Abstract
Using data from a national wound-specific electronic medical record (WoundExpert, Net Health, Pittsburgh, PA), we compared the effectiveness of a bilayered living cellular construct (BLCC) and an acellular porcine small intestine submucosa collagen dressing (SIS) for the treatment of venous leg ulcer. Data from 1,489 patients with 1,801 refractory venous leg ulcers (as defined by failure to have >40% reduction in size in the 4 weeks prior to treatment) with surface areas between 1 and 150 cm2 in size, treated between July 2009 and July 2012 at 158 wound care facilities across the US were analyzed. Patient baseline demographics and wound characteristics were comparable between groups. Kaplan-Meier–derived estimates of wound closure for BLCC (1,451 wounds) was significantly greater (p = 0.01, log-rank test) by weeks 12 (31% vs. 26%), 24 (50% vs. 41%), and 36 (61% vs. 46%), respectively, compared with SIS (350 wounds). BLCC treatment reduced the median time to wound closure by 44%, achieving healing 19 weeks sooner (24 vs. 43 weeks, p = 0.01, log-rank test). Treatment with BLCC increased the probability of healing by 29% compared with porcine SIS dressing (hazard ratio = 1.29 [95% confidence interval 1.06, 1.56], p = 0.01).
Venous leg ulcers (VLUs) are the most common type of chronic leg wound. These often painful wounds represent the majority of lower extremity ulcerations1–4 and have a lifetime prevalence that has been estimated at 1% of adults,5–8 with rates even higher among the elderly (1.69% prevalence, annually).9 VLUs also pose a significant financial burden to US payers, at an estimated annual direct medical cost of up to $18bn.10
In addition to local wound care and infection control, the standard treatment for VLUs is compression therapy. However, with standard care alone, research has shown that up to 75% of patients fail to achieve healing in a timely fashion.11 For these hard-to-heal VLUs, treatment algorithms have recommended the use of evidence-based advanced technologies to modulate and accelerate the healing process.12–14
A bilayered living cellular construct (BLCC; Apligraf, Organogenesis Inc., Canton, MA) is at present the only product approved by the FDA for the treatment of VLUs. BLCC is comprised of human neonatal keratinocytes and fibroblasts in an extracellular matrix (bovine collagen and other extracellular matrix proteins) and has been in clinical use for the treatment of VLUs since 1998. In order to gain FDA approval for the treatment of VLUs, BLCC was evaluated in a pivotal trial where it showed that when used in conjunction with compression therapy, BLCC significantly increased the percentage of patients healed by 6 months and reduced the median time to wound closure.15
Porcine small intestine submucosa (SIS; Oasis, Healthpoint, Fort Worth, TX) is an acellular wound dressing that has been cleared by the FDA for the management of VLUs. The efficacy of SIS has been evaluated in a leg ulcer clinical trial, where application of SIS with compression therapy was found to significantly increase the percent of wounds healed by 3 months compared with compression therapy alone.16
Although the efficacy of these products has been evaluated in randomized controlled clinical trials, the comparative effectiveness for treating VLUs in real-world settings has not been investigated. Although the 2009 Affordable Care Act has provided $1.1bn for comparative effectiveness research (CER), few comparative effectiveness studies have ever been performed in the wound care field.17,18 Effective treatment is the extent to which an intervention produces its intended effect in routine care conditions (real-world situations), whereas efficacious treatment provides positive results in controlled, highly constrained conditions that are optimal for obtaining favorable results in randomized controlled trials (RCTs).18,19 Thus, it is important to determine if efficacy can be translated to routine practice settings (effectiveness) to support evidence-based practice.
The purpose of this study was to investigate the comparative effectiveness of BLCC to SIS for the treatment of VLUs in real-world settings using data over a 3-year period from a large wound care–specific electronic medical record (EMR) database (WoundExpert, Net Health, Pittsburgh, PA) that is utilized by approximately 20,000 physicians in over 1,000 wound care facilities across the US.
Methods
Study design
This study is a retrospective analysis to compare the effectiveness of BLCC and SIS for the treatment of VLUs using de-identified EMRs from wound care facilities across the US in a 3-year period. The primary analyses were frequency of wound closure evaluated up to 36 months and median time to wound closure. Wound areas (cm2) were calculated from wound measurements of length and width. The final visit denoting VLU closure was not always recorded; thus, wound closure was defined as an ulcer achieving an area between 0 and 0.25 cm2.
