson, & Nagoshi, 1993; Schuckit, 1994; Schuckit & Smith, 2000; Schuckit, Smith, Anderson, & Brown, 2004), and some work (e.g., Schuckit et al., 2001) suggests that alcohol sensitivity is genetically mediated.
Another known correlate of AUD risk is heightened psychological (e.g., craving) and/or physiological (e.g., salivation) reactivity to alcohol-related cues (Collins & Brandon 2002; Curtin, Barnett, Colby, Rohsenow, & Monti, 2005; Walitzer & Sher, 1990). For example, the magnitude of reactivity to alcohol cues has been associated with dependence diagnosis (Monti et al., 1987), severity of alcoholic symptoms (e.g., Glautier & Drummond, 1994), likelihood of relapse among abstinent alcoholics (e.g., Heinz, Beck, Grusser, Grace, & Wrasse, 2009), and drinking frequency and quantity among social drinkers (e.g., Greeley, Swift, Prescott, & Heather, 1993), among other factors (see Carter & Tiffany, 1999). A core assumption underlying most cue reactivity research is that patterns of responses to alcohol-related cues vary primarily as a function of individual differences in drinking experience (i.e., a conditioning effect; see Carter & Tiffany, 1999; Stritzke, Breiner, Curtin, & Lang, 2004).
Recently, Bartholow, Henry, and Lust (2007) tested whether LS and HS individuals differ in alcohol cue-reactivity by measuring the amplitude of the P3 (or P300) component of the event-related brain potential (ERP) as participants were exposed to images depicting alcoholic and nonalcoholic beverages. Findings indicated that LS individuals showed enhanced P3 responses to alcohol cues relative to nonalcohol cues, whereas HS individuals did not. Importantly, this pattern remained even when differences in recent alcohol use and indicators of alcohol dependence were statistically controlled. This finding suggests that risk status, in addition to consumption patterns, is an important predictor of cue reactivity effects. This conclusion is analogous to findings in other relevant areas of research, such as studies showing that the reduced P3 in basic oddball paradigms often seen among alcoholics (see Porjesz et al., 2005) is due to family history of alcoholism (Begleiter, Porjesz, Bihari, & Kissin, 1984) and not to chronic alcohol abuse (Hill, Steinhauer, Zubin, & Baughman, 1988; Pfefferbaum, Ford, White, & Mathalon, 1991).
However, it remains possible that individuals at-risk for AUD simply show enhanced P3 reactivity to all arousing cues, which would suggest that previous findings (e.g., Bartholow et al., 2007; Hermann et al., 2000; Namkoong, Lee, Lee, Lee, & An, 2004) should be interpreted in terms of arousal more generally rather than alcohol-specific cue reactivity. This possibility is suggested by research indicating that substance-related cues elicit an appetitive/approach motivational state among users (see Stewart, DeWitt, & Eikelboom, 1984), and by research indicating that P3 amplitude is sensitive to both the motivational significance (Ito, Larsen, Smith, & Cacioppo, 1998; Lang, Bradley, & Cuthbert, 1997; Schupp et al., 2000; see also Nieuwenhuis, Aston-Jones, & Cohen, 2005) and the arousal properties (Delplanque, Silvert, Hot, Rigoulot, & Sequeira, 2006) of eliciting stimuli. It could be, then, that LS individuals have a generally hyper-[re]active behavioral or appetitive motivational system (Brunelle et al., 2004; Fowles 1983a; 1983b;) that predisposes them to increased risk for heavy drinking. This could occur for either of two reasons (or both). First, low sensitivity could be significantly collinear with impulsivity or related traits associated with disinhibition that appear to have a significant genetic heritability component (Hu et al., 2005; Iacono, Carlson, Elkins, & McGue, 1999). Second, enhanced appetitive motivation, whether genetically mediated or not, could lead to increased alcohol seeking and heavier drinking, resulting in acquired tolerance to alcohol’s acute effects and, hence, lower sensitivity.
Supporting this perspective, evidence indicates that some measures of alcohol sensitivity, such as heart rate reactivity to alcohol (Conrod, Peterson, & Pihl, 2001), also are associated with greater heart rate reactivity to monetary rewards (Fowles, 1983a, 1983b), personality measures of general reward-seeking (Brunelle et al., 2004), greater memory for rewarding stimuli (Bruce, Shestowsky, Mayerovitch, & Pihl, 1999) and behaviors associated with disinhibition and sensation seeking, such as gambling (Brunelle et al. 2003). These findings are complemented by research showing that the attenuated P3 effects (to non-alcohol stimuli) often associated with risk for alcoholism also generalize to other disorders of disinhibition (e.g., Bauer & Hesselbrock, 1999; Iacono, Carlson, Malone, & McGue, 2002; Patrick et al., 2006) and to sensation and reward seeking more generally (e.g., Iacono & McGue, 2006). Thus, at present, the specificity of alcohol cue reactivity among low-sensitivity individuals, as opposed to a broader sensitivity to motivationally significant stimuli more generally, is not known.
Thus, the purpose of the present study was to determine whether enhanced P3 reactivity to alcohol cues among low-sensitivity individuals is specific to alcohol-related stimuli or is generalize-able to other motivationally relevant, high-arousal stimuli. If LS individuals and their high-sensitivity HS counterparts differ in their P3 responses to numerous kinds of arousing stimuli (including alcohol), then the conclusions of previous work (Bartholow et al., 2007) showing enhanced P3 reactivity to alcohol cues as a marker of risk associated with low sensitivity would be undermined. However, if LS and HS individuals show similar P3 reactivity to other classes of highly arousing images but differ in their reactivity to alcohol cues, given the association between enhanced P3 amplitude and the motivational significance of eliciting stimuli (Ito et al., 1998; Schupp et al., 2000) this pattern would support the idea that alcohol’s rewarding properties are particularly salient to LS individuals, and suggest that P3 cue reactivity could be used as a nonreactive measure of AUD risk in the general population.
