Abstract
Aims
To evaluate fetal growth in relation to gestational weight gain in women with Type 2 diabetes.
Methods
A retrospective cohort study of 142 consecutive pregnancies in 28 women of normal weight, 39 overweight women and 75 obese women with Type 2 diabetes (pre-pregnancy BMI < 25, 25–29.9, ≥ 30 kg/m2, respectively). Gestational weight gain was categorized as excessive (exceeding the US Institute of Medicine recommendations) or as non-excessive (within or below the Institute of Medicine recommendations).
Results
Excessive and non-excessive gestational weight gain were seen in 61 (43%) and 81 women (57%) with a median (range) gestational weight gain of 14.3 (9–32) vs 7.0 (−5–16) kg (P < 0.001), respectively. Infants of women with excessive gestational weight gain were characterized by higher birth weight (3712 vs 3258 g; P = 0.001), birth weight z-score (1.14 vs -0.01, P = 0.001) and prevalence of large-for-gestational-age infants (48 vs 20%; P < 0.001). In normal weight, overweight and obese women with non-excessive gestational weight gain, the median weight gain in the first half of pregnancy was 371, 114 and 81 g/week, and in the second half of pregnancy 483, 427 and 439 g/week, respectively. In multiple linear regression analysis, gestational weight gain was associated with a higher infant birth weight z-score independent of pre-pregnancy BMI, smoking, HbA1c and insulin dose at last visit, ethnicity and parity [β=0.1 (95% CI 0.06–0.14), P < 0.001].
Conclusions
Infant birth weight was almost 0.5 kg higher in women with Type 2 diabetes and excessive gestational weight gain than in women with Type 2 diabetes and non-excessive weight gain.
Introduction
In healthy women, excessive gestational weight gain is a risk factor for fetal overgrowth as well as other perinatal and maternal complications independent of maternal BMI 1,2; therefore, the US Institute of Medicine has developed the following gestational weight gain recommendations for healthy women according to pre-pregnancy BMI: 11.5–16.0 kg for women of normal weight, 7.0–11.5 kg for overweight women and 5.0–9.0 kg for obese women 3,4 to guide pregnant women and their caregivers. In healthy women, excessive gestational weight gain according to Institute of Medicine recommendations increases the risk of large-for-gestational-age infants and macrosomia 1,5,6.
The prevalence of large-for-gestational-age infants born to women with Type 2 diabetes has been reported to be ∼50% 7,8. Excessive fetal growth is largely attributed to fetal hyperinsulinaemia induced by elevated maternal glucose levels that cross the placenta and thus stimulate fetal growth 9–11. Another risk factor for large-for-gestational-age infants of women with diabetes is maternal hypertriglyceridaemia, indicating that metabolic substrates other than glucose also play a role in the pathogenesis of fetal overgrowth 12.
Recently, we showed that a restricted total gestational weight gain, defined as ≤ 5 kg, in obese women with Type 2 diabetes resulted in more appropriate fetal growth 13. The same pattern was seen in a large register study of overweight and obese women with Type 2 diabetes, where a gestational weight gain > 9 kg was positively associated with an increased risk of infants with macrosomia 14. The impact of restricted gestational weight gain in an unselected population of women with Type 2 diabetes is unknown.
What's new?
In women with Type 2 diabetes, the impact of excessive gestational weight gain according to the Institute of Medicine's guidelines on fetal outcome was evaluated.
Infant birth weight was almost 0.5 kg higher and neonatal morbidity more prevalent in women with excessive compared with non-excessive weight gain.
Total gestational weight gain was an independent predictor of excessive fetal growth, regardless of pre-pregnancy BMI and glycaemic control during pregnancy.
The observed weekly gestational weight gain in early and late pregnancy resulting in appropriate total weight gain is given for normal weight, overweight and obese women, respectively.
The gestational weight gain in early pregnancy is mainly attributable to maternal gain of adipose tissue and increased plasma volume, while a larger part of the weight gain in late pregnancy is attributed to increased fetal load, hypertrophy of the uterus, amniotic fluid and oedema formation 3,15. The weekly gestational weight gain given for the first and second part of pregnancy may thus be a useful clinical tool in the guidance of pregnant women with Type 2 diabetes when striving for appropriate total gestational weight gain 2,13,16,17. The primary aim of the present study, therefore, was to evaluate fetal growth in relation to the total gestational weight gain in all pregnant women with Type 2 diabetes, regardless of pre-pregnancy BMI. The secondary aim was to describe the weekly gestational weight gain in early and late pregnancy resulting in appropriate total gestational weight gain according to the Institute of Medicine recommendations.
