Abstract
Molecular strategies to improve outcomes for patients with pancreatic neuroendocrine tumours (nets) have focused on targeting vascular endothelial growth factor, platelet-derived growth factor, and mtor (the mammalian target of rapamycin). This approach has led to the regulatory approval of two molecularly targeted agents for advanced pancreatic nets: sunitinib, a multi-targeted tyrosine kinase inhibitor, and everolimus, an mtor inhibitor.
Initial experience with sunitinib in advanced pancreatic net was gained from the phase iii registration trial, which used a continuous daily dosing (cdd) schedule instead of daily drug administration for 4 consecutive weeks every 6 weeks (schedule 4/2), the approved schedule for advanced renal cell carcinoma (rcc) and gastrointestinal stromal tumour (gist). Clinical experience gained with schedule 4/2 in rcc and gist shows that, using a therapy management approach, patients can start and be maintained on the recommended dose and schedule, thus optimizing treatment outcomes. Here, we discuss challenges that can potentially be faced by physicians who use sunitinib on the cdd schedule, and we use clinical data and real-life clinical experience to present therapy management approaches that support cdd in advanced pancreatic net.
Keywords: Sunitinib, cdd, pancreatic net
1. INTRODUCTION
Although pancreatic neuroendocrine tumours (nets) are infrequent malignancies that account for 1.3% of cancers arising in the pancreas1, their diagnosed incidence appears to be increasing in both the United States and Europe2,3, and improvements in therapy have led to improved survival. Approximately 90% of pancreatic nets present as nonfunctional tumours2 and, as such, are not associated with specific hormonal syndromes, leading to diagnosis at an advanced stage of the disease4. Conversely, functioning nets that ectopically secrete active peptides cause a variety of hormonal conditions5, including Zollinger– Ellison syndrome (gastrinoma), hypoglycemia (insulinoma), glucose intolerance with related symptoms (glucagonoma), and vipoma5. Thus, functioning nets are often diagnosed at earlier stages, but require particular attention for the treatment of hormone-associated syndromes.
Somatostatin analogs can relieve symptoms caused by hypersecretion of hormones from functioning tumours and can potentially have antitumour activity in selected patients6–8. For many years, standard palliative regimens have used cytotoxic treatment with the alkylating agent streptozocin in combination with doxorubicin or 5-fluorouracil for the management of advanced pancreatic nets9. The focus of biomedical research in malignant disease has shifted in recent years from cytotoxic chemotherapy to treatments aimed at molecular targets controlling biologic processes such as cell proliferation, cell death, and tumour angiogenesis.
Biologic targets that have been identified as being of potential value in pancreatic nets include mtor (the mammalian target of rapamycin), a serine–threonine kinase that stimulates cell growth, proliferation, and angiogenesis; platelet-derived growth factor; and vascular endothelial growth factor (vegf), which plays a crucial role in the development and metastasis of solid tumours10. In pancreatic nets, vegf has been found to be a key driver of angiogenesis11,12. Targeted agents that have been evaluated in advanced pancreatic nets include the mtor inhibitor everolimus (Afinitor: Novartis Pharmaceuticals, Basel, Switzerland) and sunitinib (Sutent: Pfizer, New York, NY, U.S.A.), a multi-targeted agent that inhibits several tyrosine kinases, including vegf receptors 2 and 313. Malignant pancreatic nets also express platelet-derived growth factor receptors α and β, and stem-cell factor receptor14,15, both of which are additionally targeted by sunitinib13,16.
Sunitinib has been available since 2006 for the treatment of advanced renal cell carcinoma (rcc) and imatinib-resistant gastrointestinal stromal tumour (gist), and it is now approved for patients with well-differentiated unresectable or metastatic pancreatic nets. Phase i trials with sunitinib were initially designed using discontinuous administration of sunitinib to potentially allow for recovery from the adverse events that had been described in some preclinical models. The schedule that, in the phase i program, was associated with the more favourable safety profile and an important number of responses in advanced solid tumours used sunitinib administration daily for 4 consecutive weeks in every 6 weeks (schedule 4/2)17. Schedule 4/2 was therefore selected for the development of sunitinib in phase ii and iii trials13.
As greater confidence in managing the safety of sunitinib was gained, the toxicity profile of sunitinib was observed to be characterized by rapid recovery from acute side effects and by a lack of cumulative toxicity. Thus, as more patients were treated, it appeared that sunitinib might not particularly require any discontinuation periods. A continuous daily dosing (cdd) schedule of sunitinib therefore began to be investigated in clinical trials for patients with various malignancies. The starting dose of sunitinib in the cdd schedule was calculated primarily to maintain a dose intensity similar to that used in schedule 4/2 over a 6-week period. The cdd schedule therefore uses a 37.5 mg dose of sunitinib, which results in a dose intensity equivalent to that obtained with a dose of 50 mg daily on schedule 4/2.
Currently, schedule 4/2 is recommended for patients with rcc or gist18, and a cdd schedule has been approved in both the United States and Europe for patients with pancreatic net18,19. The present review discusses the challenges that can potentially face physicians who use sunitinib on the cdd schedule and presents therapeutic and management approaches that can help to support the cdd schedule and to optimize outcomes in patients with advanced pancreatic nets.
For this review, a search for relevant articles was performed in the authors’ personal publication library and in the PubMed and ClinicalTrials.gov databases. Full-text articles identified for the review all came from English-language journals. The reference lists of identified articles were also searched for further relevant papers.
