Abstract
Objective
The degree of functional impairment and adverse developmental outcomes in individuals with attention-deficit/hyperactivity disorder (ADHD) likely reflect interplay between genes and environment. To establish whether physical exercise might reduce the level of ADHD symptoms or ADHD-related impairments, we conducted a comprehensive review of the effect of exercise in children with ADHD. Findings on the impact of exercise in animals and typically developing humans, and an overview of putative mechanisms involved are also presented to provide the context in which to understand this review.
Method
The electronic databases PubMed, OVID and Web of Knowledge were searched for all studies investigating the effect of exercise in children and adolescents with ADHD, as well as animal models of ADHD behaviours (available in January 2013). Of 2,150 initially identified records, 16 were included.
Results
Animal studies indicate that exercise, especially early in development, may be beneficial for ADHD symptom reduction. The limited research investigating the effect of exercise in children and adolescents with ADHD suggests that exercise may improve executive functioning and behavioural symptoms associated with ADHD. While animal research suggests brain-derived neurotrophic factor (BDNF) and catecholamines (CAs) play a role in mediating these effects, the association between BDNF and ADHD remains unclear in humans.
Conclusions
The potential protective qualities of exercise with regard to reducing symptoms and impairments commonly associated may hold promise for the future. Further research is needed to firmly establish whether there are clinically significant effects of exercise on the severity of ADHD symptoms, impairments and associated developmental outcomes.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a complex neurodevelopmental disorder characterised by developmentally inappropriate and impairing levels of hyperactivity, impulsivity and/or inattention.1 ADHD is associated with multiple cognitive impairments, including lower average IQ2, impairment in attentional processing, response inhibition and other aspects of executive functioning (EF)3, which share genetic/familial risks with ADHD3,4. Genetic factors play a pivotal role in the susceptibility to ADHD, with 60-75% or more of the variance in ADHD symptoms attributable to genetic variation.5,6 Environmental factors are also likely to contribute to the development of the disorder and associated emotional, behavioural and academic difficulties.7 Moreover, the effects of genetic risks on development may depend on exposure to either adverse or enriched environments leading to either negative or positive long term outcomes.8,9 Such gene-environment interactions mean that specific environments may be more or less beneficial to the long term outcomes in children with ADHD.
Several environmental risk factors have been linked to ADHD.7 Yet, less attention has been paid to protective factors that might reduce levels of ADHD symptoms and impairments associated with the disorder. We define protective factors as influences that increase adaptive functioning following environmental adversity or genetic risk. Here, we investigate exercise as a factor that may diminish an individual’s level of ADHD symptoms or the impairments associated with the disorder by modifying the effects of genetic and environmental risks on developmental outcomes. We will investigate exercise as a factor that may diminish an individual’s likelihood of developing ADHD or the impairments associated with the disorder. The idea that exercise may have protective qualities for individuals with ADHD gains support from animal research examining the impact of exercise on neural functioning, brain growth and development, as well as from human studies indicating that exercise positively affects executive functioning and inhibitory control in adults and typically developing children. Preliminary research has also been carried out to explore the utility of exercise as a protective factor or alternative treatment strategy in ADHD. Before providing a comprehensive review of the role of exercise in ADHD, we will provide the context in which to understand this review by presenting findings on the impact of exercise on cognition in animal and typically developing human research, and an overview of the putative mechanisms involved.
The neurobiology of ADHD
The precise neurobiological mechanisms underlying ADHD are poorly understood. Their complexity is likely due to the interplay of various structural, functional and developmental brain alterations in individuals with ADHD, such as abnormalities in the white matter fibres that connect grey matter regions, hypoactivity of frontal regions and aberrant or delayed brain development.10 Dysregulation of dopaminergic and noradrenergic neurotransmission has also been widely implicated in the pathophysiology of ADHD.11 The catecholaminergic system has been the key target for pharmacotherapy in ADHD.12 In molecular genetic studies of ADHD, one of the most robust findings to date is the association with the 7-repeat allele of the dopamine D4 receptor gene13, and the catecholamines (CA) are known to play a pivotal role in the regulation of psychomotor activity, motivation, inhibition, and attention14, all of which are compromised in ADHD12. (For a comprehensive review of the neurobiology and genetics of ADHD, see 10,15).
One recent theory has focused on BDNF as a factor that impacts on dopaminergic function underlying aspects of ADHD.14,16-19 The idea that BDNF interacts with the dopaminergic dysfunctions seen in individuals with ADHD was initially suggested by research showing that stimulant medications increase the expression of BDNF in the rat brain19, in addition to studies suggesting that there is an association between genetic variants in BDNF and risk for ADHD. In a community-based cohort of 1,236 Swedish individuals assessed at ages 8-9, 13-14 and 16-17 years, the presence of the Val66Met polymorphism in BDNF gene was associated with increased hyperactive-impulsive ADHD symptom counts at ages 8-9 and 13-14 years.19
However, the overall evidence for the genetic association of BDNF with ADHD is far from clear. A recent meta-analysis of published and unpublished data from four different centres (conducted as part of the International Multicentre Persistent ADHD CollaboraTion; IMpACT) investigated the association of ADHD with the most frequently reported BDNF polymorphism, Val66Met, in a total sample 1,455 ADHD adults and 2,247 sex-matched controls.20 The investigation of these four populations yielded no significant association between BDNF genotype and ADHD. Like several studies before21-23, the investigation of these four populations yielded no significant association between BDNF and ADHD, highlighting the known difficulties in replicating candidate gene association studies; potentially related to heterogeneity of the genetic effects across development or related to different clinical subgroups.
