Abstract
Intravitreal fluocinolone acetonide implant (Retisert) has a high potency, a low solubility, and a very short duration of action in the systemic circulation, enabling the steroid pellet to be small and reducing the risk of systemic side effects. Scleral melt has not been reported as a possible complication of Retisert implant. The authors describe the occurrence of scleral melt 18 months after the implantation of fluocinolone acetonide implant in a 42-year-old Caucasian woman. To the authors’ knowledge, this is the first report of this possible complication.
Keywords: Retisert, scleral melt, complication, surgical management
Introduction
Inflammatory macular edema represents one of the main causes of significant vision loss in patients with uveitis, as a result of the breakdown of the blood–retinal barrier by inflammatory mediators such as tumor necrosis factor-alpha and interleukin-1beta.1 In an effort to intercept the circulation of these mediators, corticosteroids have been used in variable delivery forms (oral, intravitreal, sub-Tenon’s administration) as a treatment modality for macular edema on the background of various types of chronic posterior uveitis.
The repeated administration nature of periocular and intravitreal steroid injections as well as the increased incidence of side effects associated both with topically and systemically administered corticosteroids2–4 led to the development of slow-release therapeutic modalities. Based on a Phase III study showing that the 34-week risk of uveitic recurrence fell significantly from 51.4% to 6.1% and visual acuity improved significantly,5,6 the Food and Drug Administration approved an intravitreal fluocinolone acetonide (FA) implant that could deliver a sustained dose of corticosteroid (Retisert; Bausch & Lomb Incorporated, Bridgewater, NJ, USA) for the treatment of the posterior uveitis.
Since 2005, the use of FA implant gained popularity among ophthalmologists. Nonetheless, the identification and management of possible intraoperative or postoperative complications associated with its use remains an ongoing process. The purpose of the present work is to report the unusual but serious postoperative complication associated with the implantation of Retisert, which to the best of our knowledge has never been reported before, and describe the surgical management of scleral melt at the site of the Retisert implantation in a patient with posterior uveitis on the background of psoriatic arthritis.
Case report
A 42-year-old Caucasian woman was referred to our department complaining of sudden vision loss, watery discharge from her right eye (RE), and pain. She had a complex past ocular history that included the implantation of Retisert in her RE to treat persistent uveitis-associated cystoid macular edema on the background of psoriatic arthritis 18 months earlier. In addition, according to her notes, she had undergone combined phacoemulsification and vitrectomy with epiretinal membrane peeling 2 years before the FA implant. One year after the Retisert implantation, she underwent right trabeculectomy to treat persistently high intraocular pressure.
On examination, the intraocular pressure (IOP) was 2 mmHg, the anterior chamber was flat, and the visual acuity was counting fingers in the RE. An anterior segment examination revealed scleral melt with active leaking (positive Seidel test) at the site of the FA implant. The patient underwent urgent surgical repair under general anesthesia. Intraoperatively, an anterior chamber maintainer was used and surgical exploration confirmed significant scleral melt at the site of implantation with leaking aqueous humor (Figure 1). A scleral patch graft was sutured over the leaking sclerotomy.
Figure 1.
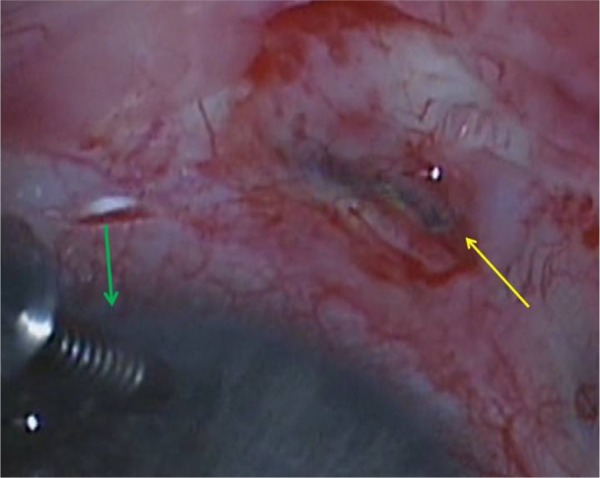
Intraoperative photo of the right eye.
Notes: Photo demonstrates an anterior chamber maintainer inferonasally (green arrow) and the leaking site of scleral melt inferotemporally (yellow arrow).
On the following day, the IOP was 9 mmHg and the fundus examination revealed that the FA implant had detached from the scleral sutures and was free floating in the vitreous cavity.
Three weeks later, the IOP was 18 mmHg, the visual acuity recovered to 20/30, and the patch graft was in good position (Figure 2). Benefits and risks of vitrectomy to remove the Retisert implant were discussed with the patient; however, she decided against surgery.
Figure 2.