2. CONSIDERATIONS FOR DEVELOPING A CDD SCHEDULE
Among the primary considerations in developing the cdd schedule were expectations that avoiding off-treatment periods would improve the activity of sunitinib in fast-growing tumours that might regrow during the washout period. Moreover, some preclinical data suggested that discontinuation of sunitinib might facilitate the emergence of resistance to sunitinib and a flare-up of tumour growth20. Furthermore, as mentioned earlier, experience with patients treated for prolonged periods was sufficient to demonstrate a lack of cumulative toxicity with sunitinib after 4 weeks of treatment. Other factors taken into account during the development of the 37.5 mg cdd schedule included pharmacokinetic and pharmacodynamic considerations. Pharmacokinetic data showed that the half-lives of sunitinib and its active metabolite were on the order of 40 and 80 hours respectively. Steady state after oral administration is thereby likely to be achieved in 1 week. A phase ii study in patients with gist21 reported that, based on mean dose-corrected trough values, the pharmacokinetics for sunitinib and its active metabolite SU12662 on a cdd schedule appear to be similar to those observed in a phase iii trial using schedule 4/222. In addition, a randomized phase ii study that compared the cdd regimen with the approved 50 mg daily dose of sunitinib on schedule 4/2 in patients with advanced rcc reported that steady-state Ctrough values for sunitinib, SU12662, and total drug were similar in patients on the cdd regimen (n = 79) and on schedule 4/2 (n = 63)23. It would therefore be expected that total plasma exposure to sunitinib would be similar for both schedule 4/2 and cdd.
Exposure–response studies in patients with rcc and gist who received sunitinib on schedule 4/2, 2/2, or 2/1 reported that time to progression and overall survival both improved with increasing sunitinib exposure. The analysis by Houk et al.24 also noted that increases in exposure increased the risk of some adverse events. In that study, the area under the concentration time-curve within the sunitinib dosing interval correlated with the incidence, but not the severity, of fatigue and with time to progression; overall survival in patients with rcc and gist also improved with increasing sunitinib exposure. Although such studies have not been performed for the cdd regimen, the experience gained using schedule 4/2 highlighted the need to initiate sunitinib at the recommended dose so as to avoid delaying the plasma steady state and subsequently to maintain patients at the recommended doses and schedules to achieve maximal clinical benefit.
In terms of the efficacy and safety profile of the cdd schedule, studies across a range of solid tumour types21,25–31 indicate that cdd is a potentially valuable sunitinib dosing schedule, showing antitumour activity with a manageable safety profile. In the randomized phase ii study that compared the 37.5 mg cdd regimen with the approved schedule 4/2 daily dose of 50 mg in patients with advanced rcc, comparable safety and quality-of-life outcomes were reported for the two arms23. However, a favourable trend for longer time to tumour progression with the schedule 4/2 daily dose of 50 mg was observed, indicating that the approved schedule 4/2 remains the starting regimen for patients with metastatic rcc23.
The greatest experience with the cdd schedule was recently reported from a phase iii trial in which 171 patients with advanced, well-differentiated pancreatic nets were randomized 1:1 to sunitinib 37.5 mg by cdd or to placebo, both with best supportive care27. Progression-free survival was inferior in the placebo group (median: 5.5 months vs. 11.4 months with sunitinib)27. The hazard ratio for progression or death with sunitinib was 0.42 (95% confidence interval: 0.26 to 0.66; p < 0.001), the hazard ratio for death alone was 0.41 (95% confidence interval: 0.19 to 0.89; p = 0.02), and the overall response rate with sunitinib was 9.3% compared with 0% with placebo. The magnitude and consistency of the observed treatment effects supported the use of sunitinib on a cdd schedule and resulted in regulatory approval of cdd sunitinib for patients with pancreatic net.
3. MAINTAINING COMPLIANCE AND CONTINUOUS EXPOSURE WITH THE CDD SCHEDULE
From our clinical experience, patients are generally comfortable taking sunitinib in daily dosing. This observation suggesting good compliance and acceptable tolerability of the cdd schedule is supported by the dose intensity achieved for sunitinib treatment in the phase iii study in patients with advanced pancreatic nets, which was maintained at a level similar to that seen with intermittent schedules27. Mean relative dose intensities (administered-to-planned dose) in the phase iii trial were 91.3% for sunitinib and 100.6% for placebo. Those results are comparable to those achieved with schedule 4/2 in pancreatic net32 and with cdd in other tumours (Table i).
TABLE I.
Dose tolerance and discontinuations related to safety in studies of sunitinib
| Study | Patients (n) | Dose (%) | Discontinuations because of adverse events (%) | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Reference | Phase | Tumour type | Intensity | Reductions | Interruptions | ||
| Continuous daily dosing (37.5 mg daily) in various cancers | |||||||
| Escudier et al., 200926 | Phase ii | rcc | 107 | 93 | 43 | 65 | 15 |
| George et al., 200921 | Phase ii | gist | 60 | nr | 23 | 77 | 7 |
| Novello et al., 200928 | Phase ii | nsclc | 47 | nr | 29.8 | 36.2 | 25.5 |
| Raymond et al., 201127 | Phase iii | Pancreatic | 86 Sunitinib | 91.3 | 31 | 30 | 17 |
| net | 85 Placebo | 100.6 | 11 | 12 | 8 | ||
| Schedule 4/2a in pancreatic net | |||||||
| Kulke et al., 200832 | Phase ii | 107 (41 carcinoid, 66 pancreatic net) | nr | 47.7 | 62.6 | 10.2 | |
Four consecutive weeks of every six weeks.
gist = gastrointestinal stromal tumour; nr = not reported; rcc = renal cell cancer; nsclc = non-small-cell lung cancer; net = neuroendocrine tumour.