An ancillary purpose of this work was to rule out the possibility that cue reactivity effects among LS individuals observed in our previous report were due to elevated levels of trait impulsivity/disinhibition. Although impulsivity can be conceptualized in terms of a number of specific facets (Whiteside & Lynam, 2001), there is also evidence that increased levels of a broad personality construct of impulsivity/disinhibition/behavioral undercontrol poses a risk for alcohol problems (see Clark, 2005; Elkins, King, McGue, & Iacono, 2006; Sher, Trull, Bartholow, & Vieth, 1999). Recently, Chen et al. (2007) found that trait impulsivity, as measured by the Barratt Impulsiveness Scale (BIS; see Patton et al., 1995), produced the same pattern of results in P3 amplitude as did alcoholism status (i.e., reduced P3 amplitude to visual oddballs with increased impulsivity). Thus, to the extent that sensitivity and impulsivity are inversely correlated, it could be that variability in cue reactivity that previously has been attributed to differences in sensitivity levels (Bartholow et al., 2007) could simply reflect risk associated with impulsivity, at least to some degree. We included the BIS in the present study to address this possibility.
Method
Participants
Participants were 47 undergraduates (25 women; ages 18–22 years) recruited from introductory psychology classes at the University of Missouri. Participants were recruited on the basis of their responses to a self-report measure of alcohol sensitivity completed as part of a mass testing protocol several weeks before the experiment. Individuals whose responses on the sensitivity measure represented the upper and lower quartiles of the distribution (stratified by sex) were invited to participate to earn course credit. Participants were further screened (via self-report) to ensure no history of major psychiatric illnesses (i.e., lack of any previous diagnoses) or head injury and for normal or corrected-to-normal vision. All participants were predominantly right-handed (Old-field, 1971). Participants were not screened for medication use, with the exception of neuroleptics (e.g., anti-seizure medications, also commonly used to treat psychiatric disorders).
Self-Report Measures
Alcohol Sensitivity Questionnaire (ASQ)
Sensitivity to the acute effects of alcohol was assessed using a 16-item self-report measure, the ASQ (see Bartholow et al., 2003; O’Neill et al., 2002; Williams et al., 2008). The ASQ includes 10 items measuring the positive or stimulating effects generally associated with the ascending limb of the blood alcohol curve (e.g., feeling “buzzed,” becoming more talkative, becoming more flirtatious). For each item, participants were first asked to indicate whether or not they had experienced the effect (e.g., “Have you ever felt ‘buzzed’ from drinking alcohol?”), and, if so, to estimate the minimum number of drinks typically required to feel the effect (“If yes, what is the minimum number of drinks you can consume before feeling ‘buzzed’?”). In addition, 6 items measured the extent to which participants experience negative or depressant effects typically associated with the descending limb of the blood alcohol concentration curve (e.g., feeling nauseous, passing out). These items are structured so that participants first indicate whether they have experienced the given effect from drinking alcohol, and, if so, to estimate the maximum number of drinks they can consume without experiencing the effect in question. A sensitivity score was calculated for each participant as the average number of drinks indicated across items (α = .94). Items that a given individual did not endorse were not used to calculate his or her sensitivity score. Given that scores on the ASQ tend to correlate with sex (i.e., men generally report lower sensitivity than women), to ensure roughly equal representation of men and women in the sample ASQ, scores were stratified by sex. Using this approach here resulted in 13 men and 12 women in the LS group, and 10 men and 12 women in the HS group. Sensitivity groups were comparable in terms of age (M = 19.1 years), education level (M = 13.5 years), and ethnicity (nearly all White/Non-Hispanic).
Typical alcohol consumption
Participants were asked to report their alcohol use within the past 3 months and past year by estimating the number of drinks they typically consume per occasion and the number of drinking occasions they typically experience per week. A composite alcohol quantity/frequency variable (AlcQF) was created by multiplying the number of typical weekly drinking occasions by estimated number of drinks typically consumed per occasion (see Table 1). Participants reported drinking on average about twice a week, and reported typically consuming 5 drinks on any one occasion. Thirty percent of participants reported having 12 or more drinks at least once in a sitting in the past 30 days. The average maximum number of drinks reported by participants in one sitting in the past 30 days was 8.85, and the average lifetime maximum number of drinks in one sitting was 13.7.
Table 1.
Means (and SDs) for Alcohol Use and Problems Variables as a Function of Alcohol Sensitivity Group
| Alcohol variables | Group
|
||
|---|---|---|---|
| LS | HS | ||
| Quantity/frequency | 14.9 (17.1) | 4.7 (3.4) | t(45) = 2.81, p < .01 |
| Negative consequences | 11.3 (8.9) | 3.2 (3.7) | t(45) = 4.04, p < .01 |
| Dependence features | 5.6 (5.6) | 1.9 (2.9) | t(45) = 3.11, p < .01 |
| Lifetime max. drinks | 18.0 (8.9) | 9.2 (4.3) | t(45) = 4.33, p < .01 |
Note. See the text for explanations of how these alcohol variables were calculated. LS = low alcohol sensitivity group; HS = high alcohol sensitivity group.
Alcohol-related negative consequences
Negative consequences of drinking alcohol were measured using a 24-item self-report questionnaire (see Hurlbut & Sher, 1992). Items on this measure were adapted from the Michigan Alcoholism Screening Test (Selzer, 1971) and from scales used by Blane (1987) and Engs (1977). Items inquired about consequences in several domains (e.g., legal, social/interpersonal, physical), for example, “Have you ever gotten hurt or injured because of drinking,” and “Have you ever gotten into trouble at work or school because of drinking?” The measure also included 9 items specifically tapping features of dependence, for example, “Have you ever had ‘the shakes’ after stopping or cutting down on drinking?” and “Have you ever continued to drink despite having a physical illness or psychological condition that gets worse with drinking?” For each item, response options included, “Never”, “Yes, but not in the past year,” “In the past year but not the past 3 months,” “Yes, in the past 3 months: once; twice; 3 times or 4 + times,” (scored 0, .3, .5, 1, 2, 3, and 5, respectively). For each participant, an overall Negative Consequences score was calculated as the sum of their responses to the full 24-item scale (α = .85), and a separate Dependence score was calculated as the sum of their responses to the 9 dependence-related items (α = .72). Table 1 presents the means of these variables as a function of sensitivity group.
Family history of alcoholism
Familial history of alcoholism was assessed using Mann, Sobell, Sobell, and Pavin’s (1985) family tree questionnaire. This measure instructs respondents to list each of their first- and second-degree relatives, and to indicate for each one whether they are (or were) a nondrinker, a nonproblem drinker, or experienced problems from drinking. For current purposes, participants were considered to be at increased familial risk if any first- or second-degree relatives were identified as having an alcohol problem (n = 26), and at low familial risk if no relatives were identified as such (n = 21). As in our previous work (Bartholow et al., 2007), familial risk and alcohol sensitivity levels were uncorrelated (r = −.04).