The conception of exercise as a potential protective factor for ADHD is suggested by animal literature investigating the positive impacts of physical activity on neurobiological mechanisms implicated in ADHD. In healthy rodents, exercise has been found to augment several factors often compromised in ADHD: neural plasticity24, cerebral blood flow25, levels of synaptic protein26 and extracellular dopamine and norepinephrine levels in the central nervous system27. Furthermore, physical exercise has been shown to lead to an increase in rodent serum and brain levels of BDNF, which is part of vital neurodevelopmental processes central to the survival and differentiation of noradrenergic28 and dopaminergic29 neurons.30-34
This upregulation of BDNF is one potential mechanism via which exercise induces its positive effects. BDNF levels in the hippocampus have been directly linked to the enhanced memory and learning processes that are observed with exercise treatments in rodents.35 One process expected to mediate the upregulation of BDNF involves epigenetic influences on gene expression. Epigenetic processes, which developmentally regulate gene expression independently of the DNA sequence via modifications to DNA, histone proteins and chromatin36, have been shown to be sensitive to a range of environmental influences37. Indeed, both acute38 and chronic39 exercise have recently been found to elicit gene expression changes linked to changes in DNA methylation. However, the strength of the association between BDNF and ADHD in humans and its potential role in eliciting the established positive effects of exercise on cognition and psychological health40 remain unclear.
The cognitive effects of exercise in humans
Beyond the animal literature, an emerging body of evidence highlights the benefits of exercise for humans.40 Research on the effect of exercise in humans has found, for example, that older adults, who were more aerobically fit or who took part in exercise programmes, demonstrated greater task-related activity in areas of the brain implicated in ADHD; namely regions of the prefrontal and parietal cortices, with the largest fitness-induced benefits occurring for executive functions.41 Furthermore, there is evidence that in humans exercise facilitates catecholaminergic neurotransmission42,43, augments serum BDNF levels31,34, enhances cognitive performance and affects cerebral structures positively by increasing cortical tissue density and brain volume41,44. A meta-analysis of 44 studies, which investigated the effects of both acute and chronic exercise on cognition in children, yielded an overall effect size (ES) of 0.32. Despite the ES being only moderate, these findings indicate a significant positive effect of exercise on cognitive performance. As the ES of exercise was greater in the children who were classified as “mentally impaired” (0.42), this moderate overall ES may point to greater beneficial effects of exercise in children with developmental disorders.45 Other more recent reviews of the effects of exercise on neurotypical children’s intelligence, cognition and academic achievement confirmed the positive effect of exercise, especially on EF40,46,47, a cognitive domain often impaired in individuals with ADHD48. Chronic and acute exercise was reported to improve inhibitory control, while the effects on attention, perception and visuo-motor coordination were more limited.
The role of exercise in ADHD
Animal and neurotypical human studies, thus, provide support for the notion that exercise enhances brain structure, cognitive performance and neural functioning. As enhanced neural functioning has been suggested to be associated with a remission of ADHD symptoms49,50, exercise may hold promise for preventing the emergence of ADHD during childhood, and as an early intervention in children presenting with high levels of ADHD symptoms, potentially inducing lasting changes in ADHD symptom severity. Our aim here, therefore, is to offer a review of the literature investigating the effect of exercise in ADHD, including cognitive impairments as well as ADHD symptoms, and to provide a rationale for further research in this area.
Selection criteria
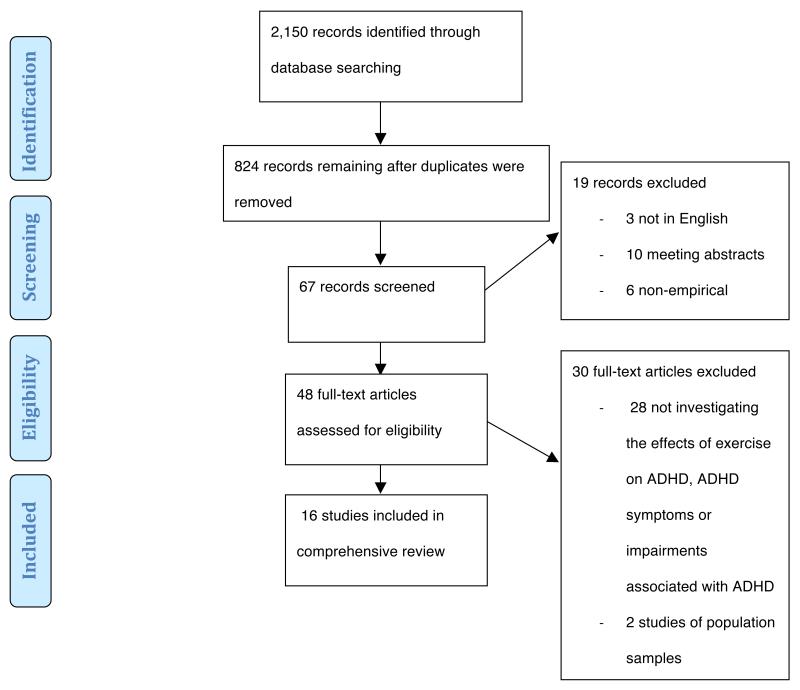
A comprehensive review was conducted to ensure a thorough search of the literature. The electronic databases PubMed, OVID and Web of Knowledge were searched. Key words searched included attention deficit hyperactivity disorder, ADHD, attention deficit disorder, ADD and hyperkinetic syndrome with either sport*, exercise or physical activity. The inclusion criteria for studies reviewed here were as follows: (a) written in English; (b) published in an internationally peer-reviewed journal before January 2013; (c) participants were children and/or adolescents diagnosed with ADHD or animal models of ADHD behaviours; (d) an empirical study (including correlational, observational and longitudinal designs) of aerobic exercise. Using these search terms and inclusion criteria, 2,150 records were identified. After screening these records for eligibility as shown in Figure 1, 16 studies were included in the comprehensive review. An overview of these studies can be found in Table 1.
Figure 1.
Flow diagram depicting the number of records identified, included and excluded in the comprehensive review, and the reasons for exclusions. Source: Moher et al. (2009)73. ADHD= attention-deficit/hyperactivity disorder.