Similarly, favourable outcomes with sunitinib in a cdd schedule were reflected in persistence with treatment and a low rate of treatment discontinuations because of adverse events or dose interruptions, particularly when compared with placebo in the phase iii study in patients with pancreatic nets (Table i)27. Compared with 4 patients randomized to placebo (5%), 19 patients randomized to sunitinib (22%) remained on the study for more than 1 year. Treatment was discontinued in 48% of patients treated with sunitinib and in 19% treated with placebo because of (early) study termination owing to disease progression (22% vs. 55% respectively) and adverse events (17% vs. 8% respectively). One or more dose interruptions (usually because of adverse events) were required for 30% of the patients in the sunitinib cdd group and for 12% in the placebo group (Table i). Those results compare favourably with results obtained using schedule 4/2 in patients with pancreatic nets or carcinoid tumours (Table i)32.
4. PRACTICAL MANAGEMENT OF TOXICITIES
As already mentioned, effective management of adverse events is essential for starting and subsequently maintaining patients at the recommended sunitinib dose and schedule, so as to optimize treatment outcomes. Experience of managing adverse events in patients receiving sunitinib was initially gained predominantly with the use of schedule 4/2 in metastatic rcc, because sunitinib has become the mainstay of treatment for most patients with that disease33; however, the recommendations for dealing with adverse events in patients receiving sunitinib are similar whether dosing is by cdd or schedule 4/2.
4.1. Gastrointestinal Toxicity
Diarrhea is one of the most common toxicities seen with sunitinib. A review of patients with rcc in medline and in the database of the American Society of Clinical Oncology34, together with a literature review and results of a structured consensus survey of German specialists33, suggests an incidence of up to 59%, although most cases are of mild-to-moderate severity. Grade 3 or 4 diarrhea (determined according to the Common Toxicity Criteria) was reported in 5% of patients with rcc receiving sunitinib34. Those proportions are matched in experiences of the cdd schedule in patients with pancreatic nets (Table ii)33. Because many patients with pancreatic net have previously undergone pancreatectomy35, that procedure could be the basis of pancreatic insufficiency and related diarrhea in some patients. In the phase iii cdd study sun 111127, 39% of patients in the placebo group experienced diarrhea of any grade, and 2% reported grade 3 or 4 diarrhea. Patients with pancreatic net who receive sunitinib might therefore require additional surveillance if they previously underwent pancreatectomy, although the significance of the effect requires further investigation.
TABLE II.
Most common adverse events with sunitinib 37.5 mg by continuous daily dosing
| Event | Event occurrence (%) | |||
|---|---|---|---|---|
|
| ||||
| Escudier et al., 200926 (rcc) | George et al., 200921 (gist) | Novello et al., 200928 (nsclc) | Raymond et al., 201127 (pancreatic net) | |
| Diarrhea | 75 | 40 | 34 | 59 |
| Nausea | 37 | 27 | 40a | 45 |
| Asthenia | 79b | 37 | 60b | 34 |
| Vomiting | 27 | 23 | 40a | 34 |
| Fatigue | 79b | 33 | 60b | 32 |
| Hair colour change | 34 | 22 | — | 29 |
| Neutropenia | 45 | 57 | 9c | 29 |
| Abdominal pain | 23 | 18 | — | 28 |
| Hypertension | 44 | 28 | 28 | 26 |
| Hand–foot syndrome | 49 | 25 | — | 23 |
| Anorexia | 42 | 20 | 17 | 22 |
| Stomatitis | 89 | 22 | 32d | 22 |
| Dysgeusia | 36 | — | 21 | 20 |
| Erythema | 17 | — | 21 | — |
Nausea and vomiting combined.
Asthenia and fatigue combined.
Only grade 3 and higher reported.
Stomatitis and mucosal inflammation.
net = neuroendocrine tumour; gist = gastrointestinal stromal tumour; rcc = renal cell cancer; nsclc = non-small-cell lung cancer.
Recommendations suggest no need for treatment interruption or dose reduction, or for intravenous fluids, in patients with rcc experiencing grade 1 or 2 diarrhea33. Patients with grade 3 or 4 diarrhea should discontinue therapy until symptoms subside to grade 1, with a dose reduction in subsequent treatment cycles34. In general, dose modification in patients receiving sunitinib according to the cdd schedule should be made in increments of 12.5 mg, noting that the maximum daily dose given in the phase iii sun 1111 study was 50 mg daily18. Table iii summarizes suggestions for managing diarrhea and other events in patients with pancreatic net receiving sunitinib.
TABLE III.
