Abstract
Lymphocytic choriomeningitis virus (LCMV) is an important cause of neurologic disease in humans. Carried and secreted principally by wild mice, LCMV covers a large geographic range and infects great numbers of people. Humans acquire LCMV disease when they come into contact with the secretions of infected mice. Because it has a strong neurotropism, the clinical signs and symptoms of LCMV infection are mostly neurologic. When the virus is acquired postnatally by children or adults, the clinical manifestations are usually those of aseptic meningitis. Most people who acquire LCMV infection during childhood or adulthood are moderately symptomatic for several weeks, but have a full recovery. A much more severe disease ensues when the infection occurs prenatally. LCMV can infect the fetal brain and retina, where it leads to substantial injury and permanent dysfunction. The possibility of LCMV infection should be considered in all babies with evidence of congenital infection, especially those with prominent neurologic signs, such as microencephaly, periventricular calcifications, and hydrocephalus.
Introduction
Lymphocytic choriomeningitis virus (LCMV) is a prevalent human pathogen that infects large numbers of people. Despite the fact that it can cause substantial neurological problems, including meningitis, encephalitis, and neurologic birth defects, neurologists are often unfamiliar with it. Perhaps there is no other pathogen about which the gap is wider between what most neurologists should know and what they do know. The goal of this article is to bridge that gap for child neurologists.
LCMV is a member of the arenaviridae family of viruses. The arenaviruses are single stranded RNA viruses that gain their name from arenosus, the Latin word for “sandy,” on the basis of the fine granularities observed within the virion when observed under an electron microscope1.
Like all arenaviruses, LCMV utilizes rodents as its principal reservoir2. The common house mouse, Mus musculus, is both the natural host and reservoir for the virus, which is transferred vertically from one generation to the next within the mouse population via intrauterine infection. Hamsters are also competent reservoirs3. Rodents that acquire LCMV transplacentally may be heavily infected with virus, but often remain asymptomatic because the virus is not cytolytic and because congenital infection in rodents provides them with immunological tolerance for the virus. Mice and hamsters infected with LCMV shed the virus in large quantities in their saliva, urine, feces, and nasal secretions throughout their lives4.
Postnatal humans typically acquire LCMV by direct contact with contaminated fomites or by inhalation of aerosolized virus. Postnatal humans can also acquire the virus via organ transplantation5,6. Congenital LCMV infection occurs when a woman acquires a primary LCMV infection during pregnancy. During maternal viremia, the virus is passed to the fetus transplacentally. The virus may also be acquired by the fetus during the intrapartum period7. Human-to-human horizontal transmission of LCMV has not been documented (except when infected tissues are transplanted).
Epidemiology
LCMV is endemic in wild mice4, 8 and likely exists as an infectious agent wherever wild mice live (which is on every continent except Antarctica). An epidemiologic study found that 9% of the mice captured in urban Baltimore are infected with LCMV9. In addition, because of the territorial nature of mice, clustering can occur where the prevalence of LCMV is much higher. Serologic studies have demonstrated that approximately five percent of American adults possess antibodies to LCMV, indicating previous exposure and infection9, 10, 11.
Humans can acquire LCMV during any season, but most LCMV infections occur during the late autumn and early winter months, reflecting the seasonal movement of mice into human homes during the cold season2.
The incidence of congenital LCMV infection is not known. The case reports of congenital LCMV demonstrate that acquisition of the virus during pregnancy can severely disrupt brain development12, 13, 14. However, because no epidemiological studies have been conducted, it is unknown whether the severely affected infants described in the case reports represent the typical outcome of gestational LCMV infection or whether they are the most severely affected cases. Information regarding the incidence of congenital LCMV infection and the spectrum of its outcome are further limited by the fact that LCMV is not one of the infectious agents for which infants with suspected congenital infection are commonly checked. (It is not one of the agents included in the TORCH acronym.) Thus, congenital LCMV infection, like many other congenital infections, might produce a spectrum of pathological effects that range from minimal to profound15. The high prevalences of infected mice and of seropositive humans suggest that congenital LCMV infection is an underdiagnosed disease and that the virus is responsible for more cases of congenital neurological and ophthalmological disease than has previously been recognized16, 17, 18.
Pathogenesis
Acquired LCMV Infection
Acquired and congenital LCMV diseases are both caused by the combination of infection with the virus and the host immune response to the infection19. In cases of acquired infection, the virus typically enters the human in an aerosolized form and is deposited in the lung, where initial viral replication occurs20. This parenchymal lung infection often manifests as interstitial lung infiltrates and lung edema early in the course of disease. The virus then enters the blood stream and travels to other organs, where further viral replication occurs. Ultimately, LCMV reaches the meninges, choroid plexus, and ventricular ependymal linings, where the virus replicates to high titers and where the inflammatory response produces the characteristic pathology and symptoms of meningitis that give the virus its name1, 2.
In acquired LCMV infection, the host immune response is both protective and deleterious. The immune response is protective in the sense that it performs the critically important function of eliminating the virus and protecting against repeat infection. However, the immune response is deleterious in the sense that tissue inflammation underlies the symptoms of disease19, 21.