Table 1.
Overview of all the studies on exercise and attention-deficit/hyperactivity disorder (ADHD) included in the comprehensive review. CBCL= Child Behaviour Checklist; CRS= Conners’ Rating Scale; EF= executive function; ES= effect size; SHR= spontaneously hypertensive rat; MPH= methylphenidate; WCST= Wisconsin Card Sorting Test; WKY = Wistar-Kyoto rat
| Sample type | Citation | Design | Intervention/Measure of physical activity | Sample size (males: females) | Mean age (SD) in years unless stated |
Cohen’s d |
|---|---|---|---|---|---|---|
| Animal literature | Hopkins et al. (2009)51 | Case-control (three-groups) |
SHR exercise group: 24h access to running wheel, 2 weeks prior to and throughout study SHR no-exercise group: no intervention WKY group: no intervention |
69 SHRs (37:32), 29 WKYs (14:15) | 8 weeks | Orienting behaviour; data not available |
| Kim et al. (2011)55 | Case-control |
SHR exercise group: low-intensity treadmill running 30 min/day, 5 times/week for 28 days SHR MPH-treated group: 1mg/kg MPH orally/day for 28 days SHR exercise and MPH-treated group SHR controls Controls |
60 (60:0) | not available “adult” | Decrease in open field activity:2.85 | |
| Robinson et al. (2011)52 | Case-control |
SHR exercise group: 24h access to running wheel, 3 weeks prior to and throughout study SHR non-exercise group: no intervention WKY controls: no intervention |
32 SHRs (16:16), 16 WKYs (8:8) | 28-32 days | Orienting behaviour; data not available | |
| Robinson et al. (2012)53 | Case-control |
SHR exercise group: 24hr access to running wheel, 3 weeks prior to and throughout study, saline-injection SHR controls: saline-injection SHR MPH-treated group: 1.0ml/kg 10min before behavioural task SHR atomoxetine-treated group: 1.0ml/kg 30min before behavioural task WKY controls WKY exercise group WKY MPH-treated group WKY atomoxetine-treated group |
74 SHRs (0:74), 32 WKYs (0:32) | 8–9 weeks | Orienting behaviour; data not available | |
| ADHD | Ahmed and Mohamed (2011)60 | Case-control, randomly assigned |
Exercise group: 3 sessions/week for 10 weeks (aerobic exercise) Controls: no intervention |
84 (54:30) | 13.9 (1.6) | Attention:0.50 Motor skills:0.59 Academic and classroom behaviour:1.1 |
| Chang et al. (2012)56 | Case-control, randomly assigned |
Exercise group: acute moderate-intensity exercise (30min) Controls: watched exercise-related video (30min) |
40 (37:3) | 10.4 (0.9) | Colour-Word Stroop:0.57 Total WCST:0.31 |
|
| Gapin et al. (2010)58 | Within-group comparison | Daily step counts and minutes/day spent in moderate-to-vigorous intensity exercise over 7 days | 18 (18:0) | 10.6 (1.5) | Tower of London task:0.57 | |
| Kang et al. (2011)59 | Case-control |
Exercise group: medication and sports therapy (12 90-min sessions over 6 weeks) Controls: medication and behaviour control education |
28 (28:0) | 8.5 (1.2) | Attention ratings:0.32 EF:0.37 Cooperativeness:0.54 |
|
| Kiluk et al. (2009)64 | Within-group comparison | CBCL indicating number of sport activities | 65 (40:25) | Range: 6-14 | Boys:1.52 Girls:1.23 |
|
| Lufi and Parish-Plass (2013)65 | Clinical comparison | 20 90-min sessions (including discussions, individual sport activity, team games) | 32 (32:0) | 10.9 (1.6) | Total CBCL:0.45 | |
| McKune et al. (2003)62 | Case-control, allocation to groups |
Exercise group: 5 1-h sessions/week for 5 weeks Controls: no intervention |
19 (13:6) | 11 (1.9) | CRS-Parent; data not available | |
| Medina et al. (2010)70 | Between-group (MPH users v. non-users) comparison | 30min aerobic exercise | 25 (25:0) | 9.5 (2.9) | Hit RT SD:0.49 | |
| Pontifex et al. (2012)57 | Case (ADHD) – control (neurotypical) and within-group comparison | Acute 20min moderate-intensity aerobic exercise | 40 (28:12) | 9.6 (0.5) | Response accuracy:0.94 | |
| Tantillo et al. (2002)66 | Case-control | Two exercise bouts (1st max. intensity, 2nd submax. intensity), one rest condition (silent cartoon); on consecutive days | 43 (21:22) | 10 (1.6) | Acoustic startle-eye-blink response latency:1.14 | |
| Verret et al. (2012)61 | Case-control |
Exercise group: 3 45-min sessions/week for 10weeks (moderate-to-vigorous aerobic, muscular and motor-skills exercise) Controls: no intervention |
21 (20:1) | 9.1 (1.1) | Attention problems:1.68 Auditory sustained attention:0.13 |
|
| Wigal et al. 200369 | Case-control | Two separate cycle ergometer sessions on different days within a week | 18 (18:0) | 8.5 (0.5) | Catecholamine response; data not available |
The effects of exercise in animal models of ADHD behaviours
The impact of voluntary exercise on attentional functioning in spontaneously hypertensive rats (SHR), an animal model of ADHD behaviours, has been investigated in three studies by the same research group. The first and second study explored exercise effects in adult51 and adolescent52 SHRs, respectively. The third study compared the effects of exercise on adult SHRs to the effects of the stimulant drugs methylphenidate (MPH) and atomoxetine (ATMX).53 In all studies, the rats in the exercise groups had free access to a shared running wheel for a few weeks prior to and throughout the study. The exercise group was compared to other SHRs with no access to a running wheel (non-exercising SHRs). Wistar-Kyoto rats (WKY) were used as an animal model of neurotypical behaviours as they are the most appropriate controls for rat models of ADHD behaviours.54 During the orienting procedure, all rats received repeated presentations of a visual stimulus (light). Hopkins et al.51 showed that non-exercising adult SHRs demonstrated more unconditioned orienting behaviour, as indicated by rearing up on the hind legs, than WKY. Exercise reduced orienting in female but not male adult SHRs.