Impulsivity
As in Chen et al. (2007), trait impulsivity was assessed using the BIS, version 10 (BIS-10; see Barratt, 1959, 1985). The BIS-10 is a 34-item self-report instrument designed to assess the personality/behavioral construct of impulsiveness. The items are believed to tap 3 related but distinct subfactors labeled motor impulsiveness (acting without thinking; e.g., “I say things without thinking”), nonplanning impulsiveness (lack of planning or considering future consequences; e.g., “I act on the spur of the moment”), and attentional impulsiveness (inability to focus attention or concentrate; e.g., “I have ‘racing thoughts’”). Participants respond to each item using a 4-point response scale (1 = Rarely/never; 2 = Sometimes; 3 = Often; 4 = Almost always/Always). The various versions of the BIS have been among the most widely used self-report measures of impulsiveness and have shown good psychometric properties (e.g., Gerbing, Ahadi, & Patton, 1987; Luengo, Carrillo-de-la-Pena, & Otero, 1991). For current purposes, responses to all BIS-10 items were summed (reversing items as appropriate) to create an overall Impulsiveness score for each participant (α = .86).1
Picture Viewing Task
Participants completed a visual oddball task that included 5 types of color images: alcoholic beverages, non-alcoholic beverages, adventure-related scenes (e.g., people riding a roller-coaster; people sky-diving), erotic scenes (men and women kissing; partial nudity), and neutral scenes (e.g., a bus; people playing chess). Beverage images were taken from the Normative Appetitive Picture System (NAPS; Breiner, Stritzke, Lang, & Patrick, 1995; Stritzke et al., 2004); the rest of the images came from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2001). Mean valence (scaled 1 = very negative to 9 = very positive) and arousal (scaled 1 = completely calm to 9 = completely excited) ratings for each picture type used here were as follows: Neutral: Mvalence = 5.02, Marousal = 2.82; Nonalcohol: Mvalence = 4.40, Marousal = 3.63; Alcohol: Mvalence = 4.42, Marousal = 4.35; Adventure: Mvalence = 7.35, Marousal = 6.89; Erotic: Mvalence = 6.89, Marousal = 5.92 (means for beverage images as reported by Briener et al., 1995; means for all other images as reported by Lang et al., 2001).2
Images were presented against a black background one at a time in sequences of 5 (i.e., trials), at least 4 of which always came from the neutral category (i.e., the context was neutral). Target images, defined here as those images from which P3 amplitudes were derived for analysis, always occurred in either the fourth or fifth position within the sequences and were equally likely to represent each of the 5 image types. There were a total of 100 trials (500 total viewed images), such that participants viewed each type of image in the target position 20 times. Participants were instructed to categorize each picture as either neutral or pleasant by pressing one of two keys (counter-balanced across participants). Each image was presented for 1,000 ms, followed by an interstimulus interval (blank screen) that varied randomly between 900 ms and 1,200 ms. Trials were separated by a 500 ms inter-trial interval during which the word “pause” appeared on the screen.
Electrophysiological Recording
Electroencephalographic (EEG) data were recorded from 28 standard scalp locations (American Encephalographic Society, 1991) using tin electrodes fixed in a stretchlycra cap (Electro-cap International, Eaton, OH). EEG was sampled continuously at 1,000 Hz (amplifier gain was set to 500 for all channels) and filtered online at .05 to 40 Hz. Scalp electrodes were referenced online to the right mastoid; an average mastoid reference was derived offline. EOG was monitored from electrodes placed above and below the left eye and 2 cm lateral to the outer canthus of each eye. Impedance was kept below 8 KΩ. Ocular artifacts (blinks) were corrected from the EEG signal off-line using a regression-based procedure (Semlitsch, Anderer, Schuster, & Presslich, 1986). Target-locked epochs containing voltage deflections ±75 microvolts (μV) were discarded prior to averaging. After artifact rejection, grand average ERP waveforms were created according to stimulus and participant conditions at each scalp electrode. For each participant, the P3 was quantified as the peak of the largest positive-going deflection between 300 to 900 ms poststimulus (mean latency = 510 ms).
Results
Analytic Approach
Three participants were dropped from analyses of behavioral responses because of missing data. One participant was dropped from analyses of ERP data because of technical difficulties with her EEG recording. Greenhouse-Geisser corrected p values are reported for all analyses involving repeated factors with more than 2 levels.
Alcohol Use and Related Behaviors
To characterize differences in alcohol-related variables between the LS and HS groups, t-tests were used to compare means for alcohol use, alcohol-related negative consequences, and features of alcohol dependence across groups. As shown in Table 1, on average, LS participants drank more, experienced more alcohol-related negative consequences, and reported more alcohol dependence-related features than did HS participants.
Target Categorization
The proportion of positive responses to targets was analyzed with a 2 (Sensitivity group) × 2 (Gender) × 5 (Target type) mixed factorial analysis of variance (ANOVA) with repeated measures on the latter factor. This analysis showed only a significant Target main effect, F(4, 168) = 61.0, p < .0001. Inspection of the means showed a predictable pattern, with erotic and adventure targets most likely to be categorized as positive (Ms = .76 & .75, respectively), followed by nonalcohol and alcohol beverage targets (Ms = .53 & .54, respectively), and finally neutral targets (M = .11). Tukey HSD follow-up comparisons showed that means for erotic and adventure targets did not differ from each other but differed from all other categories (ps < .01), alcohol and nonalcohol beverage targets did not differ from each other but differed from all other categories (ps < .01), and all differed from neutral targets (ps < .001). No other effects were significant in this analysis. Thus, LS and HS participants’ overt categorizations did not differ significantly for any of the targets, including alcohol beverages.
P3 Amplitude
To determine the distribution of the P3, initial analyses focused on data from a set of 15 scalp locations, representing electrodes at which the P3 was most visible (F3, Fz, F4, FC3, FCz, FC4, C3, Cz, C4, CP3, CPz, CP4, P3, Pz, P4), using a 5 (Target type) × 5 (Coronal location; frontal, fronto-central, central, centro-parietal, parietal) × 3 (Lateral location; left, midline, right) repeated measures ANOVA. This analysis showed a main effect of Coronal location, F(4, 180) = 107.7, p < .0001, indicating that the P3 increased linearly from more anterior (M = 6.61 μV) to more posterior scalp locations (M = 11.38 μV), as is typical (see Fabiani, Gratton, & Federmeier, 2007). Moreover, a significant Coronal location × Lateral location interaction, F(8, 360) = 10.4, p < .0001, indicated that the P3 was especially pronounced at the Pz (midline parietal) electrode (M = 12.15 μV) relative to the left-hemisphere (M = 10.83 μV) and right-hemisphere (M = 11.14 μV) parietal locations. On the basis of this finding, subsequent analyses were focused on data from electrode Pz.
