Abstract
Isolated spinach chloroplasts purified by isopycnic centrifugation in density gradients of Percoll were found to be highly intact, to be devoid of extrachloroplastic contaminations, and to retain a high rate of CO2-dependent O2 evolution.
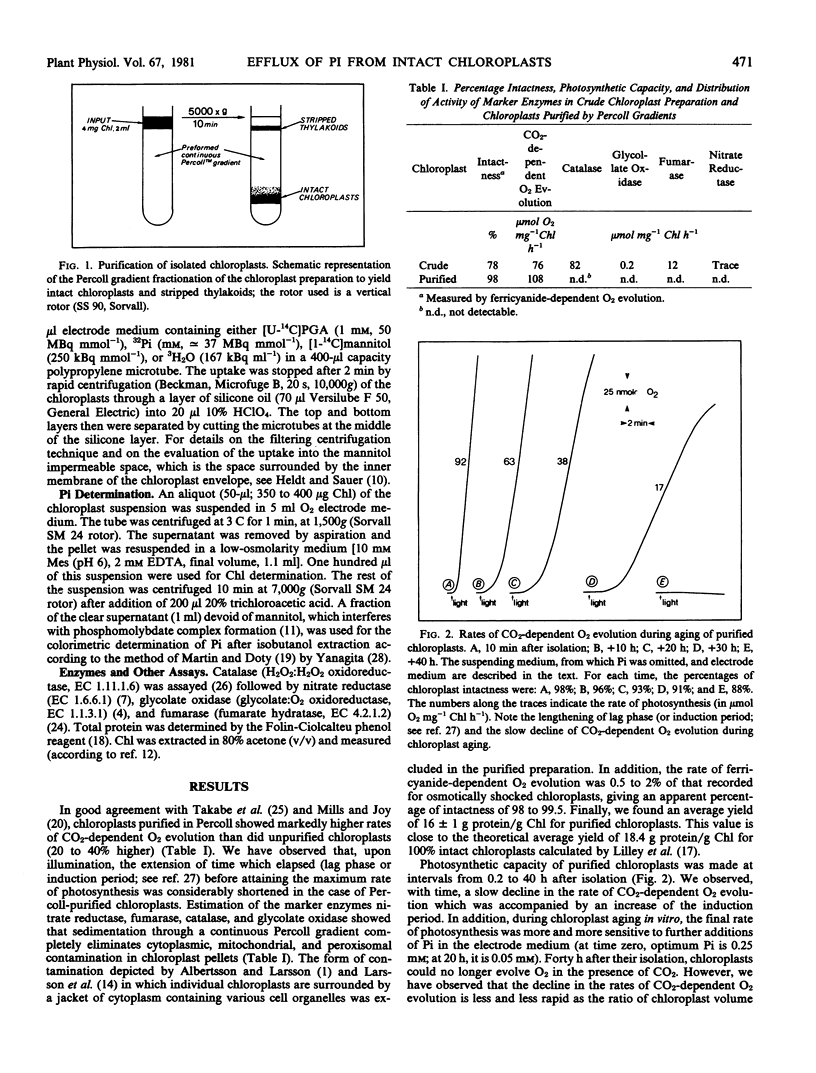
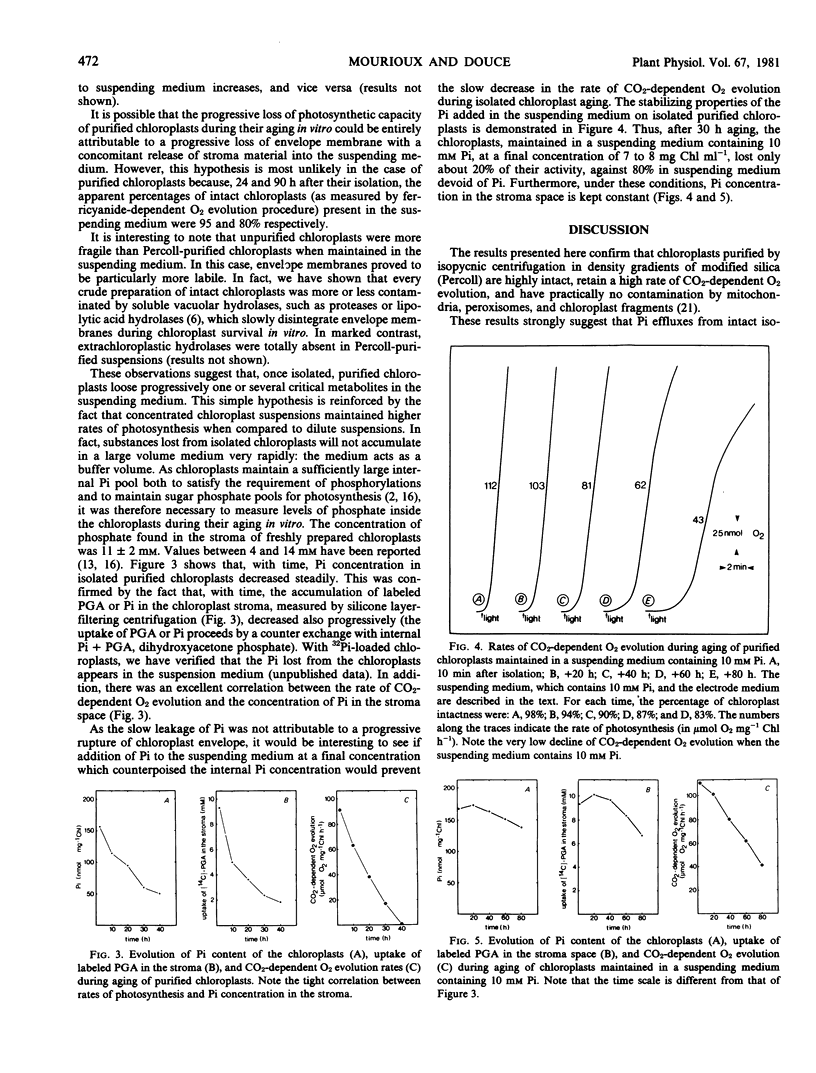
When suspended in a medium which avoided rupture of the envelope, intact purified chloroplasts progressively lost their phosphate content by passive diffusion. This led to a slow decrease in the uptake of labeled 3-phosphoglyceric acid or orthophosphate (Pi) and in the rate of CO2-dependent O2 evolution by isolated chloroplasts. Under these conditions, there was a good correlation between the rate of CO2-dependent O2 evolution and the concentration of Pi in the stroma space. Addition of Pi to the suspending medium at a final concentration of 10 millimolar, which counterpoised the slow efflux of Pi from the chloroplasts, slowed considerably the decrease in the rate of CO2-dependent O2 evolution.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertsson P. A., Larsson C. Properties of chloroplasts isolated by phase partition. Mol Cell Biochem. 1976 Jun 15;11(3):183–189. doi: 10.1007/BF01744998. [DOI] [PubMed] [Google Scholar]
- Cockburn W., Baldry C. W., Walker D. A. Some effects of inorganic phosphate on O2 evolution by isolated chloroplasts. Biochim Biophys Acta. 1967;143(3):614–624. doi: 10.1016/0005-2728(67)90067-9. [DOI] [PubMed] [Google Scholar]
- Douce R., Moore A. L., Neuburger M. Isolation and oxidative properties of intact mitochondria isolated from spinach leaves. Plant Physiol. 1977 Oct;60(4):625–628. doi: 10.1104/pp.60.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliege R., Flügge U. I., Werdan K., Heldt H. W. Specific transport of inorganic phosphate, 3-phosphoglycerate and triosephosphates across the inner membrane of the envelope in spinach chloroplasts. Biochim Biophys Acta. 1978 May 10;502(2):232–247. doi: 10.1016/0005-2728(78)90045-2. [DOI] [PubMed] [Google Scholar]