Exercising adolescent SHRs, similar to exercising female adult SHRs, exhibited levels of unconditioned orienting behaviour resembling WKY.52 Non-exercising adolescent SHRs showed higher levels of unconditioned orienting behaviour. The authors propose that this indicates their difficulty ignoring irrelevant stimuli. Treatment with MPH (0.125 mg/kg), ATMX (0.125 mg/kg), or exercise also reduced orienting behaviour in female adult SHRs to the level observed in WKY rats, with exercise just as effective as MPH or ATMX. Taken together, these findings suggest that exercise has a greater effect in females and earlier on in development. Unfortunately, ES could not be established from the data available. These findings should be confirmed using quantifiable measures of exercise and a standardised measure of attention, such as a multiple-choice serial reaction time test.
The effects of involuntary exercise and MPH on activity levels and spatial learning memory in relation to dopamine synthesis and BDNF expression have also been explored in SHRs.55 Rats in the MPH group received 1 mg/kg MPH orally once a day for 28 days, while rats in the exercise group were made to run on a treadmill for 30 minutes per day 5 times a week for 28 days. Both MPH and exercise alleviated hyperactivity effectively (see Table 1 for ES) and improved spatial learning memory; an effect seemingly mediated by the augmentation of dopamine levels and BDNF expression. These animal studies suggest that exercise could have a positive impact on symptoms of ADHD, especially early in development, potentially mediated by effects on BDNF and CAs.
The effects of exercise on individuals diagnosed with ADHD
While evidence for effects of exercise on symptoms and impairments associated with ADHD is limited, interest in novel treatment approaches is growing. It is, thus, encouraging that both chronic and acute exercise have been linked to improvements in EF in school-aged children with ADHD. One study investigating the effects of acute exercise on EF reported that 30 minutes of moderate-intensity running facilitated performance on the colour-word condition of the Stroop task and certain aspects of the Wisconsin Card Sorting Test in 20 children with ADHD randomly assigned to the exercise group, relative to 20 children with ADHD assigned to the control condition of watching an exercise-related video.56 However, it should be noted that exercise did not have an effect on all measures of EF and that ES were small to moderate. Another study looking at the impact of acute exercise used a within-participant design to assess the effect of a 20-minute moderate-intensity bout of exercise in 20 children diagnosed with ADHD and 20 healthy-matched controls.57 Following exercise, both the ADHD and the control group exhibited greater response accuracy on a version of the Eriksen flanker task, measuring inhibitory control, relative to a seated reading condition. Acute exercise, thus, seems to have at least some effect on certain tasks assessing EF. The effect also seems to be universal and not specific to ADHD.
The impact of chronic exercise on EF has been assessed by measuring the amount of moderate- to high-intensity exercise performed by children with ADHD each day over a period of one week using an accelerometer.58 Exercise quantity significantly predicted performance on the Tower of London task, and was positively associated with working memory, inhibition and information processing in 18 boys with ADHD. Similarly, a 6-week prospective trial of 12 bi-weekly sessions demonstrated that EF, as measured by the digit symbol test, was significantly improved by exercise relative to behavioural educational sessions in 28 boys with ADHD.59 Teachers also noted significant improvements in the co-cooperativeness of boys in the exercise group, compared to boys who received behavioural education. While no significant changes were found with regards to hyperactivity scores, inattention scores improved in the exercise group.
A study examining the association between attention and chronic exercise in 84 individuals with ADHD employed a moderate-intensity 10-week exercise programme of three sessions per week, which included upper limb, lower limb, trunk and neck aerobic exercises, in addition to free running.64 Individuals were randomly assigned to two equal groups: the exercise group and the control group which did not receive an exercise intervention.60 In this study, exercise significantly improved teacher ratings of attention, motor skills and academic and classroom behaviour. Correspondingly, another study assessed the effects of a 10-week moderate-to-high-intensity exercise programme on fitness, cognitive functions and ADHD-related behaviour in 10 children with ADHD and compared the effects to a no-intervention control group consisting of 11 children with ADHD. This study reported significant improvements in muscular capacities, motor skills, level of information processing and parent- and teacher-rated social, thought and attention problems following the intervention.61 The effect of chronic exercise on behaviour ratings was further investigated in a non-randomised 5-week exercise programme in 19 children with ADHD.62 While no significant group differences were found between the exercise and no-exercise control group with regards to changes in parent-rated Conners’ Rating Scale (CRS) scores, total behaviour, attention, emotional and motor skills were rated as having improved for both groups after the intervention. These findings suggest non-specific treatment effects, conceivably due to rater expectation, rather than real effects of exercise. This last view is consistent with a recent meta-analysis on non-pharmacological interventions for ADHD, which found that the ES for non-pharmacological interventions depended on the rater’s blinding status.63 While some studies of the effect of chronic exercise on EF used unblinded ratings and must be interpreted with caution, others are more convincing due to their use of relatively blinded raters, such as teachers and the objective cognitive tests they employed.