ASQ sensitivity scores correlated significantly with both BIS-10 Impulsiveness scores (r = −.33, p < .05) and AlcQF (r = −.39, p < .001), indicating that LS individuals tended to be more impulsive and to drink more than HS individuals. Given these significant associations, and the possibility that cue-reactivity differences we previously have attributed to alcohol sensitivity levels (Bartholow et al., 2007) can be explained instead by differences in impulsivity and/or recent drinking, we included BIS-10 Impulsiveness scores and AlcQF as covariates in our primary analyses of the P3 amplitude data.
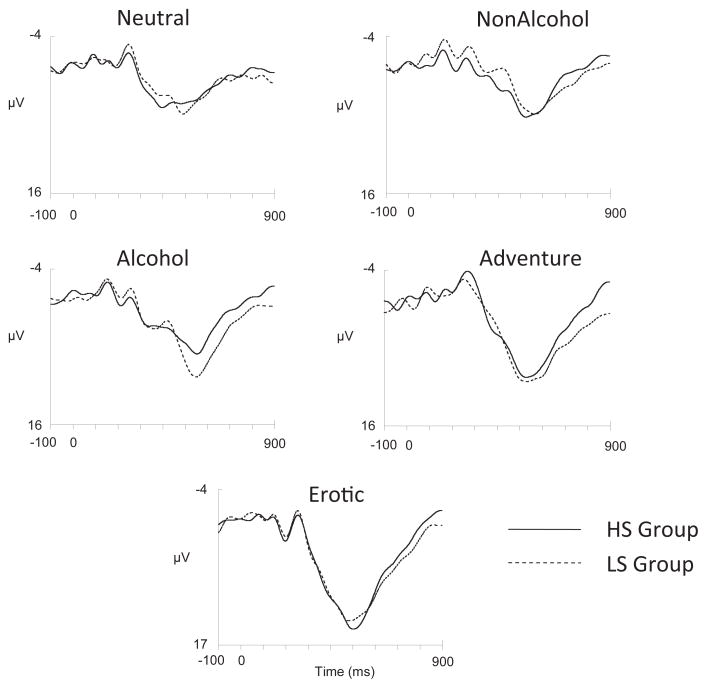
Figure 1 shows ERP waveforms elicited by the 5 types of targets at the Pz electrode as a function of sensitivity group. Peak P3 amplitude least squares means (adjusted for BIS-10 Impulsiveness and AlcQF) associated with these waveforms are given in Table 2. These data were subjected to a 2 (Sensitivity group) × 2 (Gender) × 5 (Target type) mixed factorial analysis of covariance (ANCOVA) with repeated measures on the latter factor and with BIS-10 Impulsiveness and AlcQF scores included as covariates. Two patterns are evident in Figure 1 and Table 2. First, P3 amplitude appeared largest for erotic and adventure targets and smallest for nonalcohol and neutral targets, with alcohol targets eliciting moderate P3 amplitudes. This pattern was confirmed by a significant main effect of Target type, F(4, 148) = 5.52, p < .01. Post-hoc Tukey tests indicated that the means for erotic, adventure, and alcohol targets all differed from each other (ps < .05) and all differed from nonalcohol and neutral targets (ps < .05), which did not differ from each other (p < .90).
Figure 1.
Target-locked event-related potential (ERP) waveforms elicited at the Pz electrode by each of the 5 image types as a function of alcohol sensitivity group. Time zero on the horizontal axis indicates image onset.
Table 2.
Least Squares Mean P3 Amplitudes (Adjusted for Impulsivity and Alcohol Use Variables) Elicited at Electrode Pz as a Function of Picture Type and Sensitivity Group
| Targets
|
|||||
|---|---|---|---|---|---|
| Neutral | Nonalcohol | Alcohol | Adventure | Erotic | |
| LS | 9.46a (5.05) | 9.42a (5.19) | 13.60b (4.96) | 14.30b (5.60) | 16.69c (6.43) |
| HS | 9.68a (4.31) | 9.56a (3.44) | 9.20a (4.04) | 13.58b (6.33) | 16.99c (6.00) |
Note. LS = low alcohol sensitivity group; HS = high alcohol sensitivity group. Numbers in parentheses are SDs. Means that do not share a subscript differ at p <.05. Only means for alcohol targets differed significantly between the LS and HS groups.
The second pattern suggested in Figure 1 and Table 2 is that P3 amplitudes elicited by alcohol beverages differed by sensitivity group, whereas the size of the P3 elicited by all other target types was similar for HS and LS participants. A significant Target × Sensitivity group interaction, F(4, 148) = 2.75, p < .05 (G-G adjusted), ε = .83, corroborated these patterns.3 Planned contrasts comparing the size of the P3 elicited by each target for HS and LS participants indicated that P3 amplitudes elicited by the erotic, adventure-related, nonalcoholic, and neutral targets did not differ according to sensitivity group (ts < 1). However, the P3 elicited by alcohol targets was significantly larger among LS participants than among HS participants, t(44) = 2.56, p < .02. Also, Tukey’s HSD tests showed that whereas neutral, nonalcohol and alcohol beverage targets elicited similar P3 amplitudes among HS participants (ps > .90), among LS participants the P3 elicited by alcohol targets was larger than that elicited by nonalcohol (p = .002) and neutral targets (p = .005). Finally, the interactions between Target type and Impulsiveness (F < 1.50) and Target type and AlcQF (F < 1.20) were both nonsignificant (ps > .20).
Finally, although not central to the hypotheses for this study, we conducted an exploratory analysis to examine whether P3 responses to alcohol cues were related to behavioral ratings of the pleasantness of those cues, and whether the nature of this association might differ as a function of alcohol sensitivity levels. This possibility was tested using a general linear model in which the proportion of “pleasant” responses to alcohol images was predicted from P3 amplitude elicited by alcohol targets (at electrode Pz), sensitivity group, and their interaction. This analysis produced only a significant Sensitivity group × P3 amplitude interaction, F(1, 42) = 5.42, p < .05. Post-hoc follow-up tests showed that whereas the P3 elicited by alcohol cues and the proportion of “pleasant” responses to those cues were not significantly associated among HS participants (r = −.28, p = .19), these variables were positively correlated among LS participants (r = .41, p = .046). Thus, among LS participants, a larger P3 to alcohol cues was associated with an increased likelihood that those cues would be categorized as pleasant.