Methods
We conducted a retrospective cohort study including all women with pre-pregnancy Type 2 diabetes with referral before 21 gestational weeks and a singleton pregnancy, who were followed at our Centre for Pregnant Women with Diabetes between January 2008 and February 2013. If a woman had more than one pregnancy during the study period (n = 3), only the last pregnancy was included in order to include the most recent pregnancy in this retrospective study.Women with former bariatric surgery (n = 5), thalassemia minor (n = 1), thyrotoxicosis (n = 1), Crohn's disease (n = 1) and women with missing information on gestational weight gain (n = 10) were excluded. In total, 142 women were included, of whom 81 (57%) were Nordic white women and the remaining women were from the Middle East (22%), Africa (10%), Asia (8%) and other parts of the world (3%).
The Danish Data Protection Agency approved the protocol. According to Danish law, the protocol did not need an approval from the regional ethics committee, as the study was a retrospective register study.
Pre-pregnancy BMI and gestational weight gain
Pre-pregnancy BMI was calculated using the self-reported pre-pregnancy weight and height 18 and the women were categorized as normal weight (BMI 18.5–24.9 kg/m2, n = 28), overweight (25.0–29.9 kg/m2, n = 39) or obese ( ≥ 30.0 kg/m2, n = 75). The total gestational weight gain was calculated as the difference between the last weight measured before delivery and the self-reported pre-pregnancy weight. Within the three BMI classes (normal weight, overweight and obese), total gestational weight gain was categorized based on the Institute of Medicine recommendations 3 and defined as excessive if above the Institute of Medicine recommendations (> 16.0, > 11.5 and > 9.0 kg, respectively). The weight gain in the non-excessive group was defined as appropriate if within the Institute of Medicine recommendations (11.5–16.0, 7.0–11.5 and 5.0–9.0 kg, respectively) or as insufficient if below (Fig. 1).
Figure 1.
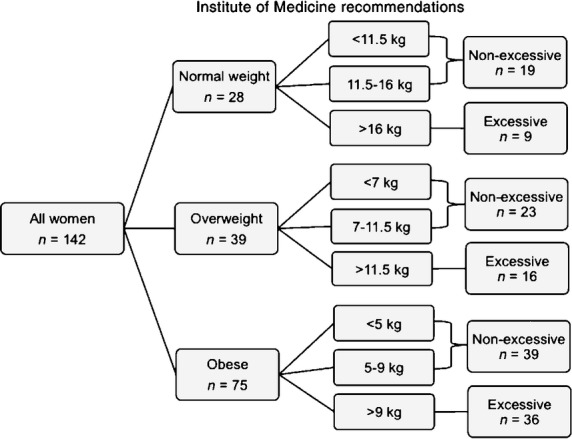
Classification of women with Type 2 diabetes according to pre-pregnancy BMI (normal, overweight and obese) and gestational weight gain categorized according to the Institute of Medicine recommendations as excessive and non-excessive in 142 women with pre-pregnancy Type 2 diabetes.
The pregnancies were divided into first and second halves, with 21 gestational weeks as the cut-off, as all women were seen at a routine visit at that time 19. The weekly gestational weight gain in the first half of pregnancy was calculated as the difference between the weights measured at first visit and at 21 gestational weeks divided by the number of weeks inbetween, with days precision. Similarly, the weekly gestational weight gain in the second half of pregnancy was calculated as the difference between the weights measured at 21 gestational weeks and at the last visit in pregnancy at 37 (26–39) gestational weeks divided by the number of weeks inbetween. At least 2 weeks of observation was required for these calculations.
Routine pregnancy care
At first pregnancy visit at 10 (5–20) gestational weeks, all women had a 1-h tailored dietary consultation, during which a low glycaemic index diet was recommended 19. Women with BMI < 30 kg/m2 were advised to gain 10–15 kg in pregnancy, whereas women with BMI ≥ 30 kg/m2 were advised to stay weight-neutral in the first half of pregnancy and thereafter to limit their total gestational weight gain to 0–5 kg 13. Moderate physical activity for ∼30 min/day was encouraged 13.
All women were offered consultations at our centre at least six times during pregnancy at ∼10, 14, 21, 28, 33 and 37 gestational weeks, where blood pressure and HbA1c were measured, and a sterile urine dipstick was screened for ketone bodies and protein 19. If ketonuria was present, carbohydrate intake was evaluated carefully and the women were eventually advised to have an additional carbohydrate intake during the evening 13. All women were seen by a diabetes caregiver every second week, either at our centre or locally. Current oral antidiabetic drugs (usually metformin) were discontinued at admission, and the women were treated with diet alone or diet and insulin. Throughout pregnancy, self-monitored plasma glucose measurements were recommended seven times daily; before and 90 min after each main meal and at bedtime 19. The targets for plasma glucose were as follows: 4.0–6.0 mmol/l preprandially, 4.0– 8.0 mmol/l 90 min post-prandially, and 6.0–8.0 mmol/l before bedtime. If these goals were not obtained with diet alone, insulin treatment was commenced with a basal-bolus regimen or biphasic insulin aspart 30 twice daily 19.
Outcome variables
Proteinuria was defined as ≥ +1 on a sterile urine dipstick or urine albumin excretion ≥ 190 mg/24 h, which corresponds to a proteinuria of 300 mg/24 h 20. Pre-eclampsia was defined as blood pressure ≥ 140/90 mmHg accompanied by proteinuria after 20 gestational weeks 19. Diabetic retinopathy was diagnosed with retinal photos at first pregnancy visit 21.