Another study focused on the association of exercise with different aspects of mental health seen in children with ADHD. In a retrospective study, participation in three or more sports was reported to be significantly associated with a reduced number of anxiety and depressive symptoms and rates of co-occurring mood disorders in children with ADHD compared to control individuals with learning disabilities.64 Yet, parent report of the number of sports a child plays is an arbitrary measure, as children playing one sport might be more dedicated to that sport and not necessarily less fit than children participating in more than one sport. As association does not imply causation, the correlation between the number of sports played and anxiety and depression symptoms could alternatively reflect that children who are less anxious are more likely to be engage in a greater number of different sports, or that depressed children have less need for social interaction and, thus, participate in fewer sports. However, a study exploring a sport-based group therapy programme in boys with ADHD and boys with other behavioural disorders supported the finding that sports participation (20 90-minute sessions over one school year) improves anxiety and other behavioural scores.65 Yet, the intervention included group discussions as well as exercise, and it remains unclear how large the effect of each aspect of the intervention was on the outcome measure. Furthermore, the lack of a neurotypical control group does not allow for conclusions about specific effects of the intervention.
To elucidate the putative mechanism underlying the effects of exercise, three studies have investigated the relationship between CA levels and exercise in individuals with ADHD. Yet, findings are inconclusive and contradictory. Among the first to investigate the impact of exercise in individuals with ADHD, Tantillo et al.66 studied the rate of spontaneous eye blinks (SEB), the acoustic startle eye blink response (ASER) and motor impersistence, as non-invasive measures sensitive to dopamine agonists, in 8- to12-year-olds with and without ADHD. Although SEB and ASER had previously been used as sensitive measures of dopamine function in children with ADHD67,68, no clear link between ASER and ADHD has been established in humans. Both the ADHD and control group underwent two bouts of exercise and one rest condition on consecutive days. The main findings were increased SEB rate, decreased latency of ASER and improved motor persistence following exercise in boys with ADHD, whereas such improvements were not observed in controls. Although the authors concluded that exercise augments dopamine levels in children with ADHD but not in neurotypical controls, the alternative interpretation is that the physiological effect is consistent across groups but emerges as significant only in those showing pathological levels of ADHD symptoms. In a more direct investigation of CA levels in response to exercise, Wigal et al.69 compared ten children with combined subtype ADHD, to eight age and gender matched controls on their CA levels following two separate cycling ergometer sessions. CA levels were measured by a radio-enzymatic technique based on the conversion of the CA to radiolabeled metanephrine and normetanephrine. This CA assay uses an extraction technique that eliminates substances that may inhibit the radio-enzymatic assay and concentrates the CA to provide a more sensitive assay. Although epinephrine and norepinephrine levels rose in both groups following the exercise sessions, the response was less strong in children with ADHD and, in contrast to controls, their dopamine levels did not increase significantly, contrary to the previous study. The most recent study that examined the relationship between high-intensity exercise, CA levels and sustained attention in 25 children with ADHD demonstrated that exercise significantly improved response time and normalised impulsivity and vigilance measures, independent of CA.70 Participants, who were divided into stimulant medication users and non-users, were assessed on Conners’ Continuous Performance Test-II (CPT) at diagnosis, after exercise, and after a 1-minute stretching session (control measure). As chronic use of MPH was associated with significant physiological and attentional effects, a comparison of stimulant users and non-users on CPT performance suggested that the improvements in cognition seen following exercise were not CA dependent. Yet, CA levels were not measured directly and no straightforward conclusion can be drawn from these findings. Furthermore, practice effects may be expected since the CPT was repeated after only a 1-minute stretching session. Although these findings must be approached cautiously due to these and other crucial study design limitations outlined below, the research investigating the effect of exercise on ADHD provides preliminary evidence of the potential for exercise to reduce emotional, behavioural and neuropsychological problems seen in children with ADHD.
Overall, based on the limited body of research conducted to date, we can conclude that exercise emerges as a potentially promising intervention for improving EF and behavioural symptoms associated with ADHD. Yet, many of the findings remain inconclusive and warrant further investigation. ES varied from small for auditory sustained attention61 to large for response accuracy57 (see Table 1). Conflicting findings have also been presented for the putative mechanism involved. This may be explained by several limitations. Concluding causality from non-randomized, retrospective and cross-sectional data is problematic. Not all of the studies reviewed here are case-control studies and those that do look at case-control differences are limited by small sample sizes and inadequate control conditions. The unblind status of the researchers and scorers may further inflate the positive effects of exercise on the various outcome variables due to the non-specific effects evoked by a child’s participation in a treatment programme. Finally, the heterogeneous nature of and various treatment approaches to ADHD are seldom taken account, complicating the interpretation and generalisability of the results.
Conclusion
ADHD is best considered in a developmental framework. One neurodevelopmental model of ADHD views the disorder as resulting from early aberrations in brain development which remain relatively static throughout an individual’s life time.71 Yet, the plasticity of the brain and its interconnected neural circuits allow for recovery from ADHD symptoms and associated impairments over the course of development. Thus, experience-dependent neurodevelopmental processes, such as synaptic pruning or myelination, if facilitated early in childhood, could be used to aid the recovery from ADHD symptoms or mitigate symptomatic escalation over development. As exercise has been found to enhance neural growth and development, and improve cognitive and behavioural functioning in neurotypical individuals and animal studies, we reviewed the literature on the effects of exercise in children and adolescents with ADHD and animal models of ADHD behaviours.
A limited number of undersized non-randomized, retrospective and cross-sectional studies have investigated the impact of exercise on ADHD and the emotional, behavioural and neuropsychological problems associated with the disorder. The findings from these studies provide some support for the notion that exercise has the potential to act as a protective factor for ADHD. Further, better-quality evidence is however needed, to firmly establish the nature and extent of positive effects of exercise on children with ADHD. Researchers will need to examine both acute and chronic effects of exercise in large, adequately powered, blind sham-controlled randomised clinical trials. In addition, follow-up studies are needed to establish whether any short-term effects are followed by longer term benefits of both acute and chronic exercise. Although it remains unclear which role, if any, BDNF plays in the pathophysiology of ADHD, enhanced neural functioning has been suggested to be associated with the reduction of remission of ADHD symptoms.49,50,72 As exercise can elicit gene expression changes mediated by alterations in DNA methylation38, the possibility emerges that some of the positive effects of exercise could be caused by epigenetic mechanisms, which may set off a cascade of processes instigated by altered gene expression that could ultimately link to a change in brain function.