Discussion
The purpose of this experiment was to determine whether the heightened P3 reactivity to alcohol cues seen in LS individuals (Bartholow et al., 2007) is specific to alcohol or more generally reflects enhanced reactivity to arousing cues. The current findings support the specificity of alcohol cue reactivity among LS individuals, suggesting that alcohol is particularly motivationally salient to individuals at risk for alcohol use disorders. This finding has important implications for understanding individual differences in alcohol sensitivity as a risk mechanism for alcohol abuse. Specifically, the current data help to rule out the possibility that alcohol sensitivity reflects an overall hyperreactive approach motivational system, which could predispose individuals to problematic alcohol involvement via behavioral disinhibition (see Bauer & Hesselbrock, 1999; Iacono & McGue, 2006; Patrick et al., 2006).
These findings also help to rule out a related possibility, specifically, that differences in P3 reactivity to alcohol cues attributed to alcohol sensitivity levels are driven by associated differences in impulsiveness or recent alcohol consumption between HS and LS participants. Although alcohol sensitivity was modestly correlated in this sample with both impulsiveness and recent alcohol use, the predicted enhancement of P3 amplitude to alcohol cues among LS individuals was observed even though these other risk indicators were included as covariates in the analysis. This finding, coupled with the lack of correlation between alcohol sensitivity levels and family history of alcoholism seen both here and previously (Bartholow et al., 2007), suggests that alcohol sensitivity is a unique predictor of differences in alcohol cue reactivity.
Recent theoretical developments associate the P3 response to motivationally significant events with the outcome of stimulus evaluation and decision making processes by the locus-coeruleus norepinephrine system (Nieuwenhuis et al., 2005). Viewed in the context of this theory, the current work adds to previous research suggesting that the P3 elicited by alcohol cues reflects their motivational significance (Bartholow et al., 2007). Although, in general, the P3 responded to cues as a function of their arousal properties (cf., Delplanque et al., 2006), only among LS participants did the P3 elicited by alcohol and nonalcohol cues differ, suggesting a particularly enhanced motivational response to alcohol among those individuals. Moreover, only among LS participants was the P3 elicited by alcohol cues associated with behavioral ratings of the pleasantness of those cues. In this context, the current findings can be viewed as consistent with existing incentive-motivational models of cue-reactivity (e.g., Carter & Tiffany, 1999), which posit that autonomic responses to drug cues reflect the activation of a generalized positive/appetitive motivational state, rather than conditioned responses to specific drugs. An interesting avenue for future research is to investigate whether LS individuals show enhanced reactivity to other drug cues, particularly for substances that often co-occur with drinking (e.g., smoking).
Beyond implications for understanding cue reactivity processes in alcohol sensitivity specifically, these and other recent data (Bartholow et al., 2007; Hermann et al., 2000; Namkoong et al., 2004) suggest that the P3 elicited by alcohol cues could represent a new endophenotype of risk for alcohol use disorder. Briefly, the endophenotype (or intermediate phenotype) approach involves identification of individual difference factors that occur between the ultimate causes (e.g., genetic variation) and ultimate outcomes (e.g., diagnosis) of clinical disorders such as alcohol dependence (see Cannon & Keller, 2006; Gottesman & Gould, 2003). Endo-phenotypes are thought to be state-independent, meaning they are manifest in affected individuals regardless of whether or not the relevant syndrome or condition (e.g., AUD) is active or has emerged. Endophenotypes are narrower in scope than clinical syndromes and therefore are more likely than clinical diagnoses to reflect the action of particular genes. The endophenotype approach is particularly useful because endophenotypes can be assessed prior to full development of a psychiatric disorder, thereby enhancing efforts at identifying at-risk individuals or groups.
Numerous previous studies support the idea that reduced P3 amplitude, elicited in very basic oddball tasks involving simple visual or auditory discriminations, is an endophenotype for alcohol abuse and other disorders of disinhibition (e.g., Begleiter et al., 1984; Carlson, Iacono, & McGue, 2004; Hesselbrock, Begleiter, Porjesz, O’Connor, & Bauer, 2001; Iacono et al., 2002; Polich, Pollock, & Bloom, 1994; Porjesz et al., 2005). In contrast, the high-risk, cue-reactivity approach used here involves elicitation of the P3 by cues that, in theory, have particular motivational significance for individuals at risk for AUD. Thus, this approach permits understanding of the P3 as a marker of risk within the conceptual framework of a neural network (i.e., the locus-coeruleus norepinephrine system) associated with evaluating the motivational significance of eliciting stimuli (Nieuwenhuis et al., 2005), providing an intuitive link between the P3 response and overt behaviors (e.g., heavy drinking) and other experiences (e.g., craving, withdrawal) associated with AUD diagnosis.
Despite the promise shown by this and other recent work (Bartholow et al., 2007; Namkoong et al., 2004) investigating the P3 response as a marker of risk, it is still far from certain that P3 reactivity to alcohol cues represents a reliable endophenotype for AUD. A number of avenues should be explored in future research to better establish the use of this measure in this regard. For example, the alcohol sensitivity construct employed here to differentiate relative levels of risk for alcohol abuse likely reflects both inherent (i.e., genetic) and acquired (i.e., learned) aspects of sensitivity. Thus, whether the P3 response can be linked specifically to genetic variation—an important consideration for establishing an endophenotype—has yet to be determined. Also, the extent to which the heightened P3 cue reactivity among alcoholics seen in other research (Hermann et al., 2000; Namkoong et al., 2004) reflects risk rather than conditioning (i.e., from heavy alcohol use) has yet to be convincingly demonstrated. One possible approach for investigating this question could be to use P3 reactivity measured among detoxified, abstinent alcoholics as a predictor of relapse. Assuming appropriate controls for exposure duration and/or symptom severity, any association between post-treatment P3 reactivity and variation in relapse-related outcomes could be attributed to pre-existing risk rather than to exposure, which would further support P3 reactivity as an endophenotype for AUD risk. Finally, it is important to note that the generalizability of the enhanced P3 response to alcohol cues among LS individuals has yet to be established using samples other than college students, who generally tend to drink more heavily compared to their peers who do not attend college (e.g., Slutske et al., 2004) and to older populations (e.g., Bartholow, Sher, & Krull, 2003), but who generally have not had the depth of drinking experience that older populations (especially alcohol-dependent individuals) have had. However, given that heavy drinking tends to be normative in college samples (see Schulenberg et al., 2001)—indeed, many college drinkers would meet diagnostic criteria for alcohol abuse or dependence (see Jackson, Sher, & Park, 2006)—and that even younger populations of problem drinkers show reliable alcohol cue reactivity (see Thomas, Drobes, & Deas, 2005), use of this population for the current research might not be as limiting for understanding alcohol abuse etiology as it first appears.