Preterm delivery was defined as birth before 37 gestational weeks 19. Caesarean section was classified as emergency when carried out < 8 h after clinical decision 13. Birth weight standard deviation score (z-score) and the prevalence of large- and small-for-gestational-age infants (i.e. infant birth weight >90th or < 10th centile, adjusted for sex and gestational age 22) were also recorded. Macrosomia was defined as an absolute infant birth weight ≥ 4000 g. The ponderal index was calculated as (birth weight in kg) / (birth length in m3).
Major congenital malformations were those causing a substantial future handicap, or those requiring surgery or leading to death. Perinatal mortality was defined as fetal death later than 22 gestational weeks or death within 1 week after delivery. Jaundice was registered when phototherapy was required, and transient tachypnoea of the newborn was defined as a need for continuous positive airway pressure for > 60 min 19. Neonatal hypoglycaemia was defined as plasma glucose < 2.5 mmol/l measured 2 h after birth 23. Our composite endpoint of perinatal morbidity was defined as the occurrence of at least one of the following complications: jaundice; transient tachypnea of the newborn; neonatal hypoglycaemia; or perinatal mortality.
Statistical analysis
Continuous variables are given as median (range) values and categorical data as number (%). Differences between two groups were analysed using the chi-squared test, Fisher's exact test or the Mann–Whitney U-test, as appropriate. A P value < 0.05 was taken to indicate statistical significance. The correlation between the self-reported pre-pregnancy weight and the first measured weight in pregnancy was performed with univariate linear regression. To control for confounders, multiple linear regression analysis was applied using gestational weight gain as the exposure variable and infant birth weight z-score as the outcome variable. Gestational weight gain was entered as either total gestational weight gain (kg) or gestational weight gain (g/week) in the first or second half of pregnancy. Based on theoretical considerations, six possible confounders were included: pre-pregnancy BMI (kg/m2); smoking (yes/no); HbA1c (%) and insulin dose (IU/kg/24-h) at last visit in pregnancy; Nordic white ethnicity (yes/no); and nulliparity (yes/no). Statistical analyses were performed using spss statistics version 20 (SPSS Inc., Chicago, IL, USA).
Results
Among 142 included women with pre-pregnancy Type 2 diabetes, 28 (20%) were normal weight, 39 (27%) overweight and 75 (53%) obese. Baseline maternal characteristics are shown in Table 1. According to the Institute of Medicine recommendations, 61 (43%) women had excessive total gestational weight gain and 81 (57%) women had non-excessive total gestational weight gain (Table 1).
Table 1.
Baseline maternal characteristics in 142 pregnant women with Type 2 diabetes according to excessive or non-excessive total gestational weight gain
| Excessive weight gain | Non-excessive weight gain | P | |
|---|---|---|---|
| Number ofwomen (%) | 61 (43) | 81 (57) | |
| Median (range) maternal age, years | 34 (20– 45) | 34 (22– 44) | 0.41 |
| Nordic white women, n (%) | 42 (69) | 39 (48) | 0.01 |
| Nulliparous, n (%) | 25 (31) | 20 (33) | 0.81 |
| Median (range) duration of diabetes, years | 3 (0.2– 9) | 3 (0.3– 11) | 0.92 |
| Smokers, n (%) | 10 (16) | 16 (20) | 0.61 |
| Median (range) pre-pregnancy weight, kg | 88.0 (53– 140) | 77.5 (50– 156) | 0.03 |
| Median (range) pre-pregnancy BMI, kg/m2 | 32.0 (19– 48) | 29.7 (20– 53) | 0.09 |
| Diabetic retinopathy, n (%) | 10 (16) | 15 (19) | 0.74 |
| Median (range) gestational age at first visit, days | 69 (36– 153) | 74 (37– 144) | 0.19 |
| Median (range) gestational age at last visit, days | 257 (203– 275) | 256 (184– 275) | 0.66 |
Excessive (>16.0, >11.5 and >9.0 kg) and non-excessive (less than excessive) gestational weight gain for normal weight, overweight and obese women, were defined according to the Institute of Medicine recommendations.
HbA1c levels at first and last visit (10 and 37 gestational weeks, respectively) in pregnancy were similar between women with excessive and non-excessive weight gain, whereas insulin therapy was more frequent, insulin doses were higher and pre-pregnancy BMI tended to be higher in women with excessive weight gain compared with women with non-excessive weight gain. The prevalence of ketonuria was low in both groups (Table 2).
Table 2.