References
- 1.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 2.Wood A, Rijsdijk F, Johnson K, et al. The relationship between ADHD and key cognitive phenotypes is not mediated by shared familial effects with IQ. Psychological Medicine. 2011;41(4):861–71. doi: 10.1017/S003329171000108X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuntsi J, Wood AC, Rijsdijk F, et al. Separation of cognitive impairments in attention-deficit/hyperactivity disorder into 2 familial factors. Archives of General Psychiatry. 2010;67(11):1159–67. doi: 10.1001/archgenpsychiatry.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuntsi J, Eley TC, Taylor A, et al. Co-occurrence of ADHD and low IQ has genetic origins. American Journal of Medical Genetics. 2004;124B(1):41–47. doi: 10.1002/ajmg.b.20076. [DOI] [PubMed] [Google Scholar]
- 5.Burt SA. Rethinking Environmental Contributions to Child and Adolescent Psychopathology: A Meta-Analysis of Shared Environmental Influences. Psychological Bulletin. 2009;135(4):608–637. doi: 10.1037/a0015702. [DOI] [PubMed] [Google Scholar]
- 6.Wood AC, Buitelaar J, Rijsdijk F, Asherson P, Kuntsi J. Rethinking shared environment as a source of variance underlying attention-deficit/hyperactivity disorder symptoms: Comment on Burt (2009) Psychological Bulletin. 2010;136(3):331–340. doi: 10.1037/a0019048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thapar A, Cooper M, Jefferies R, Stergiakouli E. What causes attention-deficit/hyperactivity disorder? Archives of Disease in Childhood. 2012;97(3):260–5. doi: 10.1136/archdischild-2011-300482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutter M. Nature, Nurture, and Development: From Evangelism through Science toward Policy and Practice. Child Development. 2002;73(1):1–21. doi: 10.1111/1467-8624.00388. [DOI] [PubMed] [Google Scholar]
- 9.Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychological Bulletin. 2009;135(6):885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- 10.Cortese S. The neurobiology and genetics of Attention-Deficit/Hyperactivity Disorder (ADHD): what every clinician should know. European Journal of Paediatric Neurology. 2012;16(5):422–33. doi: 10.1016/j.ejpn.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2011;69(12):e145–57. doi: 10.1016/j.biopsych.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 12.Minzenberg MJ. Pharmacotherapy for attention-deficit/hyperactivity disorder: from cells to circuits. Neurotherapeutics. 2012;9(3):610–21. doi: 10.1007/s13311-012-0128-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volkow ND, Wang G-J, Kollins SH, et al. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA. 2009;302(10):1084–91. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castellanos FX. Toward a pathophysiology of attention-deficit/hyperactivity disorder. Clinical Pediatrics. 1997;36(7):381–93. doi: 10.1177/000992289703600702. [DOI] [PubMed] [Google Scholar]
- 15.Faraone SV, Perlis RH, Doyle AE, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57(11):1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 16.Lanktree M, Squassina A, Krinsky M, et al. Association study of brain-derived neurotrophic factor (BDNF) and LIN-7 homolog (LIN-7) genes with adult attention-deficit/hyperactivity disorder. American Journal of Medical Genetics. 2008;147B(6):945–51. doi: 10.1002/ajmg.b.30723. [DOI] [PubMed] [Google Scholar]
- 17.Xu X, Mill J, Zhou K, Brookes K, Chen C-K, Asherson P. Family-based association study between brain-derived neurotrophic factor gene polymorphisms and attention-deficit/hyperactivity disorder in UK and Taiwanese samples. American Journal of Medical Genetics. 2007;144B(1):83–6. doi: 10.1002/ajmg.b.30406. [DOI] [PubMed] [Google Scholar]
- 18.Tsai S-J. Attention-deficit/hyperactivity disorder may be associated with decreased central brain-derived neurotrophic factor activity: clinical and therapeutic implications. Medical Hypotheses. 2007;68(4):896–9. doi: 10.1016/j.mehy.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 19.Bergman O, Westberg L, Lichtenstein P, Eriksson E, Larsson H. Study on the possible association of brain-derived neurotrophic factor polymorphism with the developmental course of symptoms of attention deficit and hyperactivity. International Journal of Neuropsychopharmacology. 2011;14(10):1367–76. doi: 10.1017/S1461145711000502. [DOI] [PubMed] [Google Scholar]
- 20.Sánchez-Mora C, Ribasés M, Ramos-Quiroga J a, et al. Meta-analysis of brain-derived neurotrophic factor p.Val66Met in adult ADHD in four European populations. American Journal of Medical Genetics. 2010;153B(2):512–23. doi: 10.1002/ajmg.b.31008. [DOI] [PubMed] [Google Scholar]
- 21.Brookes K, Xu X, Chen W, et al. The analysis of 51 genes in DSM-IV combined type attention-deficit/hyperactivity disorder: association signals in DRD4, DAT1 and 16 other genes. Molecular Psychiatry. 2006;11(10):934–53. doi: 10.1038/sj.mp.4001869. [DOI] [PubMed] [Google Scholar]
- 22.Wakefield JC. The concept of mental disorder: diagnostic implications of the harmful dysfunction analysis. World Psychiatry. 2007;6:149–156. [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J, Laurin N, Crosbie J, et al. Association study of the BDNF gene in attention-deficit/hyperactivity disorder. American Journal of Medical Genetics. 2007;144B(8):976–81. doi: 10.1002/ajmg.b.30437. [DOI] [PubMed] [Google Scholar]
- 24.Van Praag H. Neurogenesis and exercise: past and future directions. Neuromolecular Medicine. 2008;10(2):128–40. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- 25.Endres M, Gertz K, Lindauer U, et al. Mechanisms of stroke protection by physical activity. Annals of Neurology. 2003;54(5):582–90. doi: 10.1002/ana.10722. [DOI] [PubMed] [Google Scholar]