Congenital LCMV Infection
In most cases of congenital LCMV infection, the fetus acquires the virus transplacentally. Rarely, the fetus may acquire the virus during the intrapartum period, via exposure to maternal vaginal secretions or blood during maternal viremia.
After reaching the human fetus, the virus exhibits a strong tropism for the brain, where the infection produces its most common and severe pathologic effects12, 13, 22. Neuroimaging studies have demonstrated that, within the developing brain, LCMV can induce a variety of pathologic changes, including microencephaly, periventricular calcifications, hydrocephalus, cerebellar hypoplasia, focal cerebral destruction, and gyral dysplasia (figures 1 and 2).
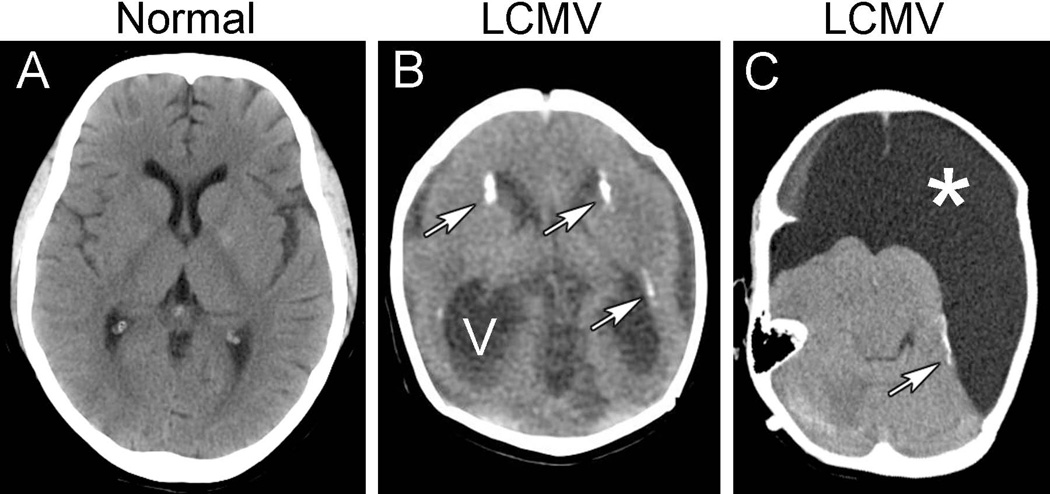
Figure 1.
Pathologic changes commonly observed in congenital LCMV infection. Shown here are head CT scans from a normal child (A) and from two children with congenital LCMV infection (B and C). B. The most common abnormalities in congenital LCMV infection include periventricular calcifications (arrows) and ventriculomegaly (V), often due either to non-communicating hydrocephalus or to cerebral atrophy. C. Some patients with congenital LCMV infection have regions of encephalomalacia (*), reflecting focal tissue destruction. In addition to massive encephalomalacia, this patient has periventricular calcifications (arrow).
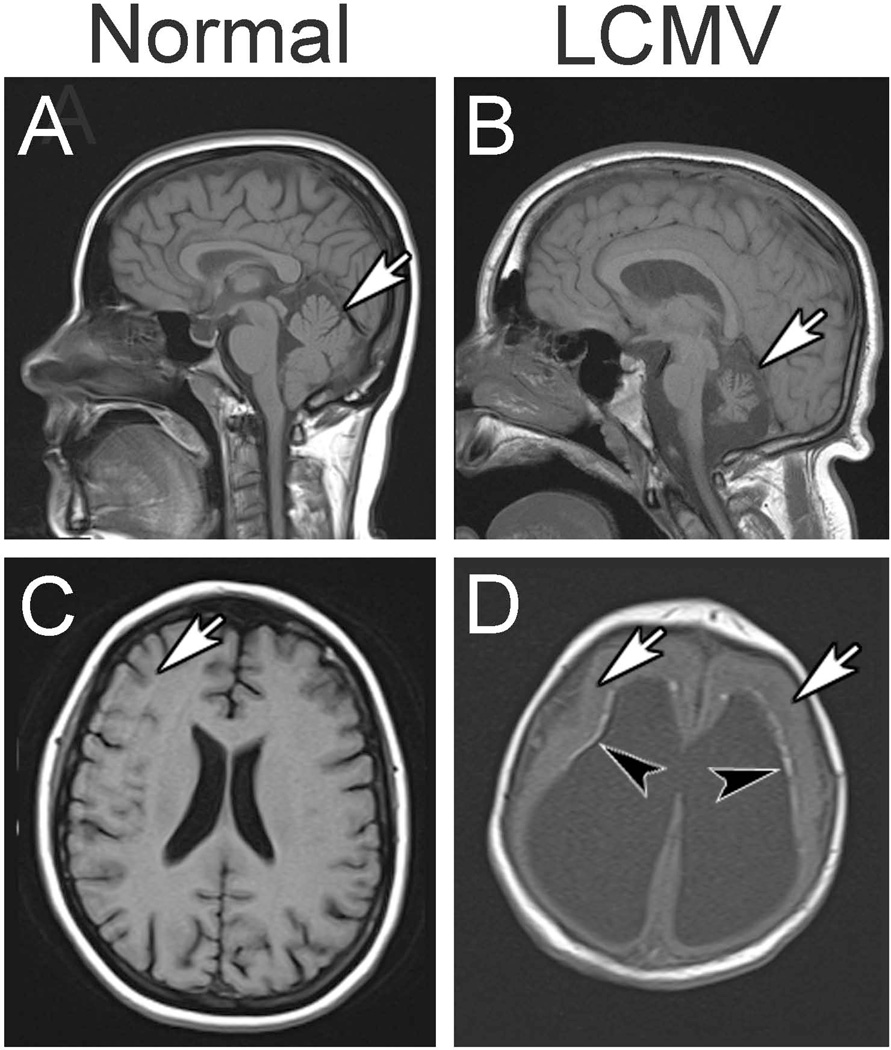
Figure 2.