Patients
Patients eligible for inclusion were those documented as receiving at least one treatment of either BLCC or SIS on a venous ulcer (partial or full thickness) with the location coded as ankle, lower leg, shin, pretibial, or calf. Included baseline wound areas were 1–150 cm2 with an ulcer duration of longer than 1 month prior to first treatment with BLCC or SIS. To assure analyses were restricted to refractory VLUs, wounds needed to have closed no more than 40% within the 4 weeks prior to first treatment with BLCC or SIS.
Wounds without baseline or follow-up area measurements were excluded as well as those where the date of BLCC or SIS treatment was unknown. Wounds were also excluded if they received either SIS or human fibroblast-derived dermal substitute (HFDS; Dermagraft, Shire Regenerative Medicine, San Diego, CA) on or within 28 days of the first treatment with BLCC or, alternatively, if they received BLCC or HFDS on or within 28 days of the first treatment with SIS. Censoring occurred for nonhealed wounds at their last visit with an area measurement. Patients were also censored at the visit where an alternate product was applied (either BLCC, SIS, or HFDS). Wounds treated with the same product >183 days after the prior application were censored at the visit where the subsequent application occurred. Other concurrent treatments such as hyperbaric oxygen (HBO2) or negative pressure wound therapy (NPWT) were allowed.
Data collection
Data were obtained from the WoundExpert EMR, which was de-identified consistent with the terms and conditions of the Health Insurance Portability and Accountability Act of 1996 (HIPAA).
Net Health provided all treatment records for any patient receiving at least one application of BLCC or SIS between July 2009 and July 2012 from the 158 centers with contracted agreements for the transfer of de-identified data for research purposes.
Treatment records included patient baseline demographics including age (years, ≤89 per HIPAA), gender, race, wound location, wound size and duration, and wound-specific information recorded at each visit including area measurements and treatments.
Statistical analysis
Descriptive data are expressed as mean (standard deviation) and median for continuous variables and n (%) for categorical variables. The level of p < 0.05 was established for the purpose of defining statistical significance. Baseline characteristics were compared using two-sample t tests and Fisher's exact two-tailed tests. The primary analyses comparing incidence of and median time to wound closure were determined by Kaplan-Meier analysis with two-tailed log-rank test. The last observation was carried forward for missing data. The hazard ratio along with its 95% confidence interval (CI) and p-value is based on a Cox proportional hazards regression model with one term for treatment group.
Results
The patient screening flow chart is shown in Figure 1. In 158 centers over a 3-year period, a total of 3702 VLUs (2,428 patients) received BLCC treatment, and 1,143 VLUs (838 patients) received SIS treatment. From these, 1,451 wounds (1,187 patients) and 350 wounds (302 patients) treated with BLCC and SIS respectively met the eligibility requirements for inclusion in the analysis.
Figure 1.
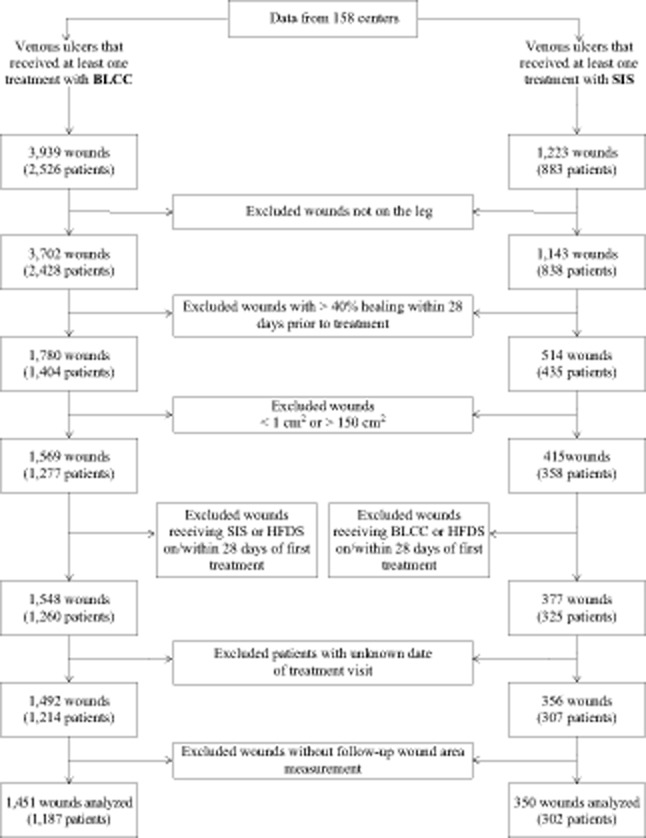
Patient and wound data screening for entry into analyses. Bilayered living cellular construct (BLCC), Apligraf; small intestine submucosa (SIS), Oasis; human fibroblast-derived dermal substitute (HFDS), Dermagraft.