In conclusion, the current results extend previous work (Bartholow et al., 2007) by showing that heightened P3 reactivity to alcohol cues among LS individuals is alcohol-specific, and does not generalize to other arousing, motivationally-relevant stimuli. Furthermore, these findings rule out the possibility that cue-reactivity differences associated with sensitivity levels are simply a proxy for differences in impulsivity. Finally, together with other previous work (Bartholow et al., 2007; Hermann et al., 2000; Namkoong et al., 2004), the current results suggest that P3 reactivity to alcohol cues could be a new, potentially sensitive endo-phenotype for alcoholism risk. Continued investigation of this possibility could lead to important advances both theoretically and in terms of risk assessment and intervention efforts.
Footnotes
Note that the newer BIS-11 (Patton, Stanford, & Barratt, 1995) is identical to the BIS-10, except that it includes 4 fewer items. For comparison purposes, we conducted all analyses that included impulsivity scores using both BIS-10 and BIS-11 scoring, and found that results were virtually identical using either scoring method.
Alcoholic beverage images included NAPS image numbers 202, 206, 212, 222, and 224; nonalcoholic beverage images included NAPS image numbers 335, 321, 322, 334, and 311. Erotic images included IAPS image numbers 4599, 4608, 4623, 4653, and 4677; Adventure images included IAPS image numbers 8185, 8179, 8370, 8030, and 8170; Neutral images included IAPS image numbers 2840, 2890, 6150, 7002, 7004, 7090, 7020, 7034,7050,2880,7160, 7161, 7179, 7185, 7187, 7233, 7235, 7950, 2850, and 9070.
An ancillary analysis including data from all 15 electrode locations examined here showed a similar pattern, but within the context of a complex, 4-way interaction involving Sensitivity group, Target type, Lateral location, and Coronal location, F(32, 1440) = 1.60, p = .02. Inspection of the means associated with this interaction indicated that, although the sensitivity group difference in P3 amplitude elicited by alcohol cues was most pronounced at Pz, this pattern was evident at all midline electrode locations and at right-hemisphere locations; the difference was smaller at left-hemisphere locations, consistent with previous research examining P3 amplitude elicited by motivationally-significant stimuli (see Cacioppo, Crites, & Gardner, 1996).
Portions of this research were presented at the 2007 annual meeting of the Society for Psychophysiological Research, Savannah, Georgia.
References
- American Encephalographic Society. Guidelines for standard electrode position nomenclature. Journal of Clinical Neurophysiology. 1991;8:200–222. [PubMed] [Google Scholar]
- Barratt ES. Anxiety and impulsiveness related to psychomotor efficiency. Perceptual and Motor Skills. 1959;9:191–198. [Google Scholar]
- Barratt ES. Impulsiveness subtraits: Arousal and information processing. In: Spence JT, Izard CE, editors. Motivation, emotion, and personality. Amsterdam: Elsevier/North Holland; 1985. pp. 137–146. [Google Scholar]
- Bartholow BD, Henry EA, Lust SA. Effects of alcohol sensitivity on P3 event-related potential reactivity to alcohol cues. Psychology of Addictive Behaviors. 2007;21:555–563. doi: 10.1037/0893-164X.21.4.555. [DOI] [PubMed] [Google Scholar]
- Bartholow BD, Pearson MA, Sher KJ, Wieman LC, Fabiani M, Gratton G. Effects of alcohol consumption and alcohol susceptibility on cognition: A psychophysiological examination. Biological Psychology. 2003;64:167–190. doi: 10.1016/s0301-0511(03)00108-x. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. P300 decrements in teenagers with conduct problems: Implications for substance abuse risk and brain development. Biological Psychiatry. 1999;46:263–272. doi: 10.1016/s0006-3223(98)00335-7. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related potentials in boys at risk for alcoholism. Science. 1984;225:1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Blane HT. Young Men’s Survey of Life Experiences. University of Chicago; NORC: 1987. Unpublished questionnaire. [Google Scholar]
- Breiner MJ, Strizke WGK, Long AR, Patrick CJ. The Normative Appetitive Picture System [photographic slides] Tallahassee: Florida State University; 1995. [Google Scholar]
- Bruce KR, Shestowsky JS, Mayerovitch JI, Pihl RO. Motivational effects of alcohol on memory consolidation and heart rate in social drinkers. Alcoholism: Clinical and Experimental Research. 1999;23:693–701. [PubMed] [Google Scholar]
- Brunelle C, Assaad J, Barrett SP, Ávila C, Conrod PJ, Tremblay RE, et al. Heightened heart rate response to alcohol intoxication is associated with a reward-seeking personality profile. Alcoholism: Clinical & Experimental Research. 2004;28:394–401. doi: 10.1097/01.alc.0000117859.23567.2e. [DOI] [PubMed] [Google Scholar]
- Brunelle C, Assaad JM, Pihl RO, Tremblay RE, Vitaro F. Ethanol induced resting cardiac reactivity as an indicator of increased risk for gambling. Psychology of Addictive Behaviors. 2003;17:83–86. doi: 10.1037/0893-164x.17.1.83. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Crites SL, Jr, Gardner WL. Attitudes to the right: Evaluative processing is associated with lateralized late positive event-related brain potentials. Personality and Social Psychology Bulletin. 1996;22:1205–1219. [Google Scholar]
- Cannon TD, Keller MC. Endophenotypes in the genetic analyses of mental disorders. Annual Review of Clinical Psychology. 2006;2:267–290. doi: 10.1146/annurev.clinpsy.2.022305.095232. [DOI] [PubMed] [Google Scholar]
- Carlson SR, Iacono WG, McGue M. P300 amplitude in non-alcoholic adolescent twin pairs who become discordant for alcoholism as adults. Psychophysiology. 2004;41:841–844. doi: 10.1111/j.1469-8986.2004.00238.x. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Chen ACH, Porjesz B, Rangaswamy M, Kamarajan C, Tang Y, Jones KA, et al. Reduced frontal lobe activity in subjects with high impulsivity and alcoholism. Alcoholism: Clinical and Experimental Research. 2007;31:156–165. doi: 10.1111/j.1530-0277.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- Clark LA. Temperament as a unifying basis for personality and psychopathology. Journal of Abnormal Psychology. 2005;114:505–521. doi: 10.1037/0021-843X.114.4.505. [DOI] [PubMed] [Google Scholar]