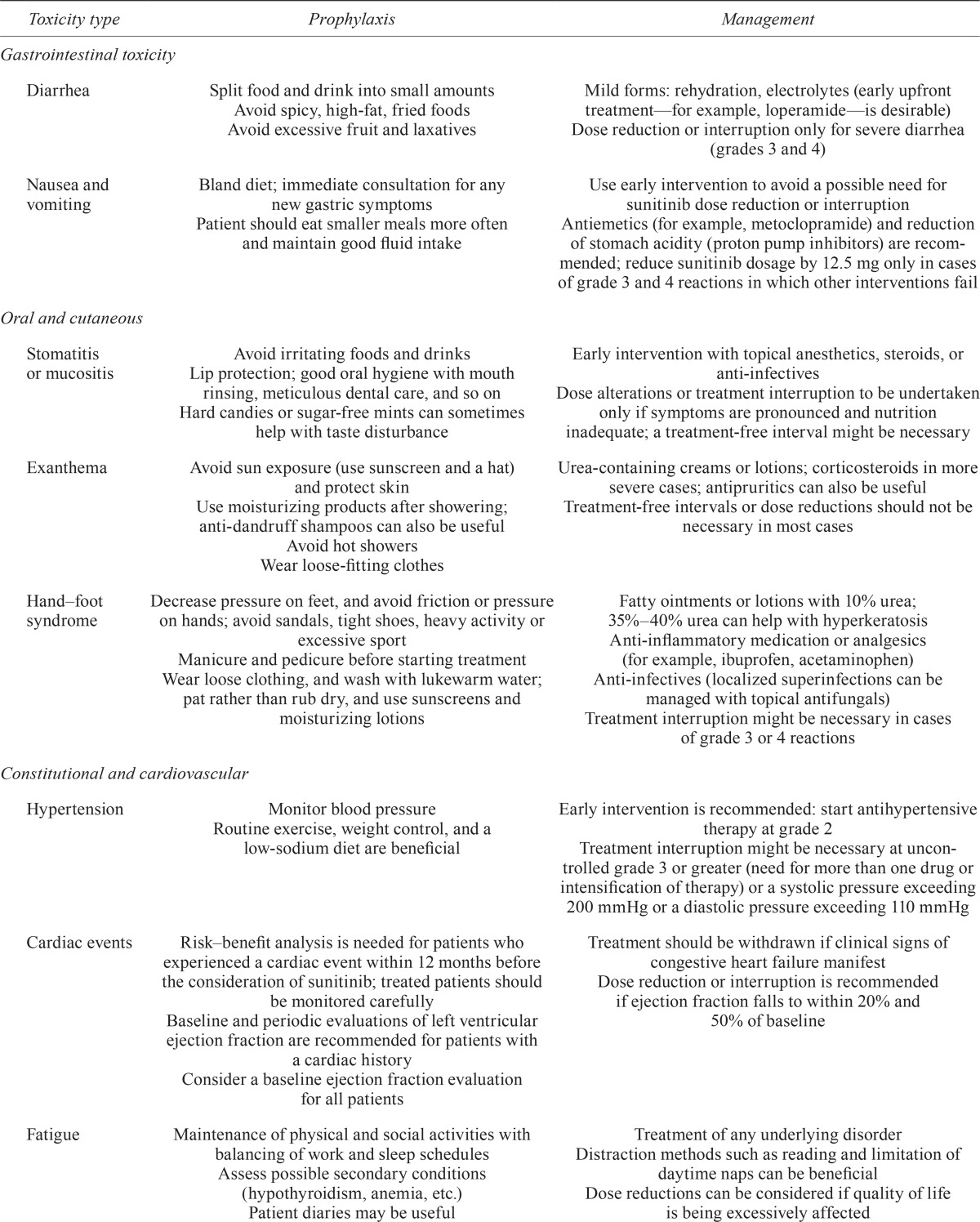
| Toxicity type | Prophylaxis | Management |
|---|---|---|
| Gastrointestinal toxicity | ||
| Diarrhea | Split food and drink into small amounts Avoid spicy, high-fat, fried foods Avoid excessive fruit and laxatives |
Mild forms: rehydration, electrolytes (early upfront treatment—for example, loperamide—is desirable) Dose reduction or interruption only for severe diarrhea (grades 3 and 4) |
| Nausea and vomiting | Bland diet; immediate consultation for any new gastric symptoms | Use early intervention to avoid a possible need for sunitinib dose reduction or interruption |
| Patient should eat smaller meals more often and maintain good fluid intake | Antiemetics (for example, metoclopramide) and reduction of stomach acidity (proton pump inhibitors) are recommended; reduce sunitinib dosage by 12.5 mg only in cases of grade 3 and 4 reactions in which other interventions fail | |
| Oral and cutaneous | ||
| Stomatitis or mucositis | Avoid irritating foods and drinks Lip protection; good oral hygiene with mouth rinsing, meticulous dental care, and so on Hard candies or sugar-free mints can sometimes help with taste disturbance |
Early intervention with topical anesthetics, steroids, or anti-infectives Dose alterations or treatment interruption to be undertaken only if symptoms are pronounced and nutrition inadequate; a treatment-free interval might be necessary |
| Exanthema | Avoid sun exposure (use sunscreen and a hat) and protect skin Use moisturizing products after showering; anti-dandruff shampoos can also be useful Avoid hot showers Wear loose-fitting clothes |
Urea-containing creams or lotions; corticosteroids in more severe cases; antipruritics can also be useful Treatment-free intervals or dose reductions should not be necessary in most cases |
| Hand–foot syndrome | Decrease pressure on feet, and avoid friction or pressure on hands; avoid sandals, tight shoes, heavy activity or excessive sport Manicure and pedicure before starting treatment Wear loose clothing, and wash with lukewarm water; pat rather than rub dry, and use sunscreens and moisturizing lotions |
Fatty ointments or lotions with 10% urea; 35%–40% urea can help with hyperkeratosis Anti-inflammatory medication or analgesics (for example, ibuprofen, acetaminophen) Anti-infectives (localized superinfections can be managed with topical antifungals) Treatment interruption might be necessary in cases of grade 3 or 4 reactions |
| Constitutional and cardiovascular | ||
| Hypertension | Monitor blood pressure Routine exercise, weight control, and a low-sodium diet are beneficial |
Early intervention is recommended: start antihypertensive therapy at grade 2 Treatment interruption might be necessary at uncontrolled grade 3 or greater (need for more than one drug or intensification of therapy) or a systolic pressure exceeding 200 mmHg or a diastolic pressure exceeding 110 mmHg |
| Cardiac events | Risk–benefit analysis is needed for patients who experienced a cardiac event within 12 months before the consideration of sunitinib; treated patients should be monitored carefully Baseline and periodic evaluations of left ventricular ejection fraction are recommended for patients with a cardiac history Consider a baseline ejection fraction evaluation for all patients |
Treatment should be withdrawn if clinical signs of congestive heart failure manifest Dose reduction or interruption is recommended if ejection fraction falls to within 20% and 50% of baseline |
| Fatigue | Maintenance of physical and social activities with balancing of work and sleep schedules Assess possible secondary conditions (hypothyroidism, anemia, etc.) Patient diaries may be useful |
Treatment of any underlying disorder Distraction methods such as reading and limitation of daytime naps can be beneficial Dose reductions can be considered if quality of life is being excessively affected |
| Laboratory tests | ||
| Hypothyroidism | Routine monitoring after baseline measurement is recommended | Continue laboratory monitoring and treat per standard practice (hormone replacement) Treatment interruption is unlikely to be necessary |
