Abstract
Background
Before the emergence of first-line combination chemotherapy, the standard of care for unresectable metastatic colorectal cancer (mcrc) was first-line monotherapy with modulated 5-fluorouracil. Several large phase iii randomized controlled trials, now completed, have assessed whether a planned sequential chemotherapy strategy—beginning with fluoropyrimidine monotherapy until treatment failure, followed by another regimen (either monotherapy or combination chemotherapy) until treatment failure—could result in the same survival benefit produced with an upfront combination chemotherapy strategy, but with less toxicity for patients.
Methods
The medline and embase databases, and abstracts from meetings of the American Society for Clinical Oncology and the European Society for Medical Oncology, were searched for reports comparing a sequential strategy of chemotherapy with an upfront combination chemotherapy in adult patients with mcrc. Publications that reported efficacy or toxicity data (or both) were included.
Results
The five eligible trials that were identified included 4532 patients. A meta-analysis of those trials demonstrates a statistically significant survival advantage for combination chemotherapy (hazard ratio: 0.92; 95% confidence interval: 0.86 to 0.99). However, the median survival advantage (3–6 weeks in most trials) is small and of questionable clinical significance. Three trials reported first-line toxicities. Upfront combination chemotherapy results in significantly more neutropenia, febrile neutropenia, thrombocytopenia, diarrhea, nausea, vomiting, and sensory neuropathy. Sequential chemotherapy results in significantly more hand–foot syndrome.
Conclusions
Given the small survival advantage associated with upfront combination chemotherapy, planned sequential chemotherapy and upfront combination chemotherapy can both be considered treatment strategies. Treatment should be chosen on an individual basis considering patient and tumour characteristics, toxicity of each strategy, and patient preference.
Keywords: Metastatic colorectal cancer, chemotherapy strategies, meta-analyses, palliative treatment, systematic reviews
1. INTRODUCTION
Colorectal cancer is the third most common cancer in men and the second most common cancer in women globally, with an estimated 1.23 million new cases in 2008. An estimated 608,000 colorectal cancer deaths occurred worldwide in 2008, representing 8% of all cancer deaths1. There is, therefore, interest in improving treatment results and quality of life (qol) for people with colorectal cancer.
The strategy most commonly used for unresectable metastatic colorectal cancer (mcrc) in Ontario is upfront combination chemotherapy with a fluoropyrimidine (5-fluorouracil or capecitabine) and either oxaliplatin or irinotecan, with or without a biologic agent2–4. Several large randomized phase iii trials, now completed, have assessed whether a planned sequential chemotherapy strategy—beginning with fluoropyrimidine monotherapy until treatment failure, followed by another regimen (either monotherapy or combination chemotherapy) until treatment failure—could result in the same survival benefit produced with an upfront combination chemotherapy strategy, but with less toxicity for patients. The combination chemotherapy strategy has been recognized as a standard of care in Canadian and international guidelines3–4. The Gastrointestinal Disease Site Group of the Program in Evidence-Based Care (pebc) decided that a systematic review of the evidence and a synthesis of the available data that could guide the treatment recommendations made by clinicians for their patients with unresectable mcrc being treated with palliative intent would be useful.
2. METHODS
2.1. Literature Search Strategy
The medline [2000 through July (week 5) 2013] and embase (2000 through week 32, 2013) databases were searched for relevant evidence. The year 2000 was chosen as the starting point because it pre-dates the approval of irinotecan and oxaliplatin for use in mcrc. The complete medline and embase literature search strategies can be found in the full guideline at https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=295210. The reference lists from retained articles were also searched for additional relevant trials. In addition, the proceedings of the 2004–2013 American Society of Clinical Oncology and the 2002–2012 European Society of Medical Oncology annual meetings were searched for abstract reports of relevant studies.
2.2. Study Selection Criteria
Articles were included if they were published English-language abstracts or full reports of randomized controlled trials comparing a sequential strategy of chemotherapy with an upfront combination chemotherapy in adult patients with mcrc and if they included at least one of the outcomes of interest. Syntheses of randomized controlled trials in the form of systematic reviews or meta-analyses were also eligible. If more than one study evaluated the same dataset, only the most recent paper was selected for inclusion.
2.3. Synthesizing the Evidence
When clinically homogenous results from two or more trials were available, the data were pooled using the Review Manager software (RevMan 5.1) provided by the Cochrane Collaboration (http://tech.cochrane.org/revman). Because the hazard ratio (hr), rather than the number of events at a certain time, is the preferred statistic for pooling time-to-event outcomes5, hrs were extracted directly from the most recently reported trial results. Variances of the hr estimates were calculated from the reported confidence intervals (cis) using the methods described by Parmar et al.5. All pooling used a random-effects model, because that model provides a more conservative estimate of effect6.
Statistical heterogeneity was calculated using the chi-square test for heterogeneity and the I2 percentage. A probability of less than or equal to 10% for the chi-square statistic (p ≤ 0.10) or an I2 greater than 50%, or both, were considered indicative of statistical heterogeneity. Results are expressed as hrs with 95% cis. When the hr is less than 1.0, patients receiving the experimental treatment have a lower probability of experiencing the event (death); conversely, when the hr is greater than 1.0, patients in the experimental arm have a higher probability of experiencing the event.