- Collins BN, Brandon TH. Effects of extinction context and retrieval cues on alcohol cue reactivity among nonalcoholic drinkers. Journal of Consulting and Clinical Psychology. 2002;70:390–397. [PubMed] [Google Scholar]
- Conrod PJ, Peterson JB, Pihl RO. Reliability and validity of alcohol-induced heart rate increase as a measure of sensitivity to the stimulant properties of alcohol. Psychopharmacology. 2001;157:20–30. doi: 10.1007/s002130100741. [DOI] [PubMed] [Google Scholar]
- Curtin JJ, Barnett NP, Colby SM, Rohsenow DJ, Monti PM. Cue reactivity in adolescents: Measurement of separate approach and avoidance reactions. Journal of Studies on Alcohol. 2005;66:332–343. doi: 10.15288/jsa.2005.66.332. [DOI] [PubMed] [Google Scholar]
- Delplanque S, Silvert L, Hot P, Rigoulot S, Sequeira H. Arousal and valence effects on event-related P3a and P3b during emotional categorization. International Journal of Psychophysiology. 2006;60:315–322. doi: 10.1016/j.ijpsycho.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Elkins IJ, King SM, McGue M, Iacono WG. Personality traits and the development of nicotine, alcohol, and illicit drug disorders: Prospective links from adolescence to young adulthood. Journal of Abnormal Psychology. 2006;115:26–39. doi: 10.1037/0021-843X.115.1.26. [DOI] [PubMed] [Google Scholar]
- Engs RC. Drinking patterns and drinking problems of college students. Journal of Studies on Alcohol. 1977;38:2144–2156. doi: 10.15288/jsa.1977.38.2144. [DOI] [PubMed] [Google Scholar]
- Fowles D. Motivational effects on heart rate and electrodermal activity: Implications for research on personality and psychopathology. Journal of Research in Personality. 1983a;17:48–71. [Google Scholar]
- Fowles D. Appetitive motivational influences on heart rate. Personality and Individual Differences. 1983b;4:393–401. [Google Scholar]
- Gerbing DW, Ahadi SA, Patton JH. Toward a conceptualization of impulsivity: Components across the behavioral and self-report domains. Multivariate Behavioral Research. 1987;22:357–379. doi: 10.1207/s15327906mbr2203_6. [DOI] [PubMed] [Google Scholar]
- Glautier S, Drummond DC. Alcohol dependence and cue reactivity. Journal of Studies on Alcohol. 1994;55:224–229. doi: 10.15288/jsa.1994.55.224. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Greeley JD, Swift W, Prescott J, Heather N. Reactivity to alcohol cues in heavy and light drinkers. Journal of Studies on Alcohol. 1993;54:359–368. doi: 10.15288/jsa.1993.54.359. [DOI] [PubMed] [Google Scholar]
- Heath AC, Madden PA, Bucholz KK, Dinwiddie SH, Slutzke WS, Bierut LJ, et al. Genetic differences in alcohol sensitivity and the inheritance of alcoholism risk. Psychological Medicine. 1999;29:1069–1081. doi: 10.1017/s0033291799008909. [DOI] [PubMed] [Google Scholar]
- Heinz A, Beck A, Grusser SM, Grace AA, Wrasse J. Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addiction Biology. 2009;14:99–107. doi: 10.1111/j.1369-1600.2008.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann MJ, Weijers HG, Wiesbeck GA, Aranda D, Boning J, Fallgater AJ. Event-related potentials and cue-reactivity in alcoholism. Alcoholism: Clinical and Experimental Research. 2000;24:1724–1729. [PubMed] [Google Scholar]
- Hesselbrock V, Begleiter H, Porjesz B, O’Connor S, Bauer L. P300 event-related potential amplitude as an endophenotype of alcoholism: Evidence from the Collaborative Study on the Genetics of Alcoholism. Journal of Biomedical Science. 2001;8:77–82. doi: 10.1007/BF02255974. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR, Zubin J, Baughman BA. Event-related potentials as markers for alcoholism risk in high density families. Alcoholism: Clinical and Experimental Research. 1988;12:545–554. doi: 10.1111/j.1530-0277.1988.tb00240.x. [DOI] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk: Neurobiological, behavioral, and environmental relations to drinking. Alcoholism: Clinical & Experimental Research. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Hurlbut SC, Sher KJ. Assessing alcohol problems in college students. Journal of American College Health. 1992;41:49–57. doi: 10.1080/07448481.1992.10392818. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson JT, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance-use disorders: Findings from the Minnesota Twin Family Study. Development and Psychopathology. 1999;11:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Malone SM, McGue M. P3 event-related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Archives of General Psychiatry. 2002;59:750–757. doi: 10.1001/archpsyc.59.8.750. [DOI] [PubMed] [Google Scholar]
- Iacono WG, McGue M. Association between P3 event-related brain potential amplitude and adolescent problem behavior. Psychophysiology. 2006;43:465–469. doi: 10.1111/j.1469-8986.2006.00422.x. [DOI] [PubMed] [Google Scholar]
- Ito TA, Larsen JT, Smith K, Cacioppo JT. Negative information weighs more heavily on the brain: The negativity bias in evaluative categorization. Journal of Personality and Social Psychology. 1998;75:887–900. doi: 10.1037//0022-3514.75.4.887. [DOI] [PubMed] [Google Scholar]
- Jackson KM, Sher KJ, Park A. Drinking among college students: Consumption and consequences. In: Galanter M, editor. Recent developments in alcoholism: Research on alcohol problems in adolescents and young adults. XVII. New York: Plenum; 2006. pp. 85–117. [DOI] [PubMed] [Google Scholar]
- Lang P, Bradley MM, Cuthbert B. Technical Report A-5. The Center for Research in Psychophysiology, University of Florida; 2001. International affective picture system (IAPS): Instruction manual and affective ratings. [Google Scholar]
- Lang P, Bradley MM, Cuthbert BN. Motivated attention: Affect, activation, and action. In: Lang P, Simons RF, Balaban M, editors. Attention and orienting: Sensory and motivational processes. Hillsdale, NJ: Erlbaum; 1997. pp. 97–136. [Google Scholar]