Neuroimaging studies reveal neuropathology induced by congenital LCMV infection. Shown here are MRI scans from normal children (left column) and children with congenital LCMV infection (right column). A. In the midsagittal plane of a normal child, the cerebellum (arrow) is large and fills the posterior fossa. B. In congenital LCMV infection, the virus can impair cerebellar growth and lead to cerebellar hypoplasia (arrow). C. In the horizontal plane of a normal child, the cerebral cortex is folded into a complex set of gyri and sulci (arrow). D. In congenital LCMV infection, the cerebral cortex is often featureless and smooth, lacking normal gyri and sulci, and reflecting a neuronal migration disturbance (arrows). This patient also has periventricular calcifications (arrowheads) and ventriculomegaly.
To elucidate the effects and mechanisms of congenital LCMV infection, an animal model of the disease has been established22, 23, 24. This animal model, in which developing laboratory rats are inoculated with LCMV, is perhaps the most powerful and useful animal model of a congenital viral infection in existence, as it replicates virtually all of the pathology observed in affected humans12, 25, 26. The animal model has shed considerable light on the pathogenesis of human congenital LCMV infection. For example, the animal model has demonstrated that LCMV exhibits a very strong tropism for neuroblasts. The presence of neuroblasts in the periventricular region of the fetal human brain explains the location of periventricular calcifications in children with congenital LCMV. The animal model has also demonstrated that LCMV infection disturbs the migration of neurons22, 25. This finding explains the gyral malformations, reflecting a neuronal migration defect, in children with congenital LCMV12, 26. Furthermore, the animal model has shown that LCMV infection triggers a robust inflammatory response, driven by cytotoxic T-lymphocytes22, 24, 25. This finding explains the lymphocytic pleocytosis in patients with LMCV meningitis and the inflammation-induced destructive cerebral lesions in children with congenital LCMV. In addition, the animal model has shown that the age of the developing host at the time of infection profoundly affects the patterns of infection and pathology within the brain25. This finding suggests that the variability in disease outcome among children with congenital LCMV infection is likely due to differences in the gestational timing of infection12, 25, 26.
Clinical Manifestations
The clinical signs and symptoms of LCMV infection depend entirely on the developmental stage of the patient at the time of infection27, 28, 29. In particular, the clinical manifestations depend on whether the infection occurs during postnatal life or during the prenatal period19.
Acquired (Postnatal) LCMV Infection
The manifestations of LCMV infection during childhood and adulthood are usually relatively mild. The infection typically consists of a brief febrile illness from which the patient fully recovers. Acquired LCMV infection is typically a biphasic illness in which the initial symptoms include fever, headache, malaise, myalgia, anorexia, nausea, and vomiting. Temporary improvement of these systemic symptoms ensues, often followed by a second phase that consists of central nervous system disease. The symptoms in this second phase are typically those of classic aseptic meningitis and include headache, photophobia, fever, vomiting, and nuchal rigidity29, 30, 31. The entire course of acquired LCMV disease is typically 1–3 weeks, though the symptoms may last for several months16.
During the initial febrile phase of the illness, laboratory abnormalities may include leukopenia, thrombocytopenia, mild elevations in liver enzymes, and infiltrates on chest radiographs. The hallmark laboratory abnormality during the second CNS phase of the illness is a CSF pleocytosis29, 32. The concentration of white blood cells in the CSF exceeds that typically observed with most other virus-induced causes of meningitis. Not unusually, the CSF in LCMV meningitis will contain hundreds to thousands of white blood cells, almost all of which are typically lymphocytes. Hypoglycorrhachia and mild elevations of CSF protein can also occur27, 29.
The clinical spectrum of acquired LCMV infection is broad. In as many as one third of infected people, the disease is asymptomatic. On the other hand, some patients develop not only CNS symptoms, but extraneural disease, as well. Pneumonitis, myocarditis, orchitis, parotitis, dermatitis, and pharyngitis have all complicated LCMV infections33. In addition, the CNS disease in some patients may be considerably more severe than just aseptic meningitis. Other CNS effects have included encephalitis, hydrocephalus, transverse myelitis, and Guillain-Barre syndrome. While acquired LCMV infection is usually mild and self-limited, it is sometimes much more severe, and fatalities from acquired LCMV infection have been reported34.