2.4. Role of the Funding Source
The pebc is supported by the Ontario Ministry of Health and Long-Term Care. All work produced by the pebc is editorially independent from the Ministry. The pebc is an initiative of the Ontario provincial cancer system, Cancer Care Ontario. Its mandate is to improve the lives of Ontarians affected by cancer through the development, dissemination, and evaluation of evidence-based products designed to facilitate clinical, planning, and policy decisions about cancer care. For more information, please visit https://www.cancercare.on.ca/toolbox/qualityguidelines/diseasesite/.
3. RESULTS
3.1. Literature Search Results
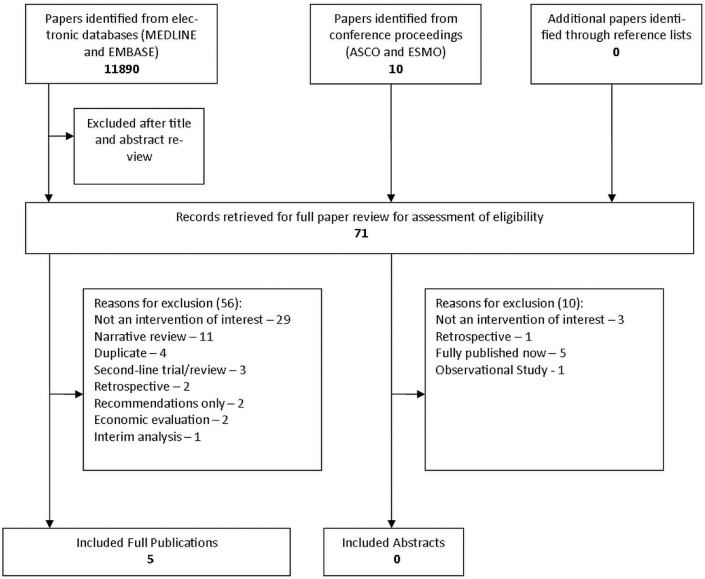
The medline search yielded 3383 hits, of which 42 were potentially relevant and were fully reviewed. Five publications were retained (Figure 1). The embase search yielded 8507 hits, of which 19 were potentially relevant and were fully reviewed. None of the 19 were retained. No abstracts from the American Society of Clinical Oncology or the European Society of Medical Oncology meetings were retained.
FIGURE 1.
The literature search results. asco = American Society of Clinical Oncology; esmo = European Society for Medical Oncology.
3.2. Trial Design and Quality
Using information provided in the trial reports, the randomized trials were assessed for key methodologic characteristics. The elements assessed were generation of the allocation sequence, allocation concealment, blinding, intention-to-treat analysis, withdrawals, loss to follow-up, funding source, statistical power calculations, length of follow-up, differences in baseline patient characteristics, and early termination.
3.3. Outcomes
3.3.1. Trial Design and Quality
All five trials7–11 involved adult patients with advanced unresectable or metastatic colorectal cancer, and all compared a planned sequential chemotherapy strategy with an upfront combination chemotherapy (Table i). None of the patients had received prior systemic therapy for advanced colorectal cancer. All patients had a World Health Organization performance status in the range 0–2. Only three studies7,8,10 reported median follow-up time. All trials were superiority trials. The focus211 trial was specifically designed for elderly and frail patients who were considered unsuitable for full-dose chemotherapy. In that trial, starting doses were 80% of standard, with the option of increasing to the full dose after 6 weeks at the discretion of the treating oncologist.
TABLE I.
Characteristics of the identified randomized controlled trials
| Reference | Patient characteristics | Treatment | Primary endpoint | Patients | Median follow-upa (months) | |
|---|---|---|---|---|---|---|
|
| ||||||
| Randomized | Evaluated | |||||
| Koopman et al., 20077 (cairo) | Colorectal cancer | Sequential: capecitabine, then irinotecan, then capecitabine–oxaliplatin | Overall survival | 410 | 401 | 31.5 |
| Advanced, not amenable to surgery | ||||||
| No previous systemic treatment for advanced disease | ||||||
| Age: ≥18 years; who ps: 0–2 | Combination: capecitabine–irinotecan, then capecitabine–oxaliplatin | 410 | 402 | |||
| Seymour et al., 20078 (focus) | Colorectal adenocarcinoma | Sequential: 5-fluorouracil, then irinotecan | 2-Year survival | 710 | 26.5 | |
| Inoperable metastatic or locoregional disease | ||||||
| No prior chemotherapy for metastatic disease | Deferred combination A: 5-fluorouracil, then 5-fluorouracil–irinotecan | 356 | ||||
| Age: >18 years; who ps: 0–2 | ||||||
| Deferred combination B: 5-fluorouracil, then 5-fluorouracil–oxaliplatin | 356 | |||||
| Combination A: 5-fluorouracil–irinotecan | 356 | |||||
| Combination B: 5-fluorouracil–oxaliplatin | 357 | |||||
| Cunningham et al., 20099 (life) | Colorectal cancer | Sequential: | 2-Year survival | 363 | 363 | Not reported |
| Distant metastases (excluding central nervous system) | 5-fluorouracil, then irinotecan | |||||
| No prior chemotherapy for metastatic disease | Combination: | 362 | 362 | |||
| Age: ≥18 years; who ps: 0–2 | 5-fluorouracil–oxaliplatin, then irinotecan | |||||
| Ducreux et al., 201110 (ffcd) | Colorectal cancer | Sequential: 5-fluorouracil, then folfox6, then folfiri | pfs for 1st- and 2nd-line treatment | 205 | 205 | 36 |
| Metastatic, not amenable to curative-intent surgery | ||||||
| No prior chemotherapy for metastatic disease | Combination: folfox6, then folfiri | 205 | 205 | |||
| Age: >18 years; who ps: 0–2 | ||||||
| Seymour et al., 201111 (focus2) | Colorectal adenocarcinoma | Sequential A: 5-fluorouracil, then 5-fluorouracil–oxaliplatin | pfs | 115 | Not reported | |
| Inoperable advanced or metastatic disease | ||||||
| No prior systemic therapy for metastatic disease | Sequential B: capecitabine, then capecitabine–oxaliplatin | 115 | ||||
| No upper or lower age limit; who ps: 0–2 | ||||||
| Patients had to be unsuitable for standard full-dose combination therapy | Combination A: 5-fluorouracil–oxaliplatin | 115 | ||||
| Combination B: capecitabine–oxaliplatin | 114 | |||||
Of patients still living.