There were no significant differences in baseline patient demographics and wound characteristics between the two treatment groups, except for number of wounds per patient (Tables 1 and 2). The majority of patients was women (54.9%), Caucasian (73.1%), and the median age was 71 years. At the first treatment application, the median wound area was 7.5 cm2 in the BLCC group and 6.0 cm2 in the SIS group, with the majority of ulcers being full thickness (87.7%). The median wound duration was 5.3 and 7.1 months in the BLCC and SIS groups, respectively.
Table 1.
Baseline patient characteristics
| Patient characteristic | BLCC treatment group (n = 1,187) | SIS treatment group (n = 302) | p-Value* |
|---|---|---|---|
| Age (years), n | 1,185 | 300 | 0.655 |
| Mean ± SD | 69.5 ± 13.9 | 69.1 ± 14.4 | |
| Median | 71.0 | 71.0 | |
| Gender, n (%) | 0.217 | ||
| Male | 551 (47.2) | 129 (43.1) | |
| Female | 616 (52.8) | 170 (56.9) | |
| Race, n (%) | 0.501 | ||
| Caucasian | 571 (73.7) | 148 (72.5) | |
| Black | 103 (13.3) | 33 (16.2) | |
| Other | 101 (13.0) | 23 (11.3) | |
| BMI (kg/m2), n | 859 | 209 | 0.460 |
| Mean ± SD | 32.8 ± 11.1 | 32.1 ± 11.0 | |
| Median | 30.5 | 29.8 | |
| Number of wounds per patient, n | 1,187 | 302 | 0.041 |
| Mean ± SD | 1.22 ± 0.6 | 1.16 ± 0.5 | |
| Median | 1.0 | 1.0 | |
| Single/multiple wounds per patient, n (%) | 0.189 | ||
| Single wound | 988 (83.2) | 261 (86.4) | |
| Multiple wounds | 199 (16.8) | 41 (13.6) |
For BMI, the p-value is from the Wilcoxon rank-sum test testing for a difference in distribution between treatments. For other categorical variables, the p-value is from a two-tailed Fisher's exact test testing for a difference in proportions between treatments. For continuous variables, the p-value is from a two-tailed, two-sample t test, testing for a difference in means between treatments.
BLCC, Apligraf; SIS, Oasis. BLCC, bilayered living cellular construct; BMI, body mass index; SD, standard deviation; SIS, small intestine submucosa.
Table 2.
Baseline wound characteristics
| Wound characteristic | BLCC treatment group (n = 1,187) | SIS treatment group (n = 302) | p-Value* |
|---|---|---|---|
| Wound area (cm2), n | 1,451 | 350 | 0.590 |
| Mean ± SD | 16.2 ± 22.1 | 15.5 ± 23.6 | |
| Median | 7.5 | 6.0 | |
| Full/partial thickness, n (%) | 0.638 | ||
| Full thickness | 1,172 (88.2) | 292 (87.2) | |
| Partial thickness | 157 (11.8) | 43 (12.8) | |
| Wound duration (months)†, n | 1,091 | 262 | 0.355 |
| Mean ± SD | 17.0 ± 56.6 | 20.1 ± 48.5 | |
| Median | 5.3 | 7.1 | |
| Wound location, n (%) | 0.479 | ||
| Ankle | 386 (26.6) | 101 (28.9) | |
| Lower leg | 957 (66.0) | 231 (66.0) | |
| Shin | 8 (0.6) | 0 (0.0) | |
| Calf | 68 (4.7) | 11 (3.1) | |
| Pretibial | 32 (2.2) | 7 (2.0) | |
| Lateral/medial, n (%) | 0.165 | ||
| Lateral | 471 (47.5) | 98 (42.2) | |
| Medial | 520 (52.5) | 134 (57.8) |
For categorical variables, the p-value is from a two-tailed Fisher's exact test testing for a difference in proportions between treatments. For continuous variables, the p-value is from a two-tailed, two sample t test, testing for a difference in means between treatments.