While the vast majority of postnatal LCMV infections are acquired from an infected rodent, the virus can also be transmitted through transplantation of infected tissue. At least three times in the past decade, groups of solid organ transplant recipients have developed signs and symptoms of infection several days following transplantation5, 6. Each cluster of patients had received tissue from a common donor. The transplant recipients developed severe disease, including encephalopathy, coagulopathy, abdominal pain, thrombocytopenia, fever, leukocytosis, and graft dysfunction. LCMV was identified as the infecting causative agent in all of these patients of all three clusters. All but one of the ten organ recipients died. The one recipient who survived received ribavirin and reduced levels of immunosuppressive therapy. Two of the three donors had no clinical or laboratory signs of infection at the time of organ donation. The third donor had a fever and CSF lymphocytosis, but was seronegative for HIV, hepatitis B and C viruses, and human T-lymphotropic virus, and met Organ Procurement Organization criteria for organ donation. He was not tested for LCMV.
The severe disease induced by LCMV in the transplant recipients almost certainly stemmed from the substantial immunosuppression – especially T-cell depletion – that followed their transplantations. T-cells play a major role in the immune response to and clearance of LCMV1, 21. The lack of T-cells, due to immunotherapy, in the organ recipients likely allowed LCMV to replicate to astronomical levels.
Congenital LCMV infection
While acquired (postnatal) LCMV infections are often mild and self-limited, this is not the case for congenital (prenatal) infections, where the consequences of infection are typically severe. Infection of the human fetus with LCMV can induce spontaneous abortion and fetal death. Among the surviving fetuses, the principal signs of congenital LCMV infection are vision impairment and brain dysfunction13, 14, 28.
A recent clinical study examined the presenting signs, radiographic findings, and clinical outcomes of children with congenital LCMV infection12. While the clinical presentations were relatively broad, the one presenting sign common to all cases of congenital LCMV infection was chorioretinitis. All children with known congenital LCMV infection have vision impairment, due to the formation of bilateral chorioretinal scars (figure 3). Most of this chorioretinal scarring is in the periphery of the fundus, although the macula may also be involved17.
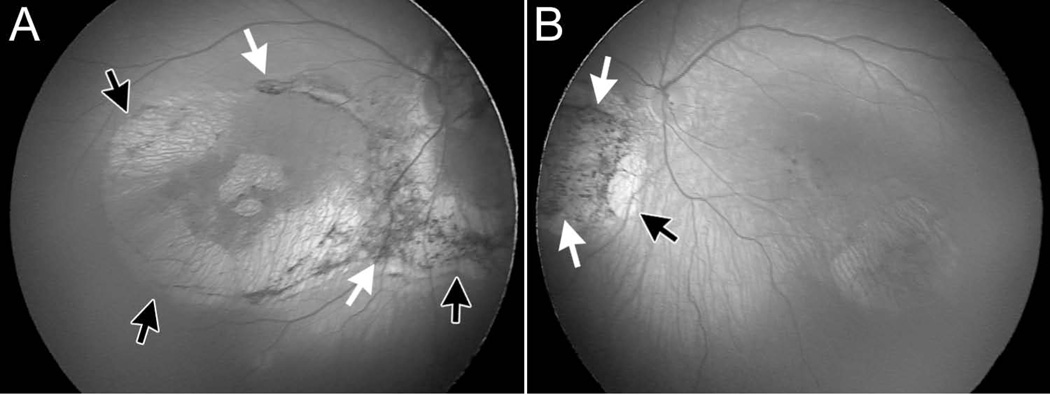
Figure 3.
Vision loss due to retinal pathology is a common feature of congenital LCMV infection. Shown here are fundal photographs from the right eye (A) and left eye (B) of a child with congenital LCMV infection. In the right eye (A), there is a large chorioretinal scar (black arrows) that surrounds the optic disk and includes the macula. Abnormal pigmentation (white arrows) abuts the chorioretinal scar in a variety of places. In the left eye (B), a chorioretinal scar (black arrow) lies inferonasal to the optic disk, and a region of abnormal pigmentation (white arrows) lies nasal to chorioretinal scar.
While the vision losses in congenital LCMV infection are often severe, it is the effect of the virus on the developing brain that causes the greatest disability12, 27, 29. Congenital LCMV infection often leads to macrocephaly or to microcephaly. The cases of macrocephaly are almost invariably due to noncommunicating hydrocephalus, reflecting inflammation and blockade of the ventricular system at the cerebral aqueduct35. Microcephaly in the setting of congenital LCMV infection is due to a virus-induced failure of brain growth and to immune-mediated destruction of infected brain tissue. Additional pathologic features often observed include periventricular calcifications, cortical dysplasia, focal cerebral destruction, and cerebellar hypoplasia12, 13.