who = World Health Organization; ps = performance status; folfox = folinic acid, 5-fluorouracil, oxaliplatin; folfiri = folinic acid, 5-fluorouracil, irinotecan; pfs = progression-free survival.
The sequential arm in all the trials consisted of a first-line fluoropyrimidine7–11. The cairo7, focus8, and li fe9 trials followed the upfront fluoropyrimidine monotherapy with second-line irinotecan monotherapy. The cairo trial also allowed for third-line combination chemotherapy (capecitabine–oxaliplatin). The ffcd10 and focus211 trials followed the upfront monotherapy with second-line combination chemotherapy. The ffcd10 trial also included another combination chemotherapy regimen as third-line treatment. The combination chemotherapy arm in all the trials began with up-front combination chemotherapy, with nothing else planned to follow8,11, although further unplanned treatment with another combination regimen7,10 or with monotherapy9 could be instituted at the discretion of the treating physician11. The focus8 trial had a third strategy called “deferred combination.” That arm consisted of first-line monotherapy with a fluoropyrimidine, followed by a combination regimen (similar to the sequential arm in focus211).
With respect to trial quality, all five trials reported on the generation of allocation sequences, described withdrawals, had industry funding, provided statistical power calculations, used intention-to-treat analysis, and had balanced baseline patient characteristics. One of the studies reported loss to follow-up10, and one of the studies was terminated early10 (data not shown; see the full guideline at https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=295210).
3.3.2. Response and Survival
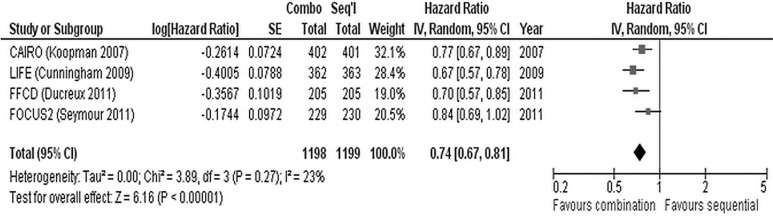
In each of the five studies7–11, the overall response rate (complete and partial responses) was significantly greater in the upfront combination chemotherapy arm than in the sequential chemotherapy arm (Table ii). Similarly, progression-free survival (pfs) was significantly greater in the combination chemotherapy arm in four of the studies7–10. In focus211, pfs was not significantly different between the treatment arms. Meta-analysis of the four trials that reported hrs for pfs7,9–11 demonstrated a significant benefit for combination chemotherapy (hr: 0.74; 95% ci: 0.67 to 0.81; p < 0.00001; Figure 2). We observed no significant heterogeneity between the trials with respect to pfs.
TABLE II.
Survival and response outcomes from the identified randomized controlled trials
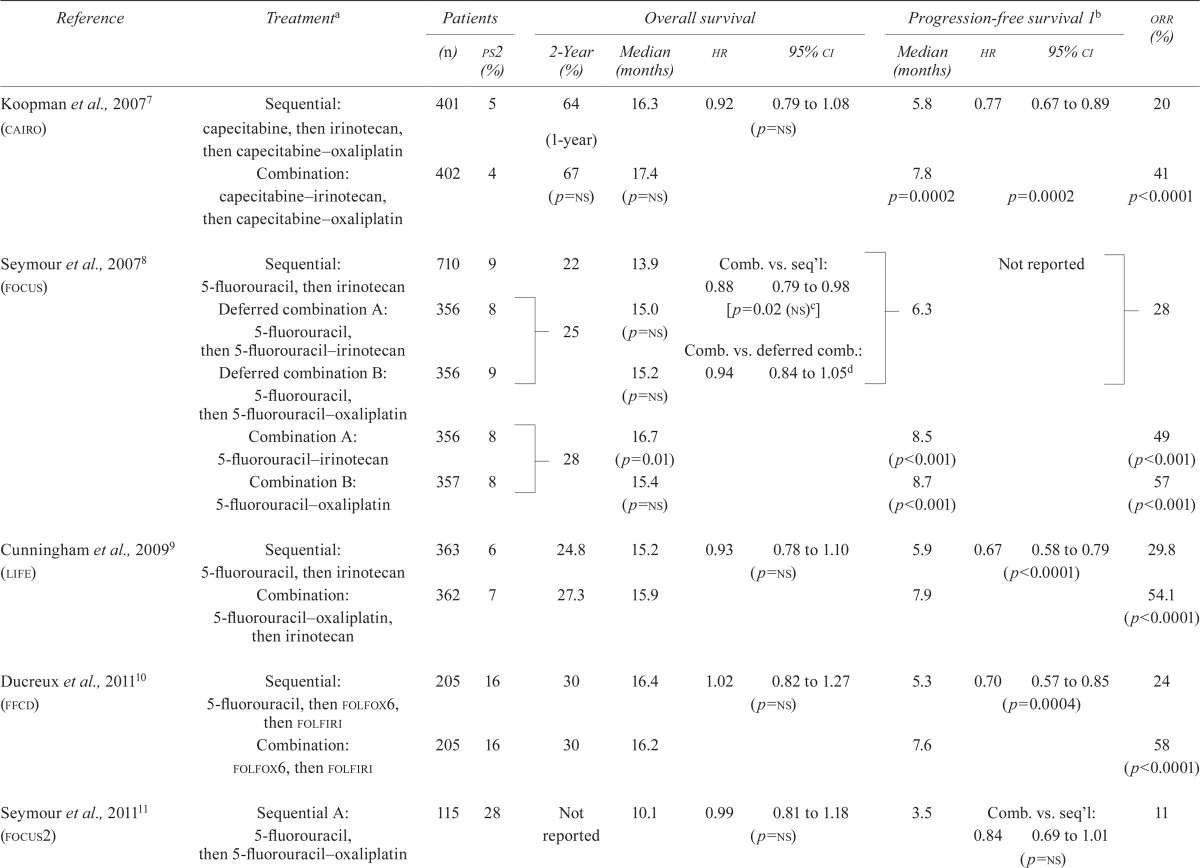
| Reference | Treatmenta | Patients | Overall survival | Progression-free survival 1b | orr (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||