Wound duration was reported in days and was converted to months according to the following: 30 days = 1 month.
BLCC, Apligraf; SIS, Oasis. BLCC, bilayered living cellular construct; SD, standard deviation; SIS, small intestine submucosa.
As shown in Table 3, the average number of treatment applications received by patients in the BLCC group was significantly lower than SIS-treated patients (p < 0.0001). A significantly higher percentage of BLCC-treated patients received a single application compared with SIS (p < 0.0001). For patients receiving multiple applications, the median interval between applications was significantly longer in the BLCC group (p < 0.0001).
Table 3.
Treatment characteristics
| Treatment characteristic | BLCC treatment group (n = 1,187) | SIS treatment group (n = 302) | p-Value* |
|---|---|---|---|
| Number of treatment applications, n | 1,451 | 350 | <0.0001 |
| Mean ± SD | 2.3 ± 1.5 | 3.8 ± 3.2 | |
| Median | 2.0 | 3.0 | |
| Single/multiple applications, n (% patients) | <0.0001 | ||
| Single | 582 (40.1) | 100 (28.6) | |
| Multiple | 869 (59.9) | 250 (71.4) | |
| Interval between applications (days), n | 869 | 250 | <0.0001 |
| Mean ± SD | 31.7 ± 24.1 | 12.6 ± 12.3 | |
| Median | 24.5 | 8.5 | |
| Number of debridements at or within 28 days prior to day 0†, n | 0.846 | ||
| 1,155 | 236 | ||
| Mean ± SD | 2.6 ± 1.3 | 2.6 ± 1.5 | |
| Median | 3.0 | 3.0 | |
| Other treatments, n (% patients) | |||
| HBO2 | |||
| Day −28 to <day 0† | 28 (1.9%) | 5 (1.4%) | 0.660 |
| Day 0 to last follow-up visit | 33 (2.3%) | 8 (2.3%) | 1.000 |
| NPWT | |||
| Day −28 to <day 0 | 25 (1.7%) | 5 (1.4%) | 0.820 |
| Day 0 to last follow-up visit | 30 (2.1%) | 8 (2.3%) | 0.836 |
For categorical variables, the p-value is from a two-tailed Fisher's exact test testing for a difference in proportions between treatments. For continuous variables, the p-value is from a two-tailed, two sample t test, testing for a difference in means between treatments.
Day 0 defined as first application visit.
BLCC, Apligraf; SIS, Oasis. BLCC, bilayered living cellular construct; HBO2, hyperbaric oxygen therapy; NPWT, negative pressure wound therapy; SD, standard deviation; SIS, small intestine submucosa.
Treatment with HBO2 or NPWT in the 28 days prior to initial BLCC or SIS application or concurrently was uncommon and comparable between treatment groups (Table 3).
Kaplan-Meier analysis of available data up to 36 months showed BLCC treatment significantly improved the median time to VLU wound closure by 44%, achieving the endpoint 19 weeks sooner than the SIS-treated patients (24 weeks for BLCC vs. 43 weeks for SIS, p = 0.01) (Figure 2). The estimated incidence of wound closure for BLCC compared with SIS was significantly improved by weeks 12 (31% vs. 26%), 24 (50% vs. 41%), and 36 (61% vs. 46%), respectively (p = 0.01) (Figure 3). BLCC treatment significantly increased the probability of wound healing by 29% compared with SIS treatment (hazard ratio = 1.29 [95% CI: 1.06, 1.56], p = 0.01).
Figure 2.