Brain function in children with congenital LCMV infection is virtually always adversely affected. However, the impairments and severity vary from case to case. Children with microencephaly and periventricular calcifications virtually always have severe mental retardation, spastic quadriparesis, and epilepsy. In contrast, patients with isolated cerebellar hypoplasia typically have ataxia and mild-to-moderate learning disabilities12.
Unlike virtually all other congenital infections, LCMV does not typically induce systemic manifestations12, 13. This lack of systemic effects reflects the strong neurotropism of the virus within the fetus22. Birth weight is typically appropriate for gestational age. Skin rashes and thrombocytopenia, which are common in several other common prenatal infections, are unusual in congenital LCMV infection. Hepatosplenomegaly is rarely observed, and serum liver enzyme levels are usually normal. Auditory deficits are rare.
Differential diagnosis
The principal differential diagnoses of congenital LCMV infection are the other infectious pathogens that can cross the placenta and damage the developing fetus13, 27, 28. These infectious pathogens are linked conceptually by the acronym “TORCHS” and include Toxoplasma gondii, rubella virus, cytomegalovirus, herpes simplex virus, and syphilis. Congential varicella virus, parechovirus, and human immunodeficiency virus could also masquerade as LCMV. Cytomegalovirus and toxoplasmosis may be particularly difficult to differentiate from LCMV, because all three of these infections can produce microencephaly, intracranial calcifications, and chorioretinitis13, 36. Although clinical clues may aid in distinguishing one congenital infection from another, definitive identification of the causative infectious agent usually requires laboratory testing, including cultures and serologic studies.
The differential diagnosis of congenital LCMV infection also includes several noninfectious entities. Chromosomal abnormalities are prominent causes of microencephaly. However, abnormalities in the structure or number of chromosomes commonly induce dysmorphic features (especially of the hands, feet, and facies) or structural abnormalities (especially of the heart or genitourinary system) that are not observed in congenital LCMV infection.
Several genetic disorders can mimic congenital LCMV infection37. In particular, Aicardi-Goutieres syndrome is an autosomal recessive disorder that often presents as neonatal encephalopathy and intracranial calcifications38. However, its progressive course and identifiable mutations in the TREX1 and RNASEH2 genes distinguish it from congenital LCMV infection.
A second disorder that mimics congenital LCMV and that may be genetic in etiology is pseudo-TORCH syndrome39. In this disorder, infants have many of the classic features of the common congenital infections that gave rise to the TORCH acronym (toxoplasmosis, rubella, cytomegalovirus, and herpes viruses). However, in pseudo-TORCH syndrome, no serological or microbiologic evidence of a congenital infection is ever identified. Because multiple siblings may be similarly affected, pseudo-TORCH syndrome is presumed to be a genetic disorder. However, no genetic locus for pseudo-TORCH syndrome has been identified. The possibility exists that pseudo-TORCH syndrome is actually an unidentified congenital infection. Congenital LCMV infection can be distinguished from pseudo-TORCH syndrome in several ways. First, most mothers of infants with congenital LCMV infection have a history of exposure to wild mice and experienced a definite “flu-like” illness during the pregnancy; these historical factors are typically absent in pseudo-TORCH syndrome. Most importantly, chorioretinitis is present in all cases of congenital LCMV infection and absent in all cases of pseudo-TORCH syndrome12, 15.
Diagnosis
Acute human LCMV infections can be diagnosed by isolation of the virus from cerebrospinal fluid. This approach may be useful in postnatal (acquired) infections, since patients typically have an ongoing LCMV infection at the time of presentation. However, by the time of birth, a baby prenatally infected with LCMV may no longer harbor the virus. Thus, congenital LCMV infection is usually diagnosed by means of serologic testing. The immunofluorescent antibody test detects both IgM and IgG and has greater sensitivity than the more widely available complement fixation method40. The immunofluorescent antibody test is commercially available, and its specificity and sensitivity make it an acceptable diagnostic tool. An even more sensitive test for the detection of congenital LCMV infection is the ELISA, which measures titers of LCMV IgG and IgM and is performed at the Centers for Disease Control and Prevention. Polymerase chain reaction has been utilized as a means of detecting LCMV mRNA in an infected infant41. The use of PCR offers exciting possibilities for both prenatal and postnatal detection of LCMV infection42. However, LCMV is not known to induce persistent infection in humans, and the time course of viral clearance from an infected human fetus is unknown. A fetus may sustain substantial brain damage from LCMV but effectively clear the virus and have no LCMV RNA to be detected by PCR in the postnatal period.
Prognosis
The prognosis for children with congenital LCMV infection is generally poor. A meta-analysis of all reported cases of congenital LCMV infection revealed a mortality rate of 35% by 21 months of age13. Of those that survive, most have severe neurodevelopmental disorders, including microcephaly, poor somatic growth, profound vision impairment, severe seizure disorders, spastic weakness, and substantial mental retardation12. However, some of these children have only moderate neurologic and mental handicaps43. Hearing is relatively spared in children with congenital LCMV infection, and developmental regression is virtually absent.