- 26.Vaynman S, Ying Z, Gomez-Pinilla F. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience. 2003;122(3):647–57. doi: 10.1016/j.neuroscience.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Fulk LJ, Stock HS, Lynn A, Marshall J, Wilson MA, Hand GA. Chronic physical exercise reduces anxiety-like behavior in rats. International Journal of Sports Medicine. 2004;25(1):78–82. doi: 10.1055/s-2003-45235. [DOI] [PubMed] [Google Scholar]
- 28.Traver S, Marien M, Martin E, Hirsch EC, Michel PP. The phenotypic differentiation of locus ceruleus noradrenergic neurons mediated by brain-derived neurotrophic factor is enhanced by corticotropin releasing factor through the activation of a cAMP-dependent signaling pathway. Molecular Pharmacology. 2006;70(1):30–40. doi: 10.1124/mol.106.022715. [DOI] [PubMed] [Google Scholar]
- 29.Hyman C, Hofer M, Barde YA, et al. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350(6315):230–2. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- 30.Rasmussen P, Brassard P, Adser H, et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Experimental Physiology. 2009;94(10):1062–9. doi: 10.1113/expphysiol.2009.048512. [DOI] [PubMed] [Google Scholar]
- 31.Seifert T, Brassard P, Wissenberg M, et al. Endurance training enhances BDNF release from the human brain. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2010;298(2):R372–7. doi: 10.1152/ajpregu.00525.2009. [DOI] [PubMed] [Google Scholar]
- 32.Tang SW, Chu E, Hui T, Helmeste D, Law C. Influence of exercise on serum brain-derived neurotrophic factor concentrations in healthy human subjects. Neuroscience Letters. 2008;431(1):62–5. doi: 10.1016/j.neulet.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 33.Ströhle A, Stoy M, Graetz B, et al. Acute exercise ameliorates reduced brain-derived neurotrophic factor in patients with panic disorder. Psychoneuroendocrinology. 2010;35(3):364–8. doi: 10.1016/j.psyneuen.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 34.Ferris LT, Williams JS, Shen C-L. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Medicine and Science in Sports and Exercise. 2007;39(4):728–34. doi: 10.1249/mss.0b013e31802f04c7. [DOI] [PubMed] [Google Scholar]
- 35.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. The European Journal of Neuroscience. 2004;20(10):2580–90. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 36.Henikoff S, Matzke MA. Exploring and explaining epigenetic effects. Trends in Genetics. 1997;13(8):293–5. doi: 10.1016/s0168-9525(97)01219-5. [DOI] [PubMed] [Google Scholar]
- 37.Cortessis VK, Thomas DC, Levine AJ, et al. Environmental epigenetics: prospects for studying epigenetic mediation of exposure-response relationships. Human Genetics. 2012;131(10):1565–89. doi: 10.1007/s00439-012-1189-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barrès R, Yan J, Egan B, et al. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metabolism. 2012;15(3):405–11. doi: 10.1016/j.cmet.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Bryan AD, Magnan RE, Hooper AEC, Harlaar N, Hutchison KE. Physical Activity and Differential Methylation of Breast Cancer Genes Assayed from Saliva: A Preliminary Investigation. Annals of Behavioral Medicine. 2012;45(1):89. doi: 10.1007/s12160-012-9411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nature reviews. Neuroscience. 2008;9(1):58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 41.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychological Science. 2003;14(2):125–30. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 42.Peyrin L, Pequignot JM, Lacour JR, Fourcade J. Relationships between catecholamine or 3-methoxy 4-hydroxy phenylglycol changes and the mental performance under submaximal exercise in man. Psychopharmacology. 1987;93(2) doi: 10.1007/BF00179932. [DOI] [PubMed] [Google Scholar]
- 43.Querido JS, Sheel AW. Regulation of cerebral blood flow during exercise. Sports Medicine. 2007;37(9):765–82. doi: 10.2165/00007256-200737090-00002. [DOI] [PubMed] [Google Scholar]
- 44.Colcombe SJ, Kramer AF, Erickson KI, et al. Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(9):3316–21. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sibley BA, Etnier JL. The Relationship Between Physical Activity and Cognition in Children: A Meta-Analysis. Pediatric Exercise Science. 2003;15:243–256. [Google Scholar]
- 46.Tomporowski PD, Davis CL, Miller PH, Naglieri JA. Exercise and Children’s Intelligen,Cognition and Academic Achievement. Educational Psychology Review. 2008;20(2):111–131. doi: 10.1007/s10648-007-9057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Best JR. Effects of physical activity on children’s executive function: Contributions of experimental research on aerobic exercise. Developmental Review. 2010;30(4):331–351. doi: 10.1016/j.dr.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biological Psychiatry. 2005;57(11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 49.Bédard A-CV, Trampush JW, Newcorn JH, Halperin JM. Perceptual and motor inhibition in adolescents/young adults with childhood-diagnosed ADHD. Neuropsychology. 2010;24(4):424–34. doi: 10.1037/a0018752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shaw P, Lerch J, Greenstein D, et al. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2006;63(5):540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- 51.Hopkins ME, Sharma M, Evans GC, Bucci DJ. Voluntary physical exercise alters attentional orienting and social behavior in a rat model of attention-deficit/hyperactivity disorder. Behavioral Neuroscience. 2009;123(3):599–606. doi: 10.1037/a0015632. [DOI] [PubMed] [Google Scholar]