Total gestational weight gain and glycaemic control in 142 pregnant women with Type 2 diabetes according to excessive or non-excessive gestational weight gain
| Excessive weight gain | Non-excessive weight gain | P | |
|---|---|---|---|
| Number of women (%) | 61 (43) | 81 (57) | |
| Weight changes, median (range) | |||
| Total weight gain, kg | 14.3 (9–32) | 7.0 (-5–16) | < 0.001 |
| Weekly weight gain, g/week | 385 (245–856) | 190 (-123–424) | < 0.001 |
| Weekly weight gain in first half of pregnancy, g/week |
347 (-1167–925) | 108 (-632–579) | < 0.001 |
| Weekly weight gain in second half of pregnancy, g/week |
626 (124–1400) | 340 (-243–857) | < 0.001 |
| HbA1c | |||
| 10 gestational weeks mmol/mol |
46 (30–130) | 49 (29–75) | 0.95 |
| % | 6.4 (4.9–13.2) | 6.6 (4.8–9.0) | |
| 21 weeks mmol/mol |
39 (27–61) | 39 (27-58) | 0.36 |
| % | 5.7 (4.6-7.7) | 5.7 (4.6–7.5) | |
| 37 weeks mmol/mol |
42 (31–64) | 41 (28– 83) | 0.12 |
| % | 6.0 (5.0–8.0) | 5.9 (4.7–9.7) | |
| Ketone bodies detected, n (%) | |||
| 10 weeks | 2 (3) | 4 (5) | 0.70 |
| 21 weeks | 0 | 0 | 1.00 |
| 37 weeks | 0 | 1 (1) | 1.00 |
| Insulin treatment, n (%) | |||
| Before first visit (10 weeks) | 21 (34) | 24 (30) | 0.54 |
| After first visit (10 weeks) | 55 (90) | 58 (72) | 0.01 |
| 37 weeks | 60 (98) | 72 (89) | 0.04 |
| Median (range) dose at first visit, IU/kg/24 h | 0.36 (0–1.1) | 0.30 (-0.15–1.48) | 0.07 |
| Median (range) dose at 37 weeks, U/kg/24 h | 1.11 (0.00–2.60) | 0.78 (0.00–2.81) | 0.003 |
Excessive (>16.0, >11.5 and >9.0 kg) and non-excessive (less than excessive) gestational weight gain for normal weight, overweight and obese women, were defined according to the Institute of Medicine recommendations.
Perinatal outcomes are shown in Table 3. In women with excessive total weight gain, the median infant birth weight was 454 g greater (P = 0.001) than the group with non-excessive weight gain. In line with this, women with excessive weight gain delivered more large-for-gestational-age infants (48 vs 20%; P < 0.001), while the prevalence of small-for-gestational-age infants was low and similar between the groups.
Table 3.
Pregnancy outcomes in 142 pregnant women with Type 2 diabetes according to excessive or non-excessive total gestational weight gain
| Excessive weight gain | Non-excessive weight gain | P | |
|---|---|---|---|
| Number of women (%) | 61 (43) | 81 (57) | |
| Pre-eclampsia, n (%) | 4 (6.6) | 2 (2.5) | 0.40 |
| Median (range) gestational age at delivery, days | 265 (206– 280) | 266 (184– 284) | 0.81 |
| Preterm delivery, n (%) | 8 (13) | 10 (12) | 0.89 |
| Emergency caesarean section, n (%) | 14 (23) | 9 (11) | 0.06 |
| Elective caesarean section, n (%) | 17 (28) | 24 (30) | 0.82 |
| Median (range) birth weight, g | 3,712 (1070– 4816) | 3258 (720– 4558) | 0.001 |
| Median (range) birth weight z-score, sd | 1.14 (-2.5– 4.7) | -0.01 (-3.5– 4.5) | 0.001 |
| Large for gestational age: >90th percentile, n (%) | 29 (48) | 16 (20) | < 0.001 |
| Small for gestational age: < 10th percentile, n (%) | 5 (8) | 11 (14) | 0.32 |
| Macrosomia: birth weight >4000 g, n (%) | 16 (26) | 2 (2.5) | < 0.001 |
| Median (range) length, cm | 52 (46– 57) | 50 (32– 56) | 0.262 |
| Median (range) ponderal index, kg/m3 | 25.9 (20.1– 32) | 25.2 (19.3– 32.4) | 0.071 |
| Composite perinatal morbidity, n (%) | 32 (53) | 29 (36) | 0.047 |
| Jaundice | 7 (12) | 9 (11) | 0.96 |
| Transient tachypnea of the Newborn |
7 (12) | 8 (10) | 0.71 |
| Neonatal hypoglycaemia | 21 (34) | 15 (19) | 0.03 |
| Perinatal mortality | 0 | 2 (3) | 0.51 |
Excessive (>16.0, >11.5 and >9.0 kg) and non-excessive (less than excessive) gestational weight gain for normal weight, overweight and obese women, were defined according to the Institute of Medicine recommendations.
Composite neonatal morbidity was defined as the occurrence of at least one of the following complications: jaundice, transient tachypnoea of the newborn, neonatal hypoglycaemia or perinatal mortality.
Higher rates of composite perinatal morbidity (53 vs 36%; P = 0.047), neonatal hypoglycaemia (34 and 19%; P = 0.03), and a trend towards more emergency caesarean sections (23 vs 11%; P = 0.06) were seen in women with excessive weight gain.