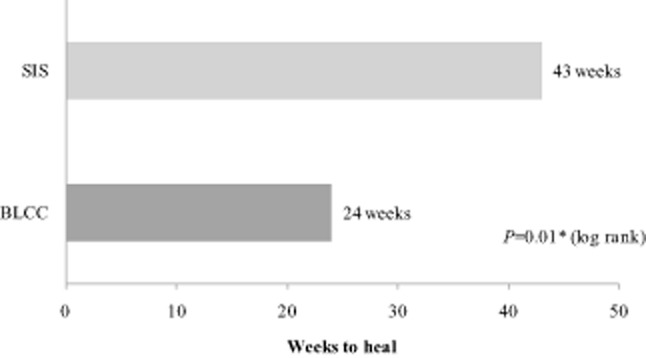
Median time to wound closure from Kaplan-Meier analysis of all available data up to 3 years. Bilayered living cellular construct (BLCC), Apligraf; small intestine submucosa (SIS), Oasis. *p-Value from a two-sided log-rank test.
Figure 3.
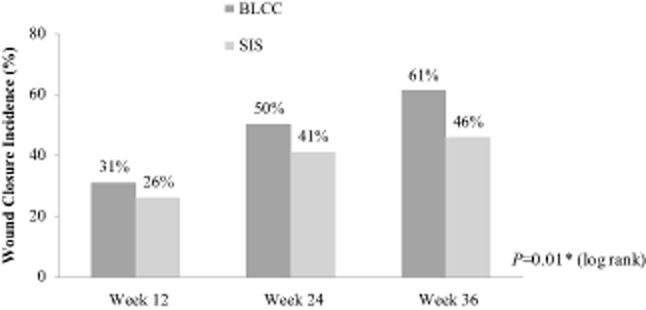
Estimated incidence of wound closure from Kaplan-Meier analysis of all available data up to 3 years. Bilayered living cellular construct (BLCC), Apligraf; small intestine submucosa (SIS), Oasis. *p-Value from a two-sided log-rank test.
Discussion
This retrospective analysis of EMR data from US wound care facilities showed that use of BLCC was more effective than SIS for the treatment of VLUs in the study population which was selected to represent a real-world setting. BLCC-treated wounds were found to have a significantly higher incidence of wound closure and reduced the median time to achieve wound closure by 44%. This study represents the largest real-world comparative effectiveness analysis of products used in the treatment of VLUs.
Efficacy reflects the degree to which an intervention produces the expected result under carefully controlled conditions. RCTs provide the best method for establishing efficacy and are considered to have high “internal validity” because of randomization, careful selection of participants, and standardized treatment protocols, all of which are intended to maximize the possibility of observing a treatment effect, if it exists.18,20,21 Although RCTs are considered level 1 evidence and the “gold standard” in determining if a product can actually work, there may be limitations in the generalizability or “external validity” of data generated.22 The strict criteria for patient inclusion (which may exclude “higher-risk” patients), rigorous monitoring, and adherence to treatment protocols may create a potentially artificial environment that may not be entirely representative of the typical patient population or the routine practice conditions where these products are utilized.18,20 Evaluating real-world effectiveness is therefore important as it does not narrowly select patient populations but evaluates an intervention as it is typically utilized in practice without intense efforts to standardize its use. Results of these pragmatic trials are often considered to be more widely applicable and complementary to RCTs as they evaluate an intervention in ordinary settings and in broader populations.23
CER is defined by the pragmatic aim of informing a specific health-care decision through the explicit comparison of clinically credible, alternative interventions in a representative study population. The Institute of Medicine issued a report in 2009 to set priorities for national CER that would support better decision making about interventions in health care.20 The report highlighted the need to perform comparisons of interventions among patients in typical patient care settings and to focus resources on the most promising approaches.24 However, despite this call for more comparative research, there remains a paucity of head-to-head comparative data available to guide clinicians.
In effectiveness studies, performance is often reduced in less homogeneous populations treated outside of expert clinical research centers and in a less rigorous fashion; thus, it is important to determine if efficacy can be translated to routine practice settings. Although direct comparisons between RCTs and the results presented here cannot be made because of differences in study design and patient populations, we found the incidence of complete wound closure for the 146 BLCC-treated patients in the pivotal RCT was 63% at week 2415 compared with 50% in this analysis. In the SIS RCT, the incidence of closure at week 12 was 55% in the 62 treated patients16 compared with 26% in this analysis.