| Hematology and liver function | Complete blood workup to be done before treatment (each cycle) | Treatment interruption or dose reduction suggested for grades 3 and 4 abnormalities Treatment withdrawal if results fail to return to normal |
| Hypophosphatemia | Electrolyte control Include serum phosphate in laboratory analyses to be done before each treatment cycle |
Alimentary substitution Treatment interruption or dose reduction should not be necessary |
Sunitinib therapy can also lead to upper gastrointestinal symptoms such as loss of appetite, epigastric pain, and nausea or vomiting. The latter two events tend to appear early in therapy and can be controlled with standard interventions (Table iii). In sun 1111 patients with pancreatic nets, nausea and vomiting were experienced by, respectively, 45% and 34% of patients receiving sunitinib (Table ii) and 29% and 30% of patients receiving placebo. Grade 3 or 4 nausea (1%) and vomiting (0%) were reported only very rarely, with an incidence that was not superior to that seen in the placebo arm; data from patients with rcc have shown rates of grade 3 vomiting of 1.6%–3.1%33. Again, management recommendations suggest a bland diet, with early use of antiemetic drugs if necessary. Sunitinib dose reduction (by 12.5 mg) should be considered only in cases of grade 3 or 4 events in which other interventions have failed.
4.2. Oral and Cutaneous Toxicity
Stomatitis has been reported to be a typical adverse reaction with sunitinib, although only in 22% of patients receiving sunitinib by cdd in the sun 1111 study, with grade 3 or 4 stomatitis being noted in 4% of patients27. In contrast to the stomatitis seen with chemotherapy, patients receiving targeted therapy often experience functional symptoms accompanied only by taste disturbances34. Symptoms should be controlled early by dietary changes and rigorous oral care (Table iii). Treatment interruptions or dose reductions are necessary only if severe mucosal toxicity develops.
Cutaneous reactions include exanthema and hand–foot syndrome (palmar–plantar erythrodysesthesia). Hand–foot syndrome presents as painful symmetrical erythematous and edematous areas on the palms and soles, often preceded or accompanied by paresthesias, tingling, or numbness. Desquamation may also be seen34,36. Management approaches include pressure relief, wearing of loose-fitting clothes, washing with lukewarm rather than hot water and patting dry rather than rubbing, and use of sunscreen and creams containing lanolin or urea36. Grade 3 or 4 hand–foot syndrome could necessitate temporary cessation of therapy, with a 12.5 mg dose reduction at restart after remission of symptoms33. Skin rashes caused by sunitinib seldom require dose reduction, and symptoms tend to decrease over time. Moisturizing skin lotions or creams used often, particularly after showering or before bedtime, can be helpful, and urea-containing lotions are useful, particularly if the skin is very dry (Table iii)33,36.
4.3. Cardiovascular Toxicity
Arterial hypertension is a class effect of vegf receptor inhibitors and was reported in 26% of patients in sun 1111, a frequency similar to that seen in patients with rcc receiving either cdd or schedule 4/223 and in studies in other tumour types in which cdd was used (Table ii)21,28. The trial by Escudier et al.26 reported a higher rate of all-grade hypertension in rcc patients receiving sunitinib by cdd (Table ii). However, the frequency of grade 3 hypertension was 11% (compared with 10% in sun 111127), and hypertension was not a major cause of treatment interruption in the trial.
Blood pressure should be monitored and treated as necessary according to local practice in patients receiving sunitinib33. Increased blood pressure can be managed without dose adjustment, although compliance with the treatment-free interval in schedule 4/2 is necessary34. Because antihypertensives might have to be reduced during the 2 weeks off sunitinib, cdd can be more manageable by avoiding the peak and trough periods of sunitinib exposure.