| (n) | ps2 (%) | 2-Year (%) | Median (months) | hr | 95% ci | Median (months) | hr | 95% ci | |||
| Koopman et al., 20077 (cairo) | Sequential: capecitabine, then irinotecan, then capecitabine oxaliplatin | 401 | 5 | 64 (1-year) | 16.3 | 0.92 | 0.79 to 1.08 | 5.8 | 0.77 | 0.67 to 0.89 | 20 |
| (p=ns) | |||||||||||
| Combination: capecitabine irinotecan, then capecitabine oxaliplatin | 402 | 4 | 67 (p=ns) | 17.4 (p=ns) | 7.8 p=0.0002 |
p=0.0002 | 41 p<0.0001 |
||||
| Seymour et al., 20078 (focus) | Sequential: 5-fluorouracil, then irinotecan | 710 | 9 | 22 | 13.9 | Comb. vs. seq’l: | 6.3 | Not reported | 28 | ||
| 0.88 | 0.79 to 0.98 | ||||||||||
| Deferred combination A: 5-fluorouracil, then 5-fluorouracil irinotecan | 356 | 8 | 25 | 15.0 (p=ns) | [p=0.02 (ns)c] | ||||||
| Comb. vs. deferred comb.: | |||||||||||
| Deferred combination B: 5-fluorouracil, then 5-fluorouracil oxaliplatin | 356 | 9 | 15.2 (p=ns) | 0.94 | 0.84 to 1.05d | ||||||
| Combination A: 5-fluorouracil irinotecan | 356 | 8 | 28 | 16.7 (p=0.01) | 8.5 (p<0.001) | 49 (p<0.001) | |||||
| Combination B: 5-fluorouracil oxaliplatin | 357 | 8 | 15.4 (p=ns) | 8.7 (p<0.001) | 57 (p<0.001) | ||||||
| Cunningham et al., 20099 (life) | Sequential: 5-fluorouracil, then irinotecan | 363 | 6 | 24.8 | 15.2 | 0.93 | 0.78 to 1.10 | 5.9 | 0.67 | 0.58 to 0.79 | 29.8 |
| (p=ns) | (p<0.0001) | ||||||||||
| Combination: 5-fluorouracil oxaliplatin, then irinotecan | 362 | 7 | 27.3 | 15.9 | 7.9 | 54.1 (p<0.0001) | |||||
| Ducreux et al., 201110 (ffcd) | Sequential: 5-fluorouracil, thenfolfox6, then folfiri | 205 | 16 | 30 | 16.4 | 1.02 | 0.82 to 1.27 | 5.3 | 0.70 | 0.57 to 0.85 | 24 |
| (p=ns) | (p=0.0004) | ||||||||||
| Combination: folfox6, then folfiri | 205 | 16 | 30 | 16.2 | 7.6 | 58 (p<0.0001) | |||||
| Seymour et al., 201111 (focus2) | Sequential A: 5-fluorouracil, then 5-fluorouracil oxaliplatin | 115 | 28 | Not reported | 10.1 | 0.99 | 0.81 to 1.18 | 3.5 | Comb. vs. seq’l: | 11 | |
| (p=ns) | 0.84 | 0.69 to 1.01 | |||||||||
| (p=ns) | |||||||||||
| Seymour et al., 201111 (focus2) | Sequential B: capecitabine, then capecitabine oxaliplatin | 115 | 30 | 11.0 | 5.2 | 14 | |||||
| Combination A: 5-fluorouracil oxaliplatin | 115 | 30 | 10.7 | 5.8 | 38 | ||||||
| Combination B: capecitabine oxaliplatin | 114 | 29 | 12.4 | 5.8 | 32 (p<0.0001) | ||||||
Planned lines of therapy shown; followed by salvage treatment at the discretion of the treating oncologist.
Time from randomization to first progression or death.
Because of adjustments made to account for multiple testing, authors set significance at p < 0.01 to confirm superiority at the 99% confidence level.