Complications in children with congenital LCMV infection are nonspecific and consist of the medical problems that commonly arise in scenarios involving ventriculoperitoneal shunts, severe seizure disorders, and static encephalopathy. These complications include shunt failure or infection, aspiration pneumonia, injuries from falls, and joint contractures.
Treatment
An effective antiviral therapy for LCMV infection has not yet been developed. Ribavirin has had mixed success in the treatment of severe infections, but is limited to off-label use and can cause substantial toxicity. However, a promising anti-viral compound is favipiravir, a pyrazine derivative with broad antiviral activity against RNA viruses. By disrupting early stages of viral replication, favipiravir has robust antiviral activity against arenaviruses44. In addition, unlike ribavirin, favipiravir has little cellular toxicity. However, to date, favipiravir’s antiviral effects against LCMV have been tested only in cell culture. Whether favipiravir could protect prenatal or postnatal humans infected with LCMV is unknown.
Prevention
There is no vaccine to prevent LCMV infection. However, steps can be taken to substantially reduce the risk of LCMV infection. Congenital LCMV infection will not occur unless a woman contracts a primary infection with LCMV while she is pregnant. Because house mice are the principal reservoir of LCMV, women can reduce their risk of contracting the virus by minimizing their exposure to the secretions and excretions of mice. This can be accomplished most effectively by eliminating cohabitation with mice. Pregnant women should also avoid contact with pet rodents, especially mice and hamsters. Laboratory personnel who work with rodents have an increased risk of infection with LCMV45. Pregnant women who work in animal care facilities or laboratories at research institutions should wear gloves, gowns, and face masks to avoid potential aerosolized or secreted LCMV.
Acquisition of LCMV from solid organ transplantation represents a grave risk to the recipient’s life. An organ donor with LCMV poses a clear and substantial risk for transmitting a fatal infection to organ recipients. Health care providers, transplant centers, and organ procurement organizations should be aware of the risks posed by LCMV and should consider LCMV in any potential donor with signs of aseptic meningitis but no identified infectious agent. The risks and benefits of offering and receiving organs from donors with possible LCMV infection should be carefully weighed6.
Acknowledgments
Supported by NIH grant NS02007, grants from the Children’s Miracle Network and the Carver Medical Research Initiative, and by the John Martin Fund for Neuroanatomical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buchmeier MJ, Zajac AJ. Lymphocytic choriomeningitis virus. In: Ahmed R, Chen I, editors. Persistent Viral Infections. New York: Wiley; 1999. pp. 575–605. [Google Scholar]
- 2.Bonthius DJ, Barton LL, Klein de Licona H, Bonthius NE, Karacay B. Arenaviruses. In: Barton LL, Friedman NR, editors. The Neurological Manifestations of Pediatric Infectious Diseases and Immunodeficiency Syndromes. Totown, NJ: Humana Press; 2008. pp. 135–150. [Google Scholar]
- 3.Biggar R, Woodall J, Walter P, Haughie G. Lymphocytic choriomeningitis outbreak associated with pet hamsters: fifty seven cases from New York state. JAMA. 1975;232:494. [PubMed] [Google Scholar]
- 4.Lehmann-Grube F. Portraits of viruses: arenaviruses. Intervirology. 1984;22:121–145. doi: 10.1159/000149543. [DOI] [PubMed] [Google Scholar]
- 5.Fischer SA, Graham MB, Kuehnert M, Kotton CN, Srinivasan A, Marty FM, et al. Transmission of lymphocytic choriomeningitis virus by organ transplantation. New Engl J Med. 2006;354:2235–2249. doi: 10.1056/NEJMoa053240. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Lymphocytic choriomeningitis virus transmitted through solid organ transplantation---Massachusetts, 2008. MMWR Morb Mortal Wkly Rep. 2008;57:799–801. [PubMed] [Google Scholar]
- 7.Komrower GM, Williams BL, Stones PB. Lymphocytic choriomeningitis in the newborn. Probable transplacental infection. Lancet. 1955;1:697–698. doi: 10.1016/s0140-6736(55)91066-7. [DOI] [PubMed] [Google Scholar]
- 8.Ambrosio AM, Feuillade MR, Gamboa GS, Maiztegui JI. Prevalence of lymphocytic choriomeningitis virus infection in a human population of Argentina. Amer J Trop Med Hyg. 1994;50:381–386. doi: 10.4269/ajtmh.1994.50.381. [DOI] [PubMed] [Google Scholar]