Infant birth weight z-score and the prevalence of large-for-gestational-age infants increased with increasing gestational weight gain, regardless of maternal BMI class (Figs2a, b and c).
Figure 2.
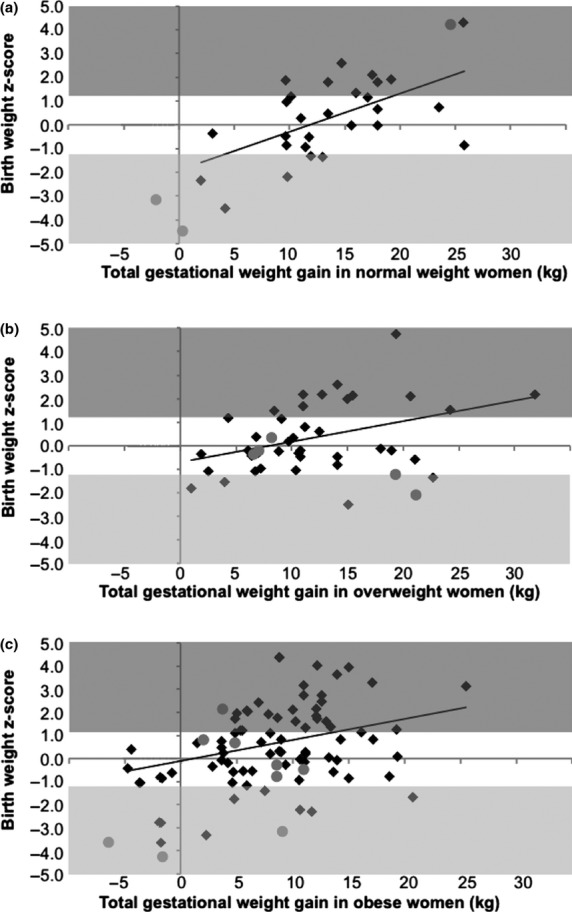
Infant birth weight z-score in relation to gestational weight gain in (a) women of normal weight, (b) overweight women and (c) obese women with Type 2 diabetes. Grey areas represent large- or small-for-gestational-age infants. The best fitted lines are depicted with β = 0.58, P = 0.001, β = 0.39, P = 0.012 and β = 0.32, P = 0.005, respectively. Grey circles, women delivered preterm; black diamonds, women delivered at term.
Total gestational weight gain was positively associated with infant birth weight z-score after adjustment for pre-pregnancy BMI, smoking, HbA1c and insulin dose at last visit, ethnicity and parity [regression coefficient β = 0.1 (95% CI 0.06 to 0.14); P < 0.001], corresponding to a 0.1 increase in infant birth weight z-score for each kg increase in total weight gain during pregnancy. The exclusion of the 21 women delivering preterm and/or developing pre-eclampsia from the analyses did not change the associations significantly.
The median weekly gestational weight gain for all women gradually increased from zero in early pregnancy and ∼400 g/week in the main part of pregnancy, to 600 g/week in the last 3 weeks of pregnancy (Fig. 3).Weekly gestational weight gain in the first and second halves of pregnancy were both significantly positively associated with birth weight z-score (P < 0.001) in multivariate linear regression.
Figure 3.
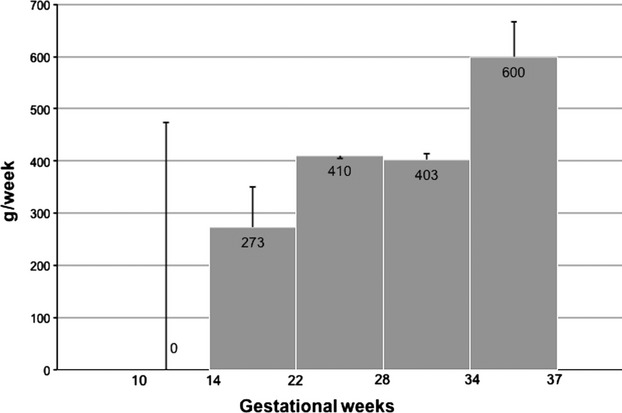
Weekly gestational weight gain from first to last pregnancy visit in 142 women with Type 2 diabetes: median and interquartile ranges.
The prevalence of small-for-gestational-age infants was similar in women with insufficient, appropriate or excessive total weight gain [20% (eight out of 41 women) vs 8% (three out of 40 women) vs 8% (five out of 61 women), respectively; P = 0.14).
In pregnancies resulting in appropriate total weight gain, the material was divided according to BMI classes (normal weight, overweight and obese). The median weight gains in the first half of pregnancy were 371 (171–579), 114 (153–608) and 81 (-308–439) g/week and for the last part of pregnancy were 483 (313–673), 427 (153–608) and 439 (169–857) g/week, in the three BMI classes, respectively.