- Luengo MA, Carrillo-de-la-Pena MT, Otero JM. The components of impulsiveness: A comparison of the I. 7 impulsiveness questionnaire and the Barratt impulsiveness scale. Personality and Individual Differences. 1991;12:657–667. [Google Scholar]
- Mann RE, Sobell LC, Sobell MB, Pavin D. Reliability of a family tree questionnaire for assessing family history of alcohol problems. Drug and Alcohol Dependence. 1985;15:61–67. doi: 10.1016/0376-8716(85)90030-4. [DOI] [PubMed] [Google Scholar]
- Monti PM, Binkoff JA, Abrams DB, Zwick WR, Nirenberg TD, Liepman MR. Reactivity of alcoholics and nonalcoholics to drinking cues. Journal of Abnormal Psychology. 1987;96:122–126. doi: 10.1037//0021-843x.96.2.122. [DOI] [PubMed] [Google Scholar]
- Namkoong K, Lee E, Lee CH, Lee BO, An SK. Increased P3 amplitudes induced by alcohol-related pictures in patients with alcohol dependence. Alcoholism: Clinical and Experimental Research. 2004;28:1317–1323. doi: 10.1097/01.alc.0000139828.78099.69. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision-making, the P3, and the locus coeruleus-norepinephrine system. Psychological Bulletin. 2005;131:510–532. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- O’Neill SE, Sher KJ, Bartholow BD. Alcohol susceptibility and tolerance in young adults. Alcoholism: Clinical and Experimental Research. 2002;26:119A. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Bernat E, Malone SM, Iacono WG, Krueger RF, McGue MK. P300 amplitude as an indicator of externalizing in adolescent males. Psychophysiology. 2006;43:84–92. doi: 10.1111/j.1469-8986.2006.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, White PM, Mathalon D. Event-related potentials in alcoholic men: P3 amplitude reflects family history but not alcohol consumption. Alcoholism: Clinical and Experimental Research. 1991;15:839–850. doi: 10.1111/j.1530-0277.1991.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Polich J, Pollock VE, Bloom FE. Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychological Bulletin. 1994;115:55–73. doi: 10.1037/0033-2909.115.1.55. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clinical Neurophysiology. 2005;116:993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Rodriguez LA, Wilson JR, Nagoshi CT. Does psychomotor sensitivity to alcohol predict subsequent alcohol use? Alcoholism: Clinical and Experimental Research. 1993;17:155–161. doi: 10.1111/j.1530-0277.1993.tb00741.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. American Journal of Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Edenberg J, Kalmijn JA, Flury TL, Smith T, Reich L, et al. A genome-wide search for genes that relate to a low level of response to alcohol. Alcoholism: Clinical and Experimental Research. 2001;25:323–329. [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. The relationships of a family history of alcohol dependence, a low level of response to alcohol and six domains of life functioning to the development of alcohol use disorders. Journal of Studies on Alcohol. 2000;61:827–835. doi: 10.15288/jsa.2000.61.827. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Anderson KG, Brown SA. Testing the level of response to alcohol: Social information processing model of alcoholism risk: A 20-year prospective study. Alcoholism: Clinical and Experimental Research. 2004;28:1881–1889. doi: 10.1097/01.alc.0000148111.43332.a5. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Smith TL, Wiesbeck GA, Kalmijn JA. The relationship between self-rating of the effects of alcohol and alcohol challenge results in ninety-eight young men. Journal of Studies on Alcohol. 1997;58:397–404. doi: 10.15288/jsa.1997.58.397. [DOI] [PubMed] [Google Scholar]
- Schulenberg J, Maggs JL, Long SW, Sher KJ, Gotham HJ, Baer JS, et al. The problem of college drinking: Insights from a developmental perspective. Alcoholism: Clinical and Experimental Research. 2001;25:473–477. [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito TA, Lang PJ. Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–261. [PubMed] [Google Scholar]
- Selzer M. The Michigan Alcoholism Screening Test: The quest for a new diagnostic instrument. American Journal of Psychiatry. 1971;172:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Trull TJ, Bartholow BD, Vieth A. Personality and alcoholism: Issues, methods and etiological processes. In: Leonard KE, Blaine HT, editors. Psychological theories of drinking and alcoholism. Vol. 2. New York: Guilford Press; 1999. pp. 54–105. [Google Scholar]
- Slutske WS, Hunt-Carter EE, Nabors-Oberg RE, Sher KJ, Bucholz KK, Madden PAF, et al. Do college students drink more than their non-college-attending peers? Evidence from a population-based longitudinal female twin study. Journal of Abnormal Psychology. 2004;113:530–540. doi: 10.1037/0021-843X.113.4.530. [DOI] [PubMed] [Google Scholar]
- Stewart J, DeWitt H, Eikelboom R. The role of conditioned and unconditioned drug effects in the self-administration of opiates and stimulants. Psychological Review. 1984;91:251–268. [PubMed] [Google Scholar]
- Stritzke WGK, Breiner MJ, Curtin JJ, Lang AR. Assessment of substance cue reactivity: Advances in reliability, specificity, and validity. Psychology of Addictive Behaviors. 2004;18:148–159. doi: 10.1037/0893-164X.18.2.148. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Drobes DJ, Deas D. Alcohol cue reactivity in alcohol-dependent adolescents. Journal of Studies on Alcohol. 2005;66:354– 360. doi: 10.15288/jsa.2005.66.354. [DOI] [PubMed] [Google Scholar]
- Walitzer KS, Sher KJ. Alcohol cue reactivity and ad lib drinking in young men at risk for alcoholism. Addictive Behaviors. 1990;15:29–46. doi: 10.1016/0306-4603(90)90005-i. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The Five Factor Model and impulsivity: Using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30:669–689. [Google Scholar]
- Williams MA, Sher KJ, Bartholow BD, Wood PK. A two-part model of alcohol sensitivity: An alternative psychometric approach to conditional response data. 2008 Manuscript submitted for publication. [Google Scholar]