Abstract
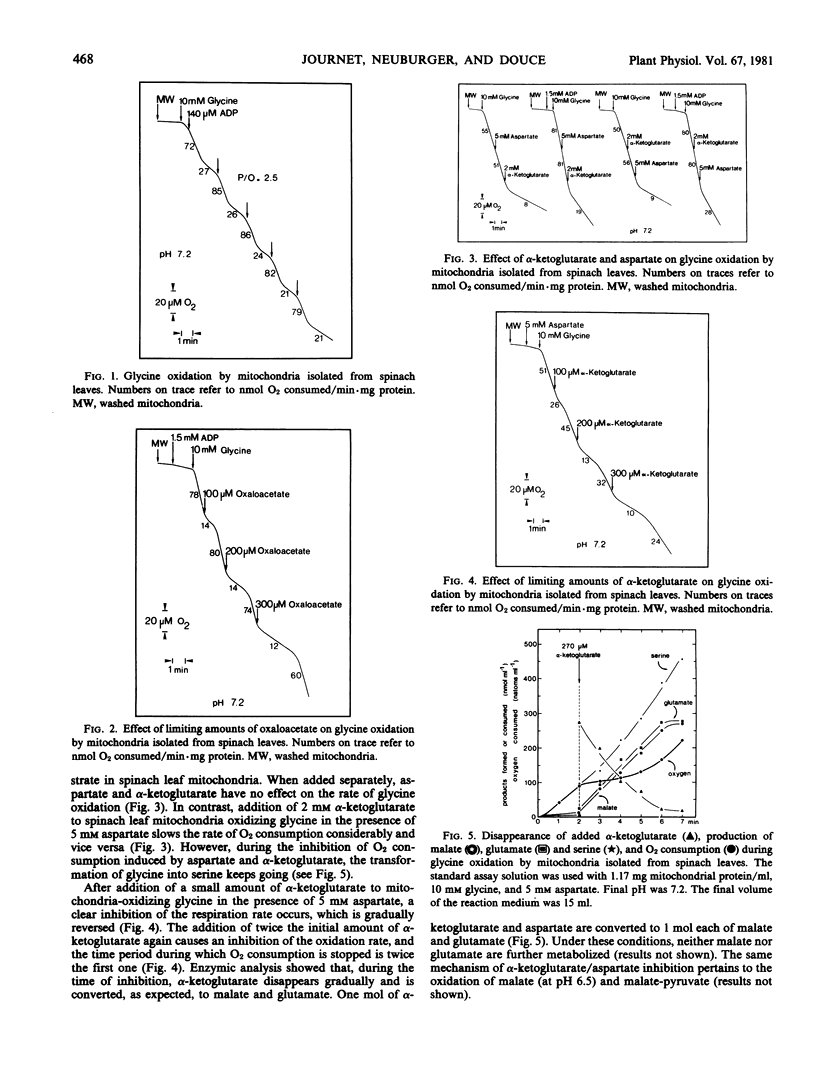
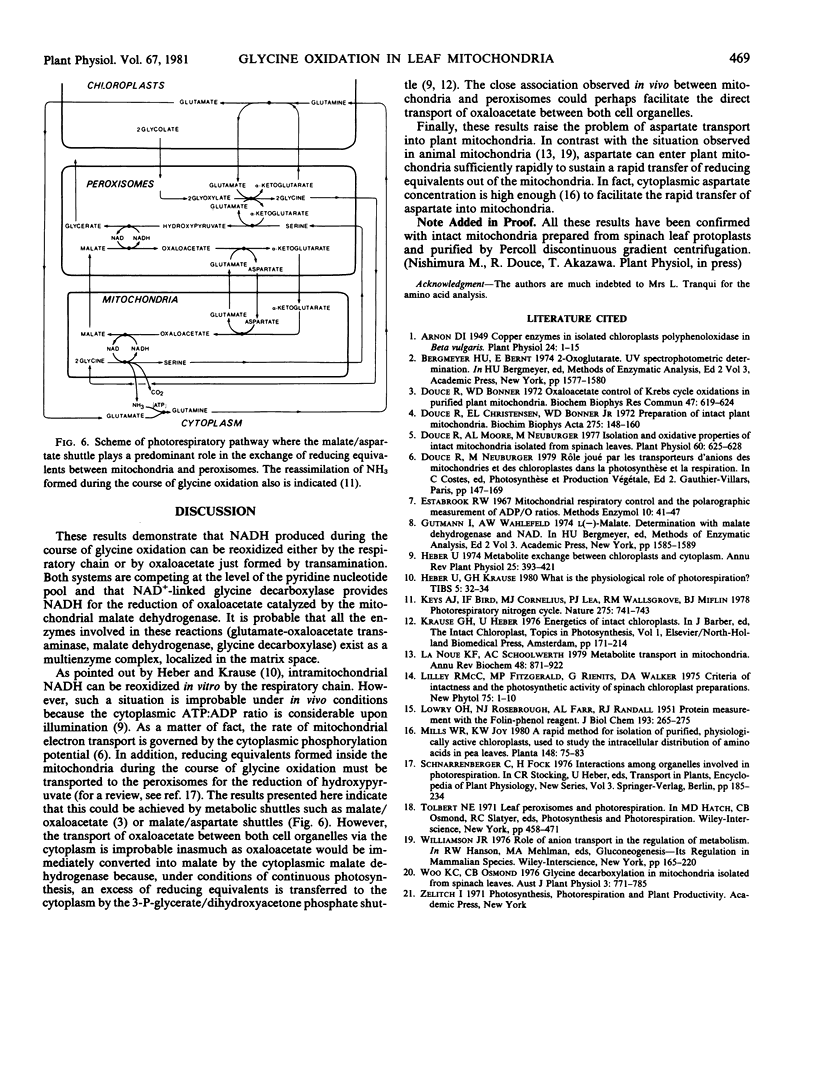
During glycine oxidation by spinach leaf mitochondria, oxygen consumption showed a strong and transient inhibition upon addition of oxaloacetate or aspartate plus α-ketoglutarate. During the course of the inhibition, aspartate and α-ketoglutarate were stoichiometrically transformed into malate and glutamate.
It is concluded that oxaloacetate formed by transamination is reduced by the malate dehydrogenase, which allows the regeneration of NAD+ for glycine oxidation and, thus, by-passes the respiratory chain. Efficiency of a malate-glutamate/aspartate-α-ketoglutarate shuttle upon illumination and under in vivo conditions is discussed.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis G. H., MacIvor J., Freeman M. Changes in the vaginal and rectal carriage of group B streptococci during pregnancy. Aust N Z J Obstet Gynaecol. 1980 Feb;20(1):32–34. doi: 10.1111/j.1479-828x.1980.tb00892.x. [DOI] [PubMed] [Google Scholar]
- Douce R., Bonner W. D., Jr Oxalacetate control of Krebs cycle oxidations in purified plant mitochondria. Biochem Biophys Res Commun. 1972 May 12;47(3):619–624. doi: 10.1016/0006-291x(72)90923-0. [DOI] [PubMed] [Google Scholar]
- Douce R., Christensen E. L., Bonner W. D., Jr Preparation of intaintact plant mitochondria. Biochim Biophys Acta. 1972 Aug 17;275(2):148–160. doi: 10.1016/0005-2728(72)90035-7. [DOI] [PubMed] [Google Scholar]
- Douce R., Moore A. L., Neuburger M. Isolation and oxidative properties of intact mitochondria isolated from spinach leaves. Plant Physiol. 1977 Oct;60(4):625–628. doi: 10.1104/pp.60.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaNoue K. F., Schoolwerth A. C. Metabolite transport in mitochondria. Annu Rev Biochem. 1979;48:871–922. doi: 10.1146/annurev.bi.48.070179.004255. [DOI] [PubMed] [Google Scholar]