Study reported a hazard ratio of 1.06 and a 90% confidence interval of 0.97 to 1.17. For the purpose of the present review, the data were inverted such that all comparisons are in the same direction. Also, the 95% confidence interval was calculated to make it consistent with the other included studies.
ps2 = having a performance status of 2; hr = hazard ratio; ci = confidence interval; orr = overall response rate; ns = nonsignificant; comb. = combination; seq’l = sequential.
FIGURE 2.
Meta-analysis of progression-free survival in four trials7,9–11. se = standard error; iv = inverse variance; ci = confidence interval.
In each of the five included studies7–11, overall survival (os) was not significantly longer for the combination than for the sequential chemotherapy strategy (Table ii). The focus study8 did report significantly greater median survival in one of the upfront combination arms (consisting of fluorouracil– irinotecan) compared with the sequential chemotherapy arm (16.7 months vs. 13.9 months, p = 0.01). However, compared with sequential chemotherapy, the other upfront combination chemotherapy arm (consisting of fluorouracil–oxaliplatin) did not result in longer survival. Moreover, a comparison of upfront combination chemotherapy and sequential chemotherapy that omitted the “deferred combination” arms did not result in a statistically significant longer survival (hr: 0.88; 95% ci: 0.79 to 0.98) because the authors of the trial used a more stringent significance level [p < 0.01 (see Table ii, footnote c)] to account for the multiple testing performed.
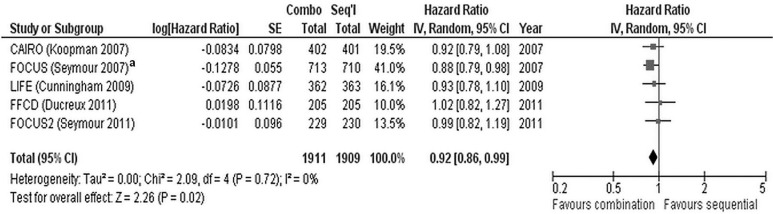
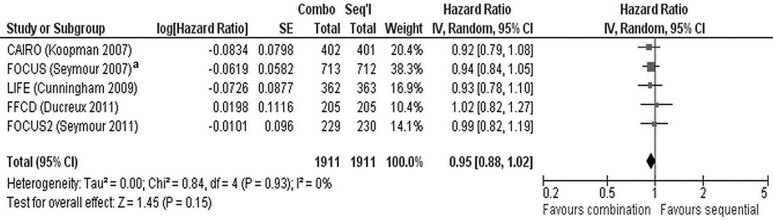
Meta-analysis of the five trials does demonstrate a significant benefit for combination chemotherapy (hr: 0.92; 95% ci: 0.86 to 0.99; p = 0.02) and no heterogeneity (I2 = 0%, p = 0.72, Figure 3). However, the meta-analysis might be problematic, in that, for the focus trial8, a hr for survival that included all the data was not available. A hr for survival was available for the comparison of the upfront combination chemotherapy (n = 713) and the sequential chemotherapy (n = 710) strategies (and was used in the meta-analysis), but the arm for the deferred combination strategy (n = 712) was not included. Thus, one third of the trial data (n = 712) were not included in that hr. A second meta-analysis (Figure 4) used the hr from a comparison of the upfront combination chemotherapy strategy (n = 713) with the deferred combination (essentially sequential) chemotherapy strategy (n = 712). The data from the sequential arm (n = 710) are not included. The second analysis demonstrated no significant survival benefit for a combination chemotherapy strategy (hr: 0.95; 95% ci: 0.88 to 1.02; p = 0.15) compared with a sequential chemotherapy strategy and no heterogeneity between the trials (I2 = 0%, p = 0.93).
FIGURE 3.
Initial meta-analysis of overall survival in five trials7–11. se = standard error; iv = inverse variance; ci = confidence interval. a Data for the upfront combination and sequential chemotherapy arms are included; data for the deferred combination chemotherapy arm are not.
FIGURE 4.
Alternate meta-analysis of overall survival in five trials7–11. se = standard error; iv = inverse variance; ci = confidence interval. a Data for the upfront combination and deferred combination chemotherapy arms are included; data for the sequential monotherapy chemotherapy arm are not.
After the initiation of focus8, standard practice changed from first-line fluorouracil to combination chemotherapy. The focus authors then decided to conduct an analysis using combination chemotherapy as the reference for the deferred combination strategy, in a type of noninferiority analysis. They used the ci from the trial to calculate a noninferiority boundary of 1.18. That analysis resulted in a hr of 0.94 and a 95% ci of 0.84 to 1.05 (results inverted to make the comparison consistent with the other trials). To conclude that one strategy is noninferior to the other, a properly designed noninferiority trial would have to be conducted.
3.3.3. Toxicity
Toxicity data are reported differently in the various trials. The life9 and focus211 trials report toxicity data over the entire trial; focus8 reports toxicities resulting from first-line treatment only. The cairo7 and ffcd10 trials report toxicity data both ways (data not shown, see the full guideline at https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=295210).
Looking at the data for specific toxicities that were reported over entire trials, the incidences of grades 3 and 4 anemia, febrile neutropenia, and thrombocytopenia were not significantly different in the sequential and upfront combination chemotherapy arms (data not shown). Neutropenia was the only hematologic toxicity that was reported in all the included trials. Two trials7,11 reported no difference in the rate of neutropenia between study arms. In the life9 trial, more cases of neutropenia were observed in the upfront combination chemotherapy arm than in the sequential chemotherapy arm (no significance level provided); and in the ffcd10 trial, significantly more cases of neutropenia were observed in the up-front combination chemotherapy arm.