VLUs are estimated to affect as many as 2.5 million US patients and have considerable negative effects on quality of life.4,25–27 The economic burden imposed by VLUs on the health-care system and payers was highlighted in a recent analysis of more than 81,000 VLU patients published by Rice et al. which estimated the direct costs to be up to $18bn annually.10 A subset of this analysis performed on the Medicare VLU patients receiving skin substitutes during the corresponding time period found that the total health-care costs were $537 per week higher for patients who were still incurring VLU-related costs (“nonhealed” ulcers) compared with those no longer incurring VLU-related costs (“healed” ulcers). Of this amount, $377 per week was for selected services considered directly related to VLU treatment.28 Therefore the 19-week difference in median healing time (week 24 vs. 43) in the current analysis should result in substantial cost savings (between $7,000 and $10,000 additional costs). This estimate likely understates the overall burden as it does not include 20% of costs not covered by Medicare. Moreover, it does not factor in important indirect costs such as those associated with quality of life or lost productivity because of patient and caregiver missed work. Although BLCC costs more per applied unit than SIS, cost savings might be realized with BLCC treatment given fewer and less frequent applications received by patients in this analysis and the considerable costs associated with having a wound remain open an additional 19 weeks. Further specifics on relative costs would require a database with more detailed information than was available for this analysis.
We recognize that this study, like all retrospective analyses, is “noisier” than a typical prospective randomized controlled study. A limitation of this study is that EMR databases often are not developed specifically for research purposes, and lack of control on the quantity of specific information exists as well as difficulties in standardizing the information collected. Although certain fields within the WoundExpert EMR were reliably and consistently completed (such as wound measurements), information regarding the type of secondary dressing or compression therapy were found to be recorded less consistently and often entered as “free text,” making reliable analyses difficult. Completion of baseline demographic information fields such as medical history, prior surgical interventions, or concomitant medications varied across centers, making certain subgroup analyses difficult. Additionally, the reporting of safety-related outcomes, adverse events, or ulcer recurrence was not possible as this information was not reliably captured within the database. Finally, given the lack of randomization, there is a possibility of bias in selection of patients for BLCC or SIS at the centers involved in patient care. However, given the number of wounds and centers providing information for the analysis, it is less likely that a uniform bias was present affecting study results.
There are also many advantages offered by the WoundExpert EMR. Electronic health-care databases are widely used to assess the comparative effectiveness of therapeutics in real-world settings29 and offer the benefit of providing large study populations and longer observation periods.30 WoundExpert provides a robust source for data as it is specifically designed for the wound care field and is used nationwide by centers treating the relevant population and utilizing the products of interest. This study analyzed data collected over a 3-year period from a large number of patients treated in facilities across the US.
It is also important to note that in this analysis, we sought to investigate outcomes in refractory “hard-to-heal” VLUs and excluded wounds that had reduced in size >40% in the prior 4 weeks, a threshold which has showed high negative predictive value (i.e., identifies wounds that will not achieve closure in a timely fashion).31 This 4-week prognostic milestone has been adopted across the field of wound care to identify patients who may benefit from intervention with advanced therapy, yet interestingly, few previous studies have employed protocols that apply this criteria for inclusion. These results therefore provide valuable information to guide treatment decisions for patients with these recalcitrant wounds.
In conclusion, these real-world data showed that BLCC, compared with SIS, significantly improved the probability, speed, and the incidence of wound closure in VLUs.
Acknowledgments
The authors thank S. William Tam PhD for preparing the manuscript draft and Biostatistical Consulting Inc. for statistical analyses. The authors also acknowledge valuable contributions from Organogenesis employees Michelle Skornicki MPH, Kate Giovino BA, and Margaret Grasso MS toward the conduct of the study and revision of the manuscript. De-identified patient data released to Organogenesis were consistent with the terms and conditions of Net Health's client contracts and the requirements of HIPAA. Net Health was not involved in any way in the analysis, interpretation, or reporting of the data.
Conflict of interest: ML Sabolinski, WA Marston, and RS Kirsner are paid consultants to Organogenesis, and NB Parsons is an employee of Organogenesis, Inc. WA Marston and RS Kirsner are paid consultants to Healthpoint.
Funding: This study funded by Organogenesis, Inc.