Although rare, cardiovascular events including heart failure, myocardial disorders, and cardiomyopathy, some of which have been fatal, have been reported during postmarketing surveillance of sunitinib18. Compared with rcc or gist patients receiving interferon alfa or placebo, rcc or gist patients treated with sunitinib more often experienced reduced left ventricular ejection fraction18, and in sun 1111, fatal cardiac failure potentially linked to study treatment was reported in 1 patient on sunitinib27. Discontinuation of therapy is recommended in any patient with clinical signs of congestive heart failure, and in the absence of congestive heart failure, dose reduction is recommended when ejection fraction declines to 20%–50% below baseline18. Caution is also advised in patients with a history of QT interval prolongation, in those taking antiarrhythmics, and in those with relevant pre-existing cardiac disease, bradycardia, or electrolyte disturbances. Periodic monitoring with electrocardiography and determination of electrolytes (magnesium, potassium) should be considered regardless of the dosing regimen used18.
4.4. Fatigue
Fatigue, which impairs quality of life over the longer term34, has previously been reported in 62% of patients with rcc18, although this adverse event is commonly associated with a variety of conditions (including cancer and endocrine disease) and with administration of cancer therapeutics generally33. In sun 1111, fatigue of any grade was reported in 32% of patients receiving sunitinib and in 27% of those receiving placebo27, although higher combined incidences of asthenia and fatigue have been reported with sunitinib cdd in other indications (Table ii). Patient information and assessment of underlying causes of fatigue are important during therapy with sunitinib. Dose interruption or reduction is necessary only when quality of life and daily activities are significantly impaired (Table iii).
4.5. Laboratory Abnormalities
Sunitinib is reported to induce neutropenia and thrombocytopenia in 50%–70% of patients, although only 3%–4% experience events of grade 3 or 4 severity36. In sun 1111, 29% of patients on sunitinib by cdd experienced neutropenia of any grade, and 12% experienced grades 3 and 4 neutropenia27. Treatment-free intervals are necessary in grades 3 and 4 cases. Hepatotoxicity has also been noted in some patients receiving sunitinib, and patients accordingly require monitoring of liver function. Grades 3 and 4 events are grounds for interruption of treatment, with discontinuation if there is no resolution18.
Thyroid function changes have been described in up to 85% of patients receiving sunitinib34,36, with up to 36% requiring treatment and approximately half of that group requiring hormone substitution34. Hypothyroidism was reported as an adverse reaction in 6 of 83 patients (7%) on sunitinib and in 1 of 82 patients (1%) in the placebo arm in sun 111127.
5. PERSONAL EXPERIENCE WITH THERAPY MANAGEMENT FOR PATIENTS WITH PANCREATIC NET
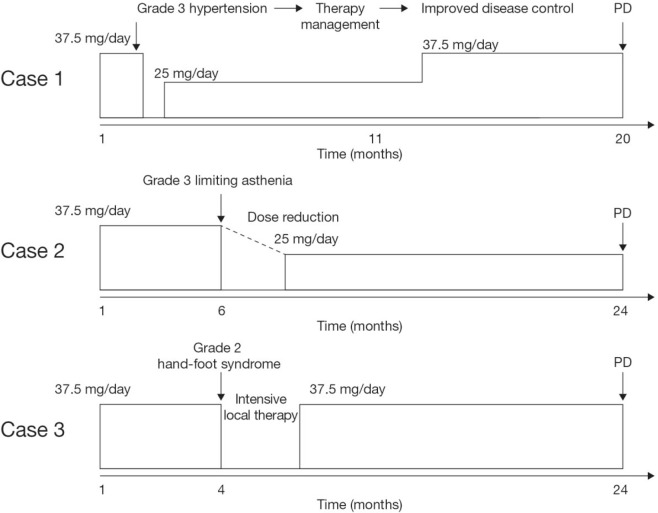
The Beaujon University Hospital clinical centre has used therapy management approaches for patients participating in the sunitinib phase iii study. Cases of grade 3 hypertension, severe asthenia, and hand–foot syndrome taken from that cohort illustrate our “real-life” approach to maintaining sunitinib treatment in such patients. Figure 1 shows the management course for each patient.
FIGURE 1.
Schematic showing the management course for patients who participated in the sunitinib phase iii study27. pd = progressive disease.
The first patient, a 58-year-old man, presented with a well-differentiated endocrine tumour in the head of the pancreas. He had a history of arterial hypertension that had been stabilized with enalapril. After diagnosis, he received adjuvant chemotherapy with 5-fluorouracil, and streptozocin was given for 6 months. Follow-up imaging at the end of chemotherapy suggested the occurrence of liver metastasis, and at 18 months’ follow-up, he became symptomatic. A significant increase in the size of the lesions was also observed. The patient then went on to receive sunitinib 37.5 mg daily by cdd as part of the phase iii trial, with stable disease (by the Response Evaluation Criteria in Solid Tumors) reported after 1 treatment cycle.
On day 8 of cycle 2, the patient experienced grade 3 hypertension (190/100 mmHg), despite receiving ongoing antihypertensive treatment. We therefore interrupted treatment and modified his antihypertensive regimen after consultation with a cardiologist (calcium inhibitor plus combined diuretic and angiotensin inhibitor). After therapy management, this patient’s blood pressure stabilized within 3 days, and after a 7-day delay, sunitinib therapy was resumed at a reduced dose of 25 mg daily. After 11 months, the dose of sunitinib was re-escalated to 37.5 mg daily because of an observed increase in tumour dimensions on radiologic imaging assessment, with no further occurrence of hypertension, allowing for 9 additional months of disease control. To summarize, management of antihypertensive therapy after a transient (1-week) interruption of sunitinib allowed for continuation of treatment for more than 1.5 years.