There were two cases of major congenital malformations: one with a curved femur requiring surgery (excessive weight gain group) and one with cardiac and renal malformations leading to stillbirth (non-excessive weight gain group). In addition, one perinatal death occurred in a large-for-gestational-age infant as a result of severe shoulder dystocia (non-excessive weight gain group).
To assess the validity of self-reported weight, the correlation between maternal weight at first pregnancy visit and self-reported pre-pregnancy weight was calculated. A strong correlation between these two varables was seen (R = 0.99, P < 0.001), with maternal weight at first pregnancy visit 1.4 (-6.4–10.1) kg higher than the self-reported weight.
Discussion
The main observation of the present study was that birth weight was almost 0.5 kg higher and neonatal morbidity was more prevalent in infants of women with Type 2 diabetes with a gestational weight gain exceeding the Institute of Medicine guidelines, compared with infants of the other women with Type 2 diabetes. In addition, total gestational weight gain was an independent predictor of excessive fetal growth regardless of pre-pregnancy BMI and glycaemic control during pregnancy.
The present study including all BMI classes of women with Type 2 diabetes extends the observations of our previous study, which was restricted to obese women with Type 2 diabetes 13, showing that low gestational weight gain is associated with a lower prevalence of large-for-gestational-age infants in obese women with Type 2 diabetes compared with women gaining > 5 kg. Our results are also consistent with a large cohort study 14 including 2310 overweight and obese women with Type 2 diabetes, which found that excessive gestational weight gain increased the risk of large-for-gestational-age infants and caesarean delivery.
Strengths of the present study are its inclusion of all consecutive pregnant women with Type 2 diabetes from a geographically well-defined area, with few exclusion criteria, and the structured collection of clinically relevant glycaemic data and weight changes during pregnancy. Both self-reported weight before pregnancy and measured weight at first pregnancy visit were recorded. As is common in clinical practice and in the Scandinavian literature 18, we chose to use maternal self-reported weight before pregnancy as the best estimate of weight before pregnancy. For accurate estimation of the weekly weight gain, the actual measured values at the same scale in the clinic were chosen for those calculations. It was reassuring that we were able to document a tight correlation between self-reported pre-pregnancy weight and the weight measured at the first pregnancy visit. Women with preterm delivery would have less time for weight gain, and pre-eclampsia might be associated with increased weight gain caused by oedema formation; however, excluding the small number of women delivering preterm or developing pre-eclampsia (13%) did not have an impact on the significant associations between gestational weight gain and fetal overgrowth.
In the present study, infant birth weight was almost 0.5 kg higher in women with excessive vs non-excessive weight gain, which is a more pronounced birth weight difference than that which has been observed with excessive weight gain according to the Institute of Medicine guidelines in healthy women 24. One explanation may be that in women with Type 2 diabetes, excessive food intake and presence of insulin resistance may result in higher plasma levels of nutrients, such as glucose and lipids compared with healthy women. These nutrients easily cross the placenta and induce fetal hyperinsulinaemia followed by excessive growth of the fetus with more fat accumulation in infants of mothers with Type 2 diabetes than in infants of women without diabetes 9–11,17.
Pre-pregnancy BMI and glycaemic control are well-known predictors of fetal overgrowth 2,10. In the present study, excessive gestational weight gain was associated with a higher infant birth weight z-score, independent of pre-pregnancy BMI, smoking, parity, ethnicity as well as HbA1c and insulin dose in late pregnancy.
Approximately every third woman was treated with insulin before first pregnancy visit and many needed insulin from early pregnancy on to achieve adequate glycaemic control. Women with non-excessive weight gain obtained strict glycaemic control with lower insulin doses than women with excessive weight gain.Whether excessive dietary intake is the driving factor for the excessive weight gain and higher insulin requirement, or whether poor glycaemic control leads to higher insulin requirement and thereby higher weight gain, is a matter of debate; however, HbA1c levels in early and late pregnancy were similar in those with excessive and those with non-excessive weight gain. Unfortunately, food records were not available for the present study. We speculate that excessive dietary intake might be the driving factor for the excessive weight gain and higher insulin dose.
Treatment with metformin may help reduce maternal weight gain during pregnancy in women with Type 2 diabetes but it does not seem to have an impact on fetal growth 25. At our centre, all oral antidiabetic drugs are discontinued at first pregnancy visit because of their potentially harmful fetal effects. A possible effect of metformin on maternal gestational weight gain and fetal growth cannot be evaluated in this study.
The weekly gestational weight gains varied by pre-pregnancy BMI class in early pregnancy, but were similar after 20 weeks, regardless of BMI class. The difference in weight gain in early pregnancy might therefore reflect less maternal gain of adipose tissue, while the weight gain in the last part of pregnancy might be more related to growth of the fetus, uterus and amniotic fluid 3,15. The weekly weight gain was highest between 34 and 37 gestational weeks, much higher than expected from the growth of the fetus, uterus and amniotic fluid and might partly reflect oedema formation. Unfortunately, we did not have any valid measure of oedema formation in this population.