The cairo study7 reported that the study arms were not significantly different with respect to grades 3 and 4 nonhematologic toxicities. Several nonhematologic toxicities (diarrhea; nausea or vomiting, or both; sensory neuropathy) occurred more often in the upfront combination chemotherapy arms in the life9 trial, although no p values were reported. Hand–foot syndrome occurred significantly more often in the sequential arm of the cairo7 trial. In the ffcd trial10, sensory neuropathy occurred significantly more often in the upfront combination chemotherapy arm. In the focus2 trial11, which included only elderly and frail participants, significantly more diarrhea and sensory neuropathy was observed in the upfront combination chemotherapy arm and significantly more hand–foot syndrome in the sequential chemotherapy arm.
With respect to hematologic toxicities in first-line treatment only, two studies7,10 reported a significantly higher incidence of grades 3 and 4 neutropenia, and one study7 reported a significantly higher incidence of febrile neutropenia in the upfront combination chemotherapy arm than in the sequential chemotherapy arm. No differences were found for anemia, and one study10 reported significantly more thrombocytopenia with the upfront combination chemotherapy strategy. The focus8 trial reported only on neutropenia. More cases of neutropenia occurred in the upfront combination chemotherapy arm, but a significance level was not provided. The nonhematologic grades 3 and 4 toxicities that occurred significantly more often in the upfront combination chemotherapy arm were diarrhea7, nausea7,10, and vomiting7,10. Significantly more grade 3 hand–foot syndrome was observed in the sequential chemotherapy arm in the cairo7 study. The focus8 trial reported more diarrhea, nausea or vomiting, and sensory neuropathy in the combination chemotherapy arm; however, significance levels were not provided.
3.3.4. Quality of Life
In the cairo7, focus8, and ffcd10 trials, qol was assessed using the European Organisation for Research and Treatment of Cancer’s Quality of Life Questionnaire C30 (qlq-C30). The authors of the cairo study reported that changes in financial problems and global health status were similar in both study arms. They also reported that decline in emotional, physical, role, and social functioning was greater in the upfront combination chemotherapy arm than in the sequential chemotherapy arm. Moreover, changes on the symptomatic scales, with the exception of pain and dyspnea, were generally greater (indicating worse symptoms) in the upfront combination chemotherapy arm. However, the only significant symptomatic change occurred on the diarrhea scale (p = 0.002), which was worse in the upfront combination chemotherapy arm7. In the focus trial8, overall qol was similar for the regimens and over time. The authors concluded that, for combination chemotherapy, there was no advantage or disadvantage at 3 and 6 months. In the ffcd10 trial, no significant differences in the global and physical dimensions were observed between the study arms. There was, however, a significant difference between the arms with respect to the emotional dimension, which favoured the upfront combination group (p = 0.009)
In the focus2 trial11, qol was assessed using the Comprehensive Health Assessment (cha) instrument. At weeks 12–14, global qol was reported to be improved in 62% of patients in the sequential chemotherapy arm and in 49% of patients in the combination chemotherapy arm (p = 0.04). Based on the cha results, the authors concluded that the addition of oxaliplatin has a detrimental effect on global qol.
The life trial9 did not report on qol.
4. DISCUSSION AND CONCLUSIONS
In the late 1990s and early 2000s, evidence emerged that, compared with fluoropyrimidine monotherapy, upfront combination chemotherapy with new cytotoxics (oxaliplatin and irinotecan) produced response and survival (pfs and os) advantages12–15 in the treatment of unresectable mcrc. Subsequently, several trials7–11 were designed to determine whether efficacy could be maintained, toxicity reduced, and qol improved by deferring introduction of the new cytotoxic agents. In the standard arms of those trials, an effective chemotherapy doublet combination was given as first-line treatment and compared with alternative strategies in which first-line therapy was a single-agent fluoropyrimidine with varying plans for subsequent administration of the remaining active therapies. With the completion and publication of those trials, our pooled analysis compares the outcomes of the alternative strategies with those of first-line combination chemotherapy.
With respect to os, upfront combination chemotherapy was not superior to planned serial administration of chemotherapy in any of the five trials7–11 individually. However, when the data are pooled meta-analytically (Figure 3), a significant survival benefit of upfront combination chemotherapy does emerge (Figure 3; hr: 0.92; 95% ci: 0.86 to 0.99; p = 0.02). The meta-analysis is somewhat problematic, in that the hr for the focus trial8 included only the comparison of the upfront combination strategy with the sequential monotherapy strategy and accounted for only 67% of the patients involved in the trial. For the present purpose, it would have been ideal for the meta-analysis to use a hr that included data for all the patients and that compared the upfront combination strategy with the arms using an upfront monotherapy strategy, but the data from the arm using the deferred combination strategy were not included in the hr. The focus trial8 does report a hr for the comparison of the upfront combination strategy with the deferred combination strategy (which is essentially a sequential strategy beginning with single-agent 5-fluorouracil). A second meta-analysis using that hr from the focus trial demonstrates that no significant survival benefit accrues to upfront combination chemotherapy compared with a sequential or deferred combination chemotherapy strategy (Figure 4; hr: 0.95; 95% ci: 0.88 to 1.02; p = 0.15). Notwithstanding the difference in statistical significance observed in the two meta-analyses, the os difference between the upfront combination and initial monotherapy strategies is likely to be of minimal clinical significance given the hrs observed in the two meta-analyses.