Glossary
- BLCC
Bilayered living cellular construct
- CER
Comparative effectiveness research
- EMR
Electronic medical record
- HBO2
Hyperbaric oxygen therapy
- HFDS
Human fibroblast-derived dermal substitute
- HIPAA
Health Insurance Portability and Accountability Act
- NPWT
Negative pressure wound therapy
- RCT
Randomized controlled trial
- SIS
Small intestine submucosa
- VLU
Venous leg ulcer
References
- 1.Valencia IC, Falabella A, Kirsner RS, Eaglstein WH. Chronic venous insufficiency and venous leg ulceration. J Am Acad Dermatol. 2001;44:401–421. doi: 10.1067/mjd.2001.111633. [DOI] [PubMed] [Google Scholar]
- 2.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fife C, Walker D, Thomson B, Carter M. Limitations of daily living activities in patients with venous stasis ulcers undergoing compression bandaging: problems with the concept of self bandaging. Wounds. 2007;19:255–257. [PubMed] [Google Scholar]
- 4.Gillespie DL Writing Group III of the Pacific Vascular Symposium 6. Kistner B, Glass C, Bailey B, Chopra A, et al. Venous ulcer diagnosis, treatment, and prevention of recurrences. J Vasc Surg. 2010;52(5 Suppl):8S–14S. doi: 10.1016/j.jvs.2010.05.068. [DOI] [PubMed] [Google Scholar]
- 5.Hankin CS, Knispel J, Lopes M, Bronstone A, Maus E. Clinical and cost efficacy of advanced wound care matrices for venous ulcers. J Manag Care Pharm. 2012;18:375–384. doi: 10.18553/jmcp.2012.18.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurz X, Kahn SR, Abenhaim L, Clement D, Norgren L, Baccaglini U, et al. Chronic venous disorders of the leg: epidemiology, outcomes, diagnosis and management. Summary of an evidence-based report of the VEINES task force. Int Angiol. 1999;18:83–102. [PubMed] [Google Scholar]
- 7.Fowkes FGR, Evans CJ, Lee AJ. Prevalence and risk factors of chronic venous insufficiency. Angiology. 2001;52(Suppl. 1):S5–15. doi: 10.1177/0003319701052001S02. [DOI] [PubMed] [Google Scholar]
- 8.The Alexander House Group. Consensus paper on venous leg ulcer. J Dermatol Surg Oncol. 1992;18:592–602. doi: 10.1111/j.1524-4725.1992.tb03513.x. [DOI] [PubMed] [Google Scholar]
- 9.Margolis DJ, Bilker W, Santanna J, Baumgarten M. Venous leg ulcer: incidence and prevalence in the elderly. J Am Acad Dermatol. 2002;46:381–386. doi: 10.1067/mjd.2002.121739. [DOI] [PubMed] [Google Scholar]
- 10.Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons N. Medical, drug, and work-loss costs of venous leg ulcers. Value Health. 2013;16:A73. doi: 10.3111/13696998.2014.903258. [DOI] [PubMed] [Google Scholar]
- 11.O'Meara S, Cullum NA, Nelson EA. Compression for venous leg ulcers (review) Cochrane Database Syst Rev. 2009;(1) doi: 10.1002/14651858.CD000265.pub2. CD000265. [DOI] [PubMed] [Google Scholar]
- 12.Warriner RA, 3rd, Carter MJ. The current state of evidence-based protocols in wound care. Plast Reconstr Surg. 2011;127(Suppl. 1):144S–153S. doi: 10.1097/PRS.0b013e31820023dc. [DOI] [PubMed] [Google Scholar]
- 13.Robson MC, Cooper DM, Aslam R, Gould LJ, Harding KG, Margolis DJ, et al. Guidelines for the treatment of venous ulcers. Wound Repair Regen. 2006;14:649–662. doi: 10.1111/j.1524-475X.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- 14.Cavorsi J, Vicari F, Wirthlin DJ, Ennis W, Kirsner R, O'Connell SM, et al. Best-practice algorithms for the use of a bilayered living cell therapy (Apligraf®) in the treatment of lower-extremity ulcers. Wound Repair Regen. 2006;14:102–109. doi: 10.1111/j.1743-6109.2006.00098.x. [DOI] [PubMed] [Google Scholar]
- 15.Falanga V, Margolis D, Alvarez O, Auletta M, Maggiacomo F, Altman M, et al. Rapid healing of venous ulcers and lack of clinical rejection with an allogeneic cultured human skin equivalent. Arch Dermatol. 1998;134:293–300. doi: 10.1001/archderm.134.3.293. [DOI] [PubMed] [Google Scholar]