In the second case, a 65-year-old woman with well-differentiated symptomatic pancreatic net and liver metastases received initial somatostatin analog therapy, followed by doxorubicin–streptozocin and then temozolomide, which required treatment interruption because of dose-limiting grade 4 thrombocytopenia. One year later, the patient entered the phase iii sunitinib trial, and stable disease (by the Response Evaluation Criteria in Solid Tumors) was noted after 2 consecutive treatment cycles. After unblinding, this patient was found to be receiving sunitinib 37.5 mg daily by cdd. After 6 cycles of treatment, an objective response (Response Evaluation Criteria in Solid Tumors) was observed on computed tomography imaging; however, the patient complained of severe cumulative asthenia and refused further cycles of treatment. The patient was advised to undergo 2 weeks’ rest from sunitinib followed by re-introduction of sunitinib at a reduced dose of 25 mg daily by cdd. Transient (2-week) interruption of sunitinib allowed the patient to recover to a good performance status. With ongoing sunitinib therapy, the patient maintained stable disease for an additional 18 months (2 years total), until disease progression and deterioration in performance status were observed.
In the third case, a 57-year-old man presented with well-differentiated pancreatic net and liver metastases and underwent duodeno-cephalic pancreatectomy. After chemotherapy (doxorubicin–streptozocin), he was enrolled in the radiant phase iii trial of everolimus and subsequently into the phase iii trial of sunitinib. After 4 cycles of sunitinib, the patient complained of difficulties in performing intensive physical activity because of foot pain. Physical examination revealed moderate hand–foot syndrome (grade 2). Sunitinib treatment was interrupted for 2 weeks, and intensive local therapy (exfoliation, topical ointments) was recommended. After complete healing within 10 days, sunitinib was reintroduced at full dose and continued without recurrence of limiting hand–foot syndrome and with sustainable disease control for more than 2 years.
6. SUMMARY
In a population with limited treatment options, targeted agents for patients with advanced pancreatic net have significantly improved progression-free survival. However, these new agents have brought with them new challenges in terms of distinct side-effect profiles.
Pharmacokinetic and clinical data from the metastatic rcc and gist setting have shown that, to optimize clinical outcomes with sunitinib, patients should be maintained at the recommended dose and schedule (schedule 4/2), with use of therapy management to minimize the likelihood or severity of adverse events leading to dose reductions. Growing clinical experience in treating patients with pancreatic net suggests that a similar approach to therapy management should be taken to ensure adherence to the recommended cdd sunitinib schedule. At our centre, therapy management approaches have included transient treatment interruption and dose modification to reduce the intensity of the adverse events, plus proactive adverse event management using appropriate prophylactic and supportive measures. In addition, radiologic imaging to assess disease status when the patient presents with side effects can be used to aid in decision-making about dose escalation.
7. ACKNOWLEDGMENTS
Medical writing support was provided by Nicola Crofts at acumed (Tytherington, U.K.) and was funded by Pfizer Inc.
8. CONFLICT OF INTEREST DISCLOSURES
ER has received compensation for a consultant/advisory role, honoraria, and research funding from Pfizer. SF has received honoraria and research funding from Pfizer and Novartis.
9. REFERENCES
- 1.Yao JC, Eisner MP, Leary C, et al. Population-based study of islet cell carcinoma. Ann Surg Oncol. 2007;14:3492–500. doi: 10.1245/s10434-007-9566-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (pnets): incidence, prognosis and recent trend toward improved survival. Ann Oncol. 2008;19:1727–33. doi: 10.1093/annonc/mdn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 4.Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology. 2008;135:1469–92. doi: 10.1053/j.gastro.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kulke MH, Bendell J, Kvols L, Picus J, Pommier R, Yao J. Evolving diagnostic and treatment strategies for pancreatic neuroendocrine tumors. J Hematol Oncol. 2011;4:29. doi: 10.1186/1756-8722-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Modlin IM, Pavel M, Kidd M, Gustafsson BI. Review article: somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine (carcinoid) tumours. Aliment Pharmacol Ther. 2010;31:169–88. doi: 10.1111/j.1365-2036.2009.04174.x. [DOI] [PubMed] [Google Scholar]
- 7.Oberg K, Kvols L, Caplin M, et al. Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Ann Oncol. 2004;15:966–73. doi: 10.1093/annonc/mdh216. [DOI] [PubMed] [Google Scholar]
- 8.Rinke A, Müller HH, Schade–Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide lar in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the promid Study Group. J Clin Oncol. 2009;27:4656–63. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 9.Moertel CG, Johnson CM, McKusick MA, et al. The management of patients with advanced carcinoid tumors and islet cell carcinomas. Ann Intern Med. 1994;120:302–9. doi: 10.7326/0003-4819-120-4-199402150-00008. [DOI] [PubMed] [Google Scholar]
- 10.Baltogiannis G, Katsios C, Roukos DH. New target therapies for patients with neuroendocrine tumors of the pancreas. Expert Rev Gastroenterol Hepatol. 2011;5:563–6. doi: 10.1586/egh.11.55. [DOI] [PubMed] [Google Scholar]