Previous studies on healthy women suggest that weight change in the first half of pregnancy rather than later on in pregnancy is an important determinant of fetal growth 26,27. In the present study, both gestational weight gains in the first and second half of pregnancy were positively associated with fetal growth. The weekly weight gain increased as pregnancy progressed in all three BMI classes (data not shown). It therefore seems reasonable to recommend a lower weekly weight gain in early compared with late pregnancy. The division according to BMI classes (normal weight, overweight and obese) makes the number of women with Type 2 diabetes in each category too small for firm recommendations of weekly weight gain; however, the data from the present study might be useful until further studies are available.
In the present study, a nonsignificant trend towards more small-for-gestational-age infants was seen in women with insufficient weight gain, but numbers were too small to make solid conclusions. In a large cohort study on overweight and obese women with Type 2 diabetes, a nonsignificant trend towards a higher prevalence of small-for-gestational-age infants in women with insufficient weight gain compared with the remaining women was also seen 14. In normal weight women without diabetes, a gestational weight gain below the Institute of Medicine recommendations is associated with infants with low birth weight ( < 2500 g) but this is not seen in overweight and obese women 28. More research on the association between maternal gestational weight gain and fetal growth is needed; however, in the meantime, we suggest that a gestational weight gain within the lower range of the values recommended by the Institute of Medicine might be appropriate for women with Type 2 diabetes 13.
Changing the main focus of care from glycaemic control only to both glycaemic control and maternal weight gain may be the paradigm shift in care of pregnant women with Type 2 diabetes that in the future will reduce the high number of large-for-gestational-age infants. The Institute of Medicine guidelines take into account the fact that women who are already overweight or obese before pregnancy have a higher risk of delivering large-for-gestational-age infants and, therefore, a lower appropriate weight gain is recommended for them than for women of normal weight 3. Using the practical guidelines from the Institute of Medicine 3, set out according to the different BMI classes, and aiming for gestational weight gain within the lowest part of the recommended range may be a valuable tool in the management of women with diabetes.
Funding sources
None.
Competing interests
None declared.
References
- 1.Dietz PM, Callaghan WM, Sharma AJ. High pregnancy weight gain and risk of excessive fetal growth. Am J Obstet Gynecol. 2009;201:51e1–6. doi: 10.1016/j.ajog.2009.04.051. [DOI] [PubMed] [Google Scholar]
- 2.Yu CK, Teoh TG, Robinson S. Obesity in pregnancy. BJOG. 2006;113:1117–1725. doi: 10.1111/j.1471-0528.2006.00991.x. [DOI] [PubMed] [Google Scholar]
- 3.Institute of Medicine and National Research Council. Weight gain during pregnancy: reexamining the guidelines. Washington, DC: The National Academies Press; 2009. [PubMed] [Google Scholar]
- 4.ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 60, March 2005. Pregestational diabetes mellitus. Obstet Gynecol. 2005;105:675–685. doi: 10.1097/00006250-200503000-00049. [DOI] [PubMed] [Google Scholar]
- 5.Hedderson MM, Weiss NS, Sacks DA, Pettitt DJ, Selby JV, Quesenberry CP, et al. Pregnancy weight gain and risk of neonatal complications: macrosomia, hypoglycemia, and hyperbilirubinemia. Obstet Gynecol. 2006;108:1153–1161. doi: 10.1097/01.AOG.0000242568.75785.68. [DOI] [PubMed] [Google Scholar]
- 6.Mochhoury L, Razine R, Kasouati J, Kabiri M, Barkat A. Body mass index, gestational weight gain, and obstetric complications in Moroccan population. J Pregnancy. 2013;2013:379461. doi: 10.1155/2013/379461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clausen TD, Mathiesen E, Ekbom P, Hellmuth E, Mandrup-Poulsen T, Damm P. Poor pregnancy outcome in women with type 2 diabetes. Diabetes Care. 2005;28:323–328. doi: 10.2337/diacare.28.2.323. [DOI] [PubMed] [Google Scholar]
- 8.Roland JM, Murphy HR, Ball V, Northcote-Wright J, Temple RC. The pregnancies of women with Type 2 diabetes: poor outcomes but opportunities for improvement. Diabet Med. 2005;22:1774–1777. doi: 10.1111/j.1464-5491.2005.01784.x. [DOI] [PubMed] [Google Scholar]
- 9.Zawiejska A, Wender-Ozegowska E, Brazert J, Sodowski K. Components of metabolic syndrome and their impact on fetal growth in women with gestational diabetes mellitus. J Physiol Pharmacol. 2008;59(Suppl. 4):5–18. [PubMed] [Google Scholar]