- 16.Mostow EN, Haraway GD, Dalsing M, Hodde JP, King D OASIS Venus Ulcer Study Group. Effectiveness of an extracellular matrix graft (OASIS Wound Matrix) in the treatment of chronic leg ulcers: a randomized clinical trial. J Vasc Surg. 2005;41:837–843. doi: 10.1016/j.jvs.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 17. The American recovery and reinvestment act of 2009. H.R. 1–63. Available at http://www.gpo.gov/fdsys/pkg/BILLS-111hr1enr/pdf/BILLS-111hr1enr.pdf (accessed May 20, 2013)
- 18.Eaglstein WH, Kirsner RS. Expectations for comparative effectiveness and efficacy research. JAMA Dermatol. 2013;149:18–19. doi: 10.1001/jamadermatol.2013.1324. [DOI] [PubMed] [Google Scholar]
- 19.Bombardier C, Maetzel A. Pharmacoeconomic evaluation of new treatments: efficacy versus effectiveness studies? Ann Rheum Dis. 1999;58(Suppl. 1):182–185. doi: 10.1136/ard.58.2008.i82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.IOM(Institute of Medicine) Initial national priorities for comparative effectiveness research. Washington, DC: The National Academies Press; 2009. [Google Scholar]
- 21.van Staa TP, Leufkens HG, Zhang B, Smeeth L. A comparison of cost effectiveness using data from randomized trials or actual clinical practice: selective cox-2 inhibitors as an example. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000194. e1000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Concato J. When to randomize, or “evidence-based medicine needs medicine-based evidence”. Pharmacoepidemiol Drug Saf. 2012;21(Suppl. 2):6–12. doi: 10.1002/pds.3245. [DOI] [PubMed] [Google Scholar]
- 23.Gandjour A. Prioritizing comparative effectiveness research: are drug and implementation trials equally worth funding? Pharmacoeconomics. 2011;29:555–561. doi: 10.2165/11588330-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.Sox HC, Greenfield S. Comparative effectiveness research: a report from the Institute of Medicine. Ann Intern Med. 2009;151:203–205. doi: 10.7326/0003-4819-151-3-200908040-00125. [DOI] [PubMed] [Google Scholar]
- 25.Phillips T, Stanton B, Provan A, Lew R. A study of the impact of leg ulcers on quality of life: financial, social, and psychologic implications. J Am Acad Dermatol. 1994;31:49–53. doi: 10.1016/s0190-9622(94)70134-2. [DOI] [PubMed] [Google Scholar]
- 26.Green J, Jester R. Health-related quality of life and chronic venous leg ulceration: part 1. Br J Community Nurs. 2009;14:S12–14. doi: 10.12968/bjcn.2009.14.Sup6.45538. S16–17. [DOI] [PubMed] [Google Scholar]
- 27.González-Consuegra RV, Verdú J. Quality of life in people with venous leg ulcers: an integrative review. J Adv Nurs. 2011;67:926–944. doi: 10.1111/j.1365-2648.2010.05568.x. [DOI] [PubMed] [Google Scholar]
- 28.Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons N. Medical, drug, and work-loss costs of venous leg ulcers. Poster presentation, Symposium on Advanced Wound Care 2013. Abstract.
- 29.Toh S, Rodríguez LAG, Hernán MA. Analyzing partially missing confounder information in comparative effectiveness and safety research of therapeutics. Pharmacoepidemiol Drug Saf. 2012;21(Suppl. 2):13–20. doi: 10.1002/pds.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motheral B, Brooks J, Clark MA, Crown WH, Davey P, Hutchins D, et al. A checklist for retrospective database studies–report of the ISPOR task force on retrospective databases. Value Health. 2003;6:90–97. doi: 10.1046/j.1524-4733.2003.00242.x. [DOI] [PubMed] [Google Scholar]
- 31.Gelfand JM, Hoffstad O, Margolis DJ. Surrogate endpoints for the treatment of venous leg ulcers. J Invest Dermatol. 2002;119:1420–1425. doi: 10.1046/j.1523-1747.2002.19629.x. [DOI] [PubMed] [Google Scholar]