- 11.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of vegf signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Inoue M, Hager JH, Ferrara N, Gerber HP, Hanahan D. vegf-a has a critical, nonredundant role in angiogenic switching and pancreatic beta cell carcinogenesis. Cancer Cell. 2002;1:193–202. doi: 10.1016/S1535-6108(02)00031-4. [DOI] [PubMed] [Google Scholar]
- 13.Faivre S, Demetri G, Sargent W, Raymond E. Molecular basis for sunitinib efficacy and future clinical development. Nat Rev Drug Discov. 2007;6:734–45. doi: 10.1038/nrd2380. [DOI] [PubMed] [Google Scholar]
- 14.Fjällskog ML, Hessman O, Eriksson B, Janson ET. Upregulated expression of pdgf receptor beta in endocrine pancreatic tumors and metastases compared to normal endocrine pancreas. Acta Oncol. 2007;46:741–6. doi: 10.1080/02841860601048388. [DOI] [PubMed] [Google Scholar]
- 15.Fjällskog ML, Lejonklou MH, Oberg KE, Eriksson BK, Janson ET. Expression of molecular targets for tyrosine kinase receptor antagonists in malignant endocrine pancreatic tumors. Clin Cancer Res. 2003;9:1469–73. [PubMed] [Google Scholar]
- 16.Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–37. [PubMed] [Google Scholar]
- 17.Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumour activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24:25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]
- 18.Pfizer Labs. Sutent—sunitinib malate capsule [prescribing information] New York, NY: Pfizer Labs; 2011. [Available online at http://labeling.pfizer.com/showlabeling.aspx?id=607; cited September 29, 2014] [Google Scholar]
- 19.European Medicines Agency (ema) Sutent [Web resource] London, UK: EMA; 2013. [Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000687/human_med_001069.jsp&mid=WC0b01ac058001d124; cited January 16, 2012] [Google Scholar]
- 20.Mancuso MR, Davis R, Norberg SM, et al. Rapid vascular regrowth in tumors after reversal of vegf inhibition. J Clin Invest. 2006;116:2610–21. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.George S, Blay JY, Casali PG, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer. 2009;45:1959–68. doi: 10.1016/j.ejca.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–38. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 23.Motzer RJ, Hutson TE, Olsen MR, et al. Randomized phase ii trial of sunitinib on an intermittent versus continuous dosing schedule as first-line therapy for advanced renal cell carcinoma. J Clin Oncol. 2012;30:1371–7. doi: 10.1200/JCO.2011.36.4133. [DOI] [PubMed] [Google Scholar]
- 24.Houk HE, Bello CL, Poland B, Rosen LS, Demetri GD, Motzer RJ. Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother Pharmacol. 2010;66:357–71. doi: 10.1007/s00280-009-1170-y. [DOI] [PubMed] [Google Scholar]
- 25.Barrios CH, Hernandez–Barajas D, Brown MP, et al. Phase ii trial of continuous once-daily dosing of sunitinib as first-line treatment in patients with metastatic renal cell carcinoma. Cancer. 2012;118:1252–9. doi: 10.1002/cncr.26440. [DOI] [PubMed] [Google Scholar]
- 26.Escudier B, Roigas J, Gillessen S, et al. Phase ii study of sunitinib administered in a continuous once-daily dosing regimen in patients with cytokine-refractory metastatic renal cell carcinoma. J Clin Oncol. 2009;27:4068–75. doi: 10.1200/JCO.2008.20.5476. [DOI] [PubMed] [Google Scholar]
- 27.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–13. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 28.Novello S, Scagliotti GV, Rosell R, et al. Phase ii study of continuous daily sunitinib dosing in patients with previously treated advanced non-small cell lung cancer. Br J Cancer. 2009;101:1543–8. doi: 10.1038/sj.bjc.6605346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carr LL, Mankoff DA, Goulart BH, et al. Phase ii study of daily sunitinib in fdg-pet–positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin Cancer Res. 2010;16:5260–8. doi: 10.1158/1078-0432.CCR-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kozloff M, Chuang E, Starr A, et al. An exploratory study of sunitinib plus paclitaxel as first-line treatment for patients with advanced breast cancer. Ann Oncol. 2010;21:1436–41. doi: 10.1093/annonc/mdp565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koeberle D, Montemurro M, Samaras P, et al. Continuous sunitinib treatment in patients with advanced hepatocellular carcinoma: a Swiss Group for Clinical Cancer Research (sakk) and Swiss Association for the Study of the Liver (sasl) multi-center phase ii trial (sakk 77/06) Oncologist. 2010;15:285–92. doi: 10.1634/theoncologist.2009-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kulke MH, Lenz HJ, Meropol NJ, et al. Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol. 2008;26:3403–10. doi: 10.1200/JCO.2007.15.9020. [DOI] [PubMed] [Google Scholar]
- 33.Grünwald V, Kalanovic D, Merseburger AS. Management of sunitinib-related adverse events: an evidence- and expert-based consensus approach. World J Urol. 2010;28:343–51. doi: 10.1007/s00345-010-0565-z. [DOI] [PubMed] [Google Scholar]
- 34.Ivanyi P, Winkler T, Ganser A, Reuter C, Grünwald V. Novel therapies in advanced renal cell carcinoma: management of adverse events from sorafenib and sunitinib. Dtsch Arztebl Int. 2008;105:232–7. doi: 10.3238/arztebl.2008.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Neuroendocrine Tumors Ver 1.2012. Fort Washington, PA: NCCN; 2012. [Current version available online at: http://www.nccn.com/files/cancer-guidelines/breast/index.html; cited January 16, 2012] [DOI] [PubMed] [Google Scholar]
- 36.Theou–Anton N, Faivre S, Dreyer C, Raymond E. Benefit–risk assessment of sunitinib in gastrointestinal stromal tumours and renal cancer. Drug Saf. 2009;32:717–34. doi: 10.2165/00002018-200932090-00003. [DOI] [PubMed] [Google Scholar]