- Heber U., Santarius K. A. Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B. 1970 Jul;25(7):718–728. doi: 10.1515/znb-1970-0714. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Sauer F. The inner membrane of the chloroplast envelope as the site of specific metabolite transport. Biochim Biophys Acta. 1971 Apr 6;234(1):83–91. doi: 10.1016/0005-2728(71)90133-2. [DOI] [PubMed] [Google Scholar]
- Jeffrey S. W., Douce R., Benson A. A. Carotenoid transformations in the chloroplast envelope. Proc Natl Acad Sci U S A. 1974 Mar;71(3):807–810. doi: 10.1073/pnas.71.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser W., Urbach W. The effect of dihydroxyacetone phosphate and 3-phosphoglycerate on O2 evolution and on the levels of ATP, ADP and Pi in isolated intact chloroplasts. Biochim Biophys Acta. 1977 Mar 11;459(3):337–346. doi: 10.1016/0005-2728(77)90035-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehner K., Heldt H. W. Dicarboxylate transport across the inner membrane of the chloroplast envelope. Biochim Biophys Acta. 1978 Mar 13;501(3):531–544. doi: 10.1016/0005-2728(78)90119-6. [DOI] [PubMed] [Google Scholar]
- Lilley R. M., Chon C. J., Mosbach A., Heldt H. W. The distribution of metabolites between spinach chloroplasts and medium during photosynthesis in vitro. Biochim Biophys Acta. 1977 May 11;460(2):259–272. doi: 10.1016/0005-2728(77)90212-2. [DOI] [PubMed] [Google Scholar]
- Morgenthaler J. J., Price C. A. Photosynthetic activity of spinach chloroplasts after isopycnic centrifugation in gradients of silica. Plant Physiol. 1974 Oct;54(4):532–534. doi: 10.1104/pp.54.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani H. Y., Barber J. An improved method for isolating chloroplasts retaining their outer membranes. Biochim Biophys Acta. 1977 Sep 14;461(3):500–512. [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- YANAGITA T. SUCCESSIVE DETERMINATIONS OF THE FREE, ACID-LABILE AND RESIDUAL PHOSPHATES IN BIOLOGICAL SYSTEMS. J Biochem. 1964 Mar;55:260–268. doi: 10.1093/oxfordjournals.jbchem.a127879. [DOI] [PubMed] [Google Scholar]