All five trials7–11 demonstrate a significantly better overall response rate for upfront combination chemotherapy (all trials, p < 0.0001). Similarly, four of the trials report significantly better pfs in the upfront combination chemotherapy arm7–10. The pfs was also superior in the upfront combination arm of focus211, although it did not reach statistical significance (hr: 0.84; 95% ci: 0.69 to 1.01; p = nonsignificant). Meta-analysis of the four trials that reported hrs for pfs8,9–11 demonstrates the overall benefit for upfront combination chemotherapy (Figure 2; hr: 0.74; 95% ci: 0.67 to 0.81; p < 0.00001). The superior pfs for combination chemotherapy occurs only in first-line treatment. During later lines of treatment, pfs was not significantly different between treatment arms7,10 (data not shown).
In three studies, qol was assessed using the qlq-C30 instrument7,8,10. The authors of the cairo study7 reported similar changes in financial problems and global health status in both arms of their study. They also reported that the decline in emotional, physical, role, and social functioning was generally greater in the upfront combination chemotherapy arm than in the sequential chemotherapy arm. Moreover, changes on the symptomatic scales, with the exception of pain and dyspnea, were generally greater (that is, symptoms were worse) in the upfront combination chemotherapy arm. However, the only significant symptomatic change was seen on the diarrhea scale (p = 0.002), with diarrhea being worse in the upfront combination chemotherapy arm7. In the focus trial8, overall qol was similar between the regimens and over time. In the ffcd10 trial, no significant differences between the trial arms were observed with respect to the global and physical dimensions. There was, however, a significant difference between the arms with respect to the emotional dimension, which favoured the upfront combination group (p = 0.009). In the focus2 trial11, qol was assessed using the cha instrument, and the authors reported improved global qol at weeks 12–14 in 62% of patients in the sequential chemotherapy arm and in 49% of patients in the combination chemotherapy arm (p = 0.04). Based on the cha data, the authors concluded that the addition of oxaliplatin has a detrimental effect on global qol. Overall, qol was at least as good— and, for some dimensions, better—with a sequential chemotherapy strategy.
With respect to hematologic toxicities in first-line treatment only (data not shown, three studies reporting), significantly greater grades 3 and 4 toxicity was reported for neutropenia7,10, febrile neutropenia7, and thrombocytopenia10 in the upfront combination chemotherapy arm than in the sequential chemotherapy arm. The nonhematologic grades 3 and 4 toxicities of diarrhea7, nausea7,10, and vomiting7,10 occurred significantly more often in the upfront combination chemotherapy arm. In the cairo7 study, significantly more grade 3 hand–foot syndrome occurred in the sequential chemotherapy arm. The focus8 trial reported more diarrhea, nausea or vomiting, and sensory neuropathy in the upfront combination chemotherapy arm; however, significance levels were not provided. Those results are not surprising, in that toxicity would be higher for upfront combination chemotherapy than for initial monotherapy.
There has been criticism16 that the trials comparing upfront combination and sequential chemotherapy strategies achieve a considerably lower median survival than most other recent trials. There are several possible explanations for that discrepancy. All five trials included in the systematic review enrolled patients who were less fit and would likely not be candidates for curative surgery if the first-line chemotherapy had been sufficiently successful. In fact, in several of the trials, recruiting physicians were specifically asked not to enrol patients they thought might become operable if they responded well enough to first-line chemotherapy. Moreover, focus211 included only frail and elderly patients, a population that is traditionally underrepresented in clinical trials. Such patients would also never be considered for resection. A performance status of 2 was more frequent among the patients in the trials used in the meta-analysis (4%–30%, Table ii) than among patients in other trials (2%–8%)17. It is therefore not surprising that the median survival in the studies included in the present review is lower than that seen in contemporaneous trials. Another possible explanation is the use, in some of the trials, of capecitabine and irinotecan, which, compared with folfir i (leucovorin–5-fluorouracil–irinotecan), have been shown to be associated with inferior survival18 and toxicity profiles18,19. Additionally, the superior pfs seen with first-line combination chemotherapy in the trials included in our systematic review is not maintained over subsequent lines of treatment.
That observation, combined with a lack of superior survival in the comparison of upfront combination chemotherapy with sequential chemotherapy suggests that the survival benefit seen in other recent trials might be attributable to an inadequate use of salvage treatments in the monotherapy arm. One other possible explanation relates to the number of patients in the sequential strategies who were actually exposed to all the effective drugs that they were planned to be exposed to. In the five trials, only 36%–61% of patients in the sequential arms received all planned lines of therapy and, therefore, exposure to all planned effective drugs. It is notable that, in the ffcd trial, which used only folfiri chemotherapy and which had appropriate access to all three cancer drugs, os was similar to that seen in other recent trials.
Our systematic review considered trials conducted in an era before targeted therapies were included as part of mcrc treatment. We now have good evidence that the addition of biologics has further improved outcomes in first-line treatment; the current Ontario standard is chemotherapy and bevacizumab20–23. The addition of bevacizumab to a single-agent fluoropyrimidine has been shown to be safe and effective20,21,24. As the authors of both the focus and the ffcd trials point out, bevacizumab and fluoropyrimidine monotherapy is generally reserved for patients thought to be unfit for combination therapy. They suggest that the results of their trials support extending that approach to patients receiving upfront monotherapy in a sequential approach8,10. However, given that none of the randomized trials included in the present analysis used biologics, definitive statements about the integration of biologics into a sequential strategy cannot be made at this time.