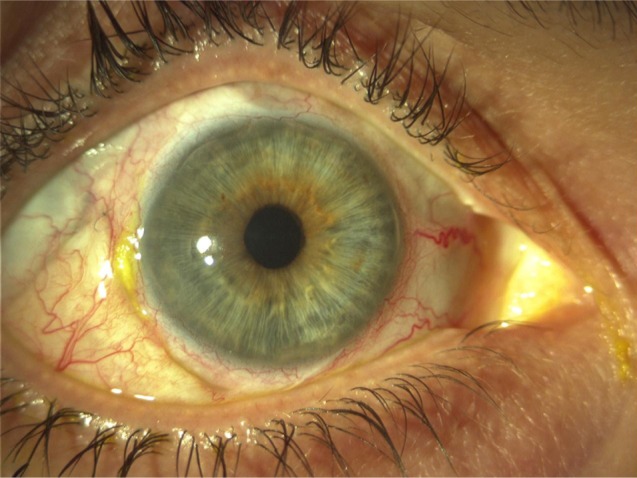
Anterior segment image of the right eye 3 weeks postoperatively.
Note: Image demonstrates good position of the scleral patch graft inferotemporally.
Two years later, visual acuity remained 20/30 with no recurrence of the cystoid macular edema, and IOP within normal limits, without additional medication.
Discussion
FA has a high potency, a low solubility, and a very short duration of action in the systemic circulation, enabling the steroid pellet to be small and reducing the risk of systemic side effects.
The implant consists of a tablet encased in a silicone elastomer cup containing a release orifice and a polyvinyl alcohol membrane positioned between the tablet and the orifice. The silicone elastomer cup assembly is attached to a polyvinyl alcohol suture tab with silicone adhesive, which is supported to the sclera with sutures. The most common side effects of Retisert are cataract formation (all patients require cataract surgery within 3 years) and significantly raised ocular pressure (40% of patients require trabeculectomy surgery). More rare side effects are vitreous hemorrhage, retinal detachment, vitreous bands, scleral thinning over the implant, and hypotony (IOP <6 mmHg in 9.4% of implanted eyes).2,6–9
Scleral melt has not been reported as a possible complication of FA implant. In a study aiming to evaluate whether intravitreal FA implantation (Retisert) leads to scleral thinning, Taban et al10 reported that FA implant appears to lead to overall statistically nonsignificant scleral thinning with few exceptions.
In our case, significant scleral melt developed 18 months after the implantation of FA implant, which to the best of our knowledge has never been reported before. Of course, the patient had previously undergone vitrectomy with epiretinal membrane peel before FA implant as well as a trabeculectomy post-Retisert implantation. It is possible that these procedures could represent an additional factor for the significant scleral melt observed in our patient, although no significant inflammation or infection had been recorded post-vitrectomy or post-trabeculectomy. Scleral patch graft appeared to be adequate for the surgical management in our case. No adverse events were observed to be associated with the presence of the free-floating implant in the vitreous cavity.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Gallagher MJ, Yilmaz T, Cervantes-Castañeda RA, Foster CS. The characteristic features of optical coherence tomography in posterior uveitis. Br J Ophthalmol. 2007;91:1680–1685. doi: 10.1136/bjo.2007.124099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brumm MV, Nguyen QD. Fluocinolone acetonide intravitreal sustained release device – a new addition to the armamentarium of uveitic management. Int J Nanomedicine. 2007;2:55–64. doi: 10.2147/nano.2007.2.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130:492–513. doi: 10.1016/s0002-9394(00)00659-0. [DOI] [PubMed] [Google Scholar]
- 4.Vavvas D, Foster CS. Immunomodulatory medications in uveitis. Int Ophthalmol Clin. 2004;44:187–203. doi: 10.1097/00004397-200404430-00016. [DOI] [PubMed] [Google Scholar]
- 5.Jaffe GJ, Martin D, Callanan D, et al. Fluocinolone Acetonide Uveitis Study Group Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis: thirty-four-week results of a multicenter randomized clinical study. Ophthalmology. 2006;113:1020–1027. doi: 10.1016/j.ophtha.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 6.Fluocinolone acetonide ophthalmic – Bausch & Lomb: fluocinolone acetonide Envision TD implant. Drugs R D. 2005;6:116–119. doi: 10.2165/00126839-200506020-00007. [DOI] [PubMed] [Google Scholar]
- 7.Taban M, Lowder CY, Kaiser PK. Outcome of fluocinolone acetonide implant (Retisert) reimplantation for chronic noninfectious posterior uveitis. Retina. 2008;28(9):1280–1288. doi: 10.1097/IAE.0b013e31817d8bf2. [DOI] [PubMed] [Google Scholar]
- 8.Lim LL, Smith JR, Rosenbaum JT. Retisert. Curr Opin Investig Drugs. 2005;6(11):1159–1167. [PubMed] [Google Scholar]
- 9.Berger BB, Mendoza W. Sclerotomy closure for Retisert implant. Retina. 2013;33(2):436–438. doi: 10.1097/IAE.0b013e3182759fdb. [DOI] [PubMed] [Google Scholar]
- 10.Taban M, Lowder CY, Ventura AA, et al. Scleral thickness following fluocinilone acetonide implant (retisert) Ocul Immunol Inflamm. 2010;18(4):305–313. doi: 10.3109/09273941003658292. [DOI] [PubMed] [Google Scholar]