- 52.Robinson AM, Hopkins ME, Bucci DJ. Effects of physical exercise on ADHD-like behavior in male and female adolescent spontaneously hypertensive rats. Developmental Psychobiology. 2011;53(4):383–90. doi: 10.1002/dev.20530. [DOI] [PubMed] [Google Scholar]
- 53.Robinson AM, Eggleston RL, Bucci DJ. Physical exercise and catecholamine reuptake inhibitors affect orienting behavior and social interaction in a rat model of attention-deficit/hyperactivity disorder. Behavioral Neuroscience. 2012;126(6):762–71. doi: 10.1037/a0030488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sagvolden T, Johansen EB. Rat models of ADHD. Current Topics in Behavioral Neurosciences. 2012;9:301–15. doi: 10.1007/7854_2011_126. [DOI] [PubMed] [Google Scholar]
- 55.Kim H, Heo H-I, Kim D-H, et al. Treadmill exercise and methylphenidate ameliorate symptoms of attention-deficit/hyperactivity disorder through enhancing dopamine synthesis and brain-derived neurotrophic factor expression in spontaneous hypertensive rats. Neuroscience Letters. 2011;504(1):35–9. doi: 10.1016/j.neulet.2011.08.052. [DOI] [PubMed] [Google Scholar]
- 56.Chang Y-K, Liu S, Yu H-H, Lee Y-H. Effect of acute exercise on executive function in children with attention-deficit/hyperactivity disorder. Archives of Clinical Neuropsychology. 2012;27(2):225–37. doi: 10.1093/arclin/acr094. [DOI] [PubMed] [Google Scholar]
- 57.Pontifex MB, Saliba BJ, Raine LB, Picchietti DL, Hillman CH. Exercise Improves Behavioral, Neurocognitive, and Scholastic Performance in Children with Attention-Deficit/Hyperactivity Disorder. The Journal of Pediatrics. 2012;162(3):543–551. doi: 10.1016/j.jpeds.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gapin J, Etnier JL. The relationship between physical activity and executive function performance in children with attention-deficit/hyperactivity disorder. Journal of Sport and Exercise Psychology. 2010;32(6):753–63. doi: 10.1123/jsep.32.6.753. [DOI] [PubMed] [Google Scholar]
- 59.Kang KD, Choi JW, Kang SG, Han DH. Sports therapy for attention, cognitions and sociality. International Journal of Sports Medicine. 2011;32(12):953–9. doi: 10.1055/s-0031-1283175. [DOI] [PubMed] [Google Scholar]
- 60.Ahmed G, Mohamed S. Effect of Regular Aerobic Exercise on Behavioural, Cognitive and Psychological Response in Patients with Attention-Deficit/Hyperactivity Disorder. Life Science Journal. 2011;8(2):366–371. [Google Scholar]
- 61.Verret C, Guay M-C, Berthiaume C, Gardiner P, Béliveau L. A physical activity program improves behavior and cognitive functions in children with ADHD: an exploratory study. Journal of Attention Disorders. 2012;16(1):71–80. doi: 10.1177/1087054710379735. [DOI] [PubMed] [Google Scholar]
- 62.McKune AJ, Pautz J, Lombard J. Behavioural response to exercise in children with attention-deficit/hyperactivity disorder. Sports Medicine. 2003;15(3):17–21. [Google Scholar]
- 63.Sonuga-Barke EJS, Brandeis D, Cortese S, et al. Nonpharmacological Interventions for ADHD: Systematic Review and Meta-Analyses of Randomized Controlled Trials of Dietary and Psychological Treatments. The American Journal of Psychiatry. 2013;170(3):275–89. doi: 10.1176/appi.ajp.2012.12070991. [DOI] [PubMed] [Google Scholar]
- 64.Kiluk BD, Weden S, Culotta VP. Sport participation and anxiety in children with ADHD. Journal of Attention Disorders. 2009;12(6):499–506. doi: 10.1177/1087054708320400. [DOI] [PubMed] [Google Scholar]
- 65.Lufi D, Parish-Plass J. Sport-Based Group Therapy Program for Boys with ADHD or with Other Behavioral Disorders. Child and Family Behavior Therapy. 2011;33(3):217–230. [Google Scholar]
- 66.Tantillo M, Kesick CM, Hynd GW, Dishman RK. The effects of exercise on children with attention-deficit/hyperactivity disorder. Medicine and Science in Sports and Exercise. 2002;34(2):203–212. doi: 10.1097/00005768-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 67.Caplan R, Guthrie D, Komo S. Blink rate in children with attention-deficit/hyperactivity disorder. Biological Psychiatry. 1996;39(12):1032–8. doi: 10.1016/0006-3223(95)00315-0. [DOI] [PubMed] [Google Scholar]
- 68.Goldstein DJ, Blumenthal TD. Startle eyeblink elicitation in attention-deficit-disordered children using low-intensity acoustic stimuli. Perceptual and Motor Skills. 1995;80(1):227–31. doi: 10.2466/pms.1995.80.1.227. [DOI] [PubMed] [Google Scholar]
- 69.Wigal SB, Nemet D, Swanson JM, et al. Catecholamine response to exercise in children with attention-deficit/hyperactivity disorder. Pediatric Research. 2003;53(5):756–61. doi: 10.1203/01.PDR.0000061750.71168.23. [DOI] [PubMed] [Google Scholar]
- 70.Medina J, Netto TLB, Muszkat M, et al. Exercise impact on sustained attention of ADHD children, methylphenidate effects. Attention Deficit and Hyperactivity Disorders. 2010;2(1):49–58. doi: 10.1007/s12402-009-0018-y. [DOI] [PubMed] [Google Scholar]
- 71.Halperin JM, Schulz KP. Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychological Bulletin. 2006;132(4):560–581. doi: 10.1037/0033-2909.132.4.560. [DOI] [PubMed] [Google Scholar]
- 72.Schulz KP, Newcorn JH, Fan J, Tang CY, Halperin JM. Brain activation gradients in ventrolateral prefrontal cortex related to persistence of ADHD in adolescent boys. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(1):47–54. doi: 10.1097/01.chi.0000145551.26813.f9. [DOI] [PubMed] [Google Scholar]
- 73.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. British Medical Journal. 2009;339(1):b2535–b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]