- 9.Childs JE, Glass GE, Korch GW, Ksiazek TG, Leduc JW. Lymphocytic choriomeningitis virus infection and house mouse (Mus musculus) distribution in urban Baltimore. Amer J Trop Med Hyg. 1992;47:27–34. doi: 10.4269/ajtmh.1992.47.27. [DOI] [PubMed] [Google Scholar]
- 10.Riera L, Castillo E, Del Carmen Saavedra M, Priotto J, Sottosanti J, Polop J, Ambrosio AM. Serological study of LCMV in an inner city of Argentina. J Med Virol. 2005;76:285–289. doi: 10.1002/jmv.20357. [DOI] [PubMed] [Google Scholar]
- 11.Stephensen CB, Blount SR, Lanford RE, Holmes KV, Montali RJ, Fleenor ME, Shaw JF. Prevalence of serum antibodies against lymphocytic choriomeningitis virus in selected populations from two U.S. cities. J Med Virol. 1992;38:27–31. doi: 10.1002/jmv.1890380107. [DOI] [PubMed] [Google Scholar]
- 12.Bonthius DJ, Wright R, Tseng B, Barton L, Marco E, Karacay B, Larsen PD. Congenital lymphocytic choriomeningitis virus infection: spectrum of disease. Ann Neurol. 2007;62:347–355. doi: 10.1002/ana.21161. [DOI] [PubMed] [Google Scholar]
- 13.Wright R, Johnson D, Neumann M, Ksiazek TG, Rollin P, Keech RV, Bonthius DJ, et al. Congenital lymphocytic choriomeningitis virus syndrome: A disease that mimics congenital toxoplasmosis or cytomegalovirus infection. Pediatrics. 1997;100:1–6. doi: 10.1542/peds.100.1.e9. [DOI] [PubMed] [Google Scholar]
- 14.Barton LL, Peters CJ, Ksiazek TG. Lymphocytic choriomeningitis virus: an unrecognized teratogenic pathogen. Emerg Inf Dis. 1995;1:152–153. doi: 10.3201/eid0104.950410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonthius DJ. Diagnosed cases of congenital LCMV infection: tip of the iceberg? Ann Neurol. 2008;64:356. [Google Scholar]
- 16.Jahrling PB, Peters CJ. Lymphocytic choriomeningitis virus: a neglected pathogen of man. Arch Pathol Lab Med. 1992;116:486–488. [PubMed] [Google Scholar]
- 17.Mets MB, Barton LL, Khan AS, Ksiazek TG. Lymphocytic choriomeningitis virus: an underdiagnosed cause of congenital chorioretinitis. Am J Ophthalmol. 2000;130:209–215. doi: 10.1016/s0002-9394(00)00570-5. [DOI] [PubMed] [Google Scholar]
- 18.Barton LL, Mets MB. Congenital lymphocytic choriomeningitis virus infection: decade of rediscovery. Clin Infect Dis. 2001;33:370–374. doi: 10.1086/321897. [DOI] [PubMed] [Google Scholar]
- 19.Bonthius DJ. Lymphocytic choriomeningitis virus: A prenatal and postnatal threat. Advances Pediatrics. 2009;56:75–86. doi: 10.1016/j.yapd.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Danes L, Benda R, Fuchsova M. Experimental inhalation with the lymphocytic choriomeningitis virus (WE strain) of the monkeys of the Macacus cynomolgus and Macacus rhesus species. Bratisl Lek Listy. 1963;43:21–34. [PubMed] [Google Scholar]
- 21.Buchmeier MJ, Bowen MD, Peters CJ. Arenaviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Field’s Virology. 4th edition. Philadelphia: Lippicottt, Williams, and Wilkins; 2001. pp. 1635–1668. [Google Scholar]
- 22.Bonthius DJ, Mahoney JC, Buchmeier MJ, Taggard DA. Critical role for glial cells in the propagation and spread of lymphocytic choriomeningitis virus in the developing rat brain. J Virol. 2002;76:6618–6635. doi: 10.1128/JVI.76.13.6618-6635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monjan AA, Cole GA, Nathanson N. Pathogenesis of LCM disease in the rat. In: Lehmann F, editor. Lymphocytic Choriomeningitis Virus and Other Arenaviruses. Philadelphia: Springer-Verlag; 1973. pp. 195–206. [Google Scholar]
- 24.Monjan AA, Gilden DH, Cole GA, Nathanson N. Cerebellar hypoplasia in neonatal rats caused by lymphocytic choriomeningitis virus. Science. 1971;171:194–196. doi: 10.1126/science.171.3967.194. [DOI] [PubMed] [Google Scholar]
- 25.Bonthius DJ, Nichols B, Harb H, Mahoney J, Karacay B. Lymphocytic choriomeningitis virus infection of the developing brain: critical role of host age. Ann Neurol. 2007;62:356–374. doi: 10.1002/ana.21193. [DOI] [PubMed] [Google Scholar]
- 26.Bonthius DJ, Perlman S. Congenital viral infections of the brain: lessons learned from lymphocytic choriomeningitis virus in the neonatal rat. PLoS Pathogens. 2007;3:1541–1550. doi: 10.1371/journal.ppat.0030149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonthius DJ. Lymphocytic choriomeningitis virus (LCMV) In: Kleigman RM, Stanton BF, St Geme J, Schor N, Behrman RE, editors. Nelson Textbook of Pediatrics. 19th Edition. Philadelphia: Elsevier; 2011. pp. 1153–1154. Also available at www.expertconsult.com. [Google Scholar]