- 10.Fowden A. Endocrine regulation of fetal growth. Reprod Fertil Dev. 1995;7:351–363. doi: 10.1071/rd9950351. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen J. Weight and length at birth of infants of diabetic mothers. Acta Endocrinologica. 1954;16:330–342. doi: 10.1530/acta.0.0160330. [DOI] [PubMed] [Google Scholar]
- 12.Schaefer-Graf UM, Graf K, Kulbacka I, Kjos SL, Dudenhausen J, Vetter K, et al. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care. 2008;31:1858–1863. doi: 10.2337/dc08-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asbjornsdottir B, Rasmussen SS, Kelstrup L, Damm P, Mathiesen ER. Impact of Restricted Maternal weight gain on fetal growth and perinatal morbidity in obese women with Type 2 diabetes. Diabetes Care. 2013;36:1102–1106. doi: 10.2337/dc12-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yee LM, Cheng YW, Inturrisi M, Caughey AB. Effect of gestational weight gain on perinatal outcomes in women with type 2 diabetes mellitus using the 2009 Institute of Medicine guidelines. Am J Obstet Gynecol. 2011;205:257e1–6. doi: 10.1016/j.ajog.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehrenberg HM, Huston-Presley L, Catalano PM. The influence of obesity and gestational diabetes mellitus on accretion and the distribution of adipose tissue in pregnancy. Am J Obstr Gynecol. 2003;189:944–948. doi: 10.1067/s0002-9378(03)00761-0. [DOI] [PubMed] [Google Scholar]
- 16.Nohr EA, Vaeth M, Baker JL, Sorensen T, Olsen J, Rasmussen KM. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. Am J Clin Nutr. 2008;87:1750–1759. doi: 10.1093/ajcn/87.6.1750. [DOI] [PubMed] [Google Scholar]
- 17.Harper LM, Shanks AL, Odibo AO, Colvin R, Macones GA, Cahill AG. Gestational weight gain in insulin-resistant pregnancies. J Perinatol. 2013;33:929–933. doi: 10.1038/jp.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen DM, Damm P, Sorensen B, Molsted-Pedersen L, Westergaard JG, Ovesen P, et al. Pregnancy outcome and prepregnancy body mass index in 2459 glucose-tolerant Danish women. Am J Obstr Gynecol. 2003;189:239–244. doi: 10.1067/mob.2003.441. [DOI] [PubMed] [Google Scholar]
- 19.Secher AL, Ringholm L, Andersen HU, Damm P, Mathiesen ER. The effect of real-time continuous glucose monitoring in pregnant women with diabetes: a randomized controlled trial. Diabetes Care. 2013;36:1877–1883. doi: 10.2337/dc12-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen LR, Damm P, Mathiesen ER. Improved pregnancy outcome in type 1 diabetic women with microalbuminuria or diabetic nephropathy: effect of intensified antihypertensive therapy? Diabetes Care. 2009;32:38–44. doi: 10.2337/dc08-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vestgaard M, Ringholm L, Laugesen CS, Rasmussen KL, Damm P, Mathiesen ER. Pregnancy-induced sight-threatening diabetic retinopathy in women with Type 1 diabetes. Diabet Med. 2010;27:431–435. doi: 10.1111/j.1464-5491.2010.02958.x. [DOI] [PubMed] [Google Scholar]
- 22.Maršál K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatrica. 1996;85:843–848. doi: 10.1111/j.1651-2227.1996.tb14164.x. [DOI] [PubMed] [Google Scholar]
- 23.Cordua S, Secher AL, Ringholm L, Damm P, Mathiesen ER. Real-time continuous glucose monitoring during labour and delivery in women with Type 1 diabetes - observations from a randomized controlled trial. Diabet Med. 2013;30:1374–1381. doi: 10.1111/dme.12246. [DOI] [PubMed] [Google Scholar]
- 24.Waters TP, Huston-Presley L, Catalano PM. Neonatal body composition according to the revised institute of medicine recommendations for maternal weight gain. J Clin Endocrinol Metab. 2012;97:3648–3654. doi: 10.1210/jc.2012-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rowan JA, Gao W, Hague WM, McIntyre HD. Glycemia and its relationship to outcomes in the metformin in gestational diabetes trial. Diabetes Care. 2010;33:9–16. doi: 10.2337/dc09-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li R, Haas JD, Habicht J-P. Timing of the influence of maternal nutritional status during pregnancy on fetal growth. Am J Hum Biol. 1998;10:529–539. doi: 10.1002/(SICI)1520-6300(1998)10:4<529::AID-AJHB13>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 27.Neufeld LM, Haas JD, Grajeda R, Martorell R. Changes in maternal weight from the first to second trimester of pregnancy are associated with fetal growth and infant length at birth. Am J Clin Nutr. 2004;79:646–652. doi: 10.1093/ajcn/79.4.646. [DOI] [PubMed] [Google Scholar]
- 28.Siega-Riz AM, Viswanathan M, Moos MK, Deierlein A, Mumford S, Knaack J, et al. A systematic review of outcomes of maternal weight gain according to the Institute of Medicine recommendations: birthweight, fetal growth, and postpartum weight retention. Am J Obstr Gynecol. 2009;201:339e1–14. doi: 10.1016/j.ajog.2009.07.002. [DOI] [PubMed] [Google Scholar]