- 28.Bonthius DJ. Congenital lymphocytic choriomeningitis virus infection. In: Gilman S, editor. MedLink-Neurobase. 12th Edition. San Diego: Arbor Publishing Corp.; (in press). Also available at www.medlink.com. [Google Scholar]
- 29.Bonthius DJ, Karacay B. Meningitis and encephalitis in children: an update. Neurol Clin. 2002;20:1013–1038. doi: 10.1016/s0733-8619(02)00016-6. [DOI] [PubMed] [Google Scholar]
- 30.Asnis DS, Muana O, Kim DG, Garcia M, Rollin PE, Slavinski S. Lymphocytic choriomeningitis virus meningitis, New York, NY, USA, 2009. Emerg Infect Dis. 2010;16:328–330. doi: 10.3201/eid1602.091347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barton LL, Hyndman NJ. Lymphocytic choriomeningitis virus: reemerging central nervous system pathogen. Pediatrics. 2000;105:E35. doi: 10.1542/peds.105.3.e35. [DOI] [PubMed] [Google Scholar]
- 32.Folk S, Steinbecker S, Windmeyer J, Macneil A, Campbell S, Rollin PE. Lymphocytic choriomeningitis with severe manifestations, Missouri, USA. Emerg Inf Dis. 2011;17:1973–1974. doi: 10.3201/eid1710.110911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis JM, Utz JP. Orchitis, parotitis and meningoencephalitis due to lymphocytic choriomeningitis virus. N Engl J Med. 1961;265:776–780. doi: 10.1056/NEJM196110192651604. [DOI] [PubMed] [Google Scholar]
- 34.Meyer HM, Jr, Johnson RT, Crawford IP, Dascomb HE, Rogers NG. Central nervous system syndromes of "viral" etiology: a study of 713 cases. Am J Med. 1960;29:334–347. doi: 10.1016/0002-9343(60)90029-2. [DOI] [PubMed] [Google Scholar]
- 35.Larsen PD, Chartrand SA, Tomashek KM, Hauser LG, Ksiazek TG. Hydrocephalus complicating lymphocytic choriomeningitis virus infection. Ped Infect Dis J. 1993;12:528–531. doi: 10.1097/00006454-199306000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Bale JF, Murph JR. Congenital infections and the nervous system. Pediatr Clin North Amer. 1992;39:669–690. doi: 10.1016/s0031-3955(16)38370-5. [DOI] [PubMed] [Google Scholar]
- 37.Sanchis A, Cervero L, Bataller A, Tortajada JL, Huguet J, Crow YJ, et al. Genetic syndromes mimic congenital infections. J Pediatr. 2005;146:701–705. doi: 10.1016/j.jpeds.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 38.Rice G, Patrick T, Parmar R, Taylor CF, Aeby A, Aicardi J, et al. Clinical and molecular phenotype of Aicardi-Goutieres syndrome. Amer J Hum Genet. 2007;81:713–725. doi: 10.1086/521373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vivarelli R, Grosso S, Cioni M, Galluzzi P, Monti L, Morgese G, Balestri P. Pseudo-TORCH syndrome or Baraitser-Reardon syndrome: diagnostic criteria. Brain Devel. 2001;23:18–23. doi: 10.1016/s0387-7604(00)00188-1. [DOI] [PubMed] [Google Scholar]
- 40.Lehmann-Grube F, Kallay M, Ibscher B, Schwartz R. Serologic diagnosis of human infections with lymphocytic choriomeningitis virus: comparative evaluation of seven methods. J Med Virol. 1979;4:125–136. doi: 10.1002/jmv.1890040207. [DOI] [PubMed] [Google Scholar]
- 41.Enders G, Varho-Gobel M, Lohler J, Terletskain-Ladwig E, Eggers M. Congenital lymphocytic choriomeningitis virus infection: an underdiagnosed disease. Ped Infect Dis J. 1999;18:652–655. doi: 10.1097/00006454-199907000-00020. [DOI] [PubMed] [Google Scholar]
- 42.Cordey S, Sahli R, Moraz M-L, Estrade C, Morandi L, Cherpillod P, et al. Analytical validation of a lymphocytic choriomeningitis virus real-time RT-PCR assay. J Virol Meth. 2011;177:118–122. doi: 10.1016/j.jviromet.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 43.Larsen PD, Wright R. Early clinical manifestations and long-term outcome in children with symptomatic congenital lymphocytic choriomeningitis virus infection. Neurology. 2001;56:A39–A40. (Abstract). [Google Scholar]
- 44.Mendenhall M, Russell A, Juelich T, Messina EL, Smee DF, Freiberg AN, et al. T-705 (Favipiravir) inhibition of arenavirus replication in cell culture. Antimicrob Agents Chemother. 2011;55:782–787. doi: 10.1128/AAC.01219-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dykewicz CA, Dato VM, Fisher-Hoch SP. Lymphocytic choriomeningitis outbreak associated with nude mice in a research institute. JAMA. 1992;267:1349–1353. [PubMed] [Google Scholar]