The ultimate goal for the treatment of mcrc is to improve survival duration and qol for patients. Compared with upfront combination chemotherapy strategies, strategies that initially use monotherapy are less toxic, improve qol in some trials, produce no clinically significant detriment in os, and are an acceptable option for patients with unresectable mcrc. Appropriate patient selection is important; upfront combination chemotherapy might be more appropriate for patients with rapidly progressing, highly symptomatic, or bulky life-threatening visceral disease, given the higher overall response rates seen with that strategy.
In conclusion, based on currently available evidence, the use of sequential chemotherapy in the palliative treatment of mcrc is an appropriate option for some patients and should be part of an informed discussion between patients with unresectable mcrc and their medical oncologists. Given the advances in targeted therapy for mcrc, more studies of sequential strategies incorporating biologic agents are needed.
5. ACKNOWLEDGMENTS
The pebc is supported by the Ontario Ministry of Health and Long-Term Care. All work produced by the pebc is editorially independent from its funding source.
6. CONFLICT OF INTEREST DISCLOSURES
The following conflicts of interest are declared: TA has received more than $5000 in a single year from consulting fees, honoraria, or other support from Sanofi–Aventis, Roche, and Pfizer. He has received research grant support from Sanofi–Aventis, Roche, and Pfizer and has been a co-investigator in mcrc trials, providing an opinion piece for the The Globe and Mail and acting as the Ontario Medical Association vice-chair for the section on Hematology and Medical Oncology. The remaining authors have no financial conflicts of interest to disclose.
7. REFERENCES
- 1.Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008: Cancer Incidence and Mortality Worldwide [Web resource] Ver. 2.0. Lyon, France: International Agency for Research on Cancer; 2010. [Current version available online at: http://globocan.iarc.fr; cited December 2, 2013] [Google Scholar]
- 2.Welch S, Spithoff K, Rumble RB, Maroun J, on behalf of the Gastrointestinal Cancer Disease Site Group Bevacizumab combined with chemotherapy for patients with advanced colorectal cancer: a systematic review. Ann Oncol. 2010;21:1152–62. doi: 10.1093/annonc/mdp533. [DOI] [PubMed] [Google Scholar]
- 3.Vickers M, Samson B, Colwell B, et al. Eastern Canadian colorectal cancer conference: setting the limits of resectable disease. Curr Oncol. 2010;17:70–7. doi: 10.3747/co.v17i3.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Cutsem E, Nordlinger B, Cervantes A, on behalf of the esmo Guidelines Working Group Advanced colorectal cancer: esmo clinical practice guidelines for treatment. Ann Oncol. 2010;21(suppl 5):v92–7. doi: 10.1093/annonc/mdq273. [DOI] [PubMed] [Google Scholar]
- 5.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–34. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8. [Erratum in: Stat Med 2004;23:1817] [DOI] [PubMed] [Google Scholar]
- 6.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 7.Koopman M, Antonini NF, Douma J, et al. Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (cairo): a phase iii randomised controlled trial. Lancet. 2007;370:135–42. doi: 10.1016/S0140-6736(07)61086-1. [DOI] [PubMed] [Google Scholar]
- 8.Seymour MT, Maughan TS, Ledermann JA, et al. Different strategies of sequential and combination chemotherapy for patients with poor prognosis advanced colorectal cancer (mrc focus): a randomised controlled trial. Lancet. 2007;370:143–52. doi: 10.1016/S0140-6736(07)61087-3. [Erratum in: Lancet 2007;370:566] [DOI] [PubMed] [Google Scholar]
- 9.Cunningham D, Sirohi B, Pluzanska A, et al. Two different first-line 5-fluorouracil regimens with or without oxaliplatin in patients with metastatic colorectal cancer. Ann Oncol. 2009;20:244–50. doi: 10.1093/annonc/mdn638. [DOI] [PubMed] [Google Scholar]
- 10.Ducreux M, Malka D, Mendiboure J, et al. on behalf of the Fédération Francophone de Cancérologie Digestive (ffcd) 2000-05 Collaborative Group Sequential versus combination chemotherapy for the treatment of advanced colorectal cancer (ffcd 2000-05): an open-label, randomised, phase 3 trial. Lancet Oncol. 2011;12:1032–44. doi: 10.1016/S1470-2045(11)70199-1. [DOI] [PubMed] [Google Scholar]
- 11.Seymour MT, Thompson LC, Wasan HS, et al. on behalf of the focus2 Investigators and the National Cancer Research Institute Colorectal Cancer Clinical Studies Group Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (mrc focus2): an open-label, randomised factorial trial. Lancet. 2011;377:1749–59. doi: 10.1016/S0140-6736(11)60399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–47. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 13.Giacchetti S, Perpoint B, Zidani R, et al. Phase iii multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil–leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18:136–47. doi: 10.1200/JCO.2000.18.1.136. [DOI] [PubMed] [Google Scholar]
- 14.Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–7. doi: 10.1016/S0140-6736(00)02034-1. [Erratum in: Lancet 2000;355:1372] [DOI] [PubMed] [Google Scholar]
- 15.Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905–14. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 16.Schmoll HJ, Sargent D. Single agent fluorouracil for first-line treatment of advanced colorectal cancer as standard? Lancet. 2007;370:105–7. doi: 10.1016/S0140-6736(07)61062-9. [DOI] [PubMed] [Google Scholar]
- 17.Colucci G, Gebbia V, Paoletti G, et al. Phase iii randomized trial of folfiri versus folfox4 in the treatment of advancer colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia Meridionale. J Clin Oncol. 2005;23:4866–75. doi: 10.1200/JCO.2005.07.113. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs CS, Marshall J, Mitchell E, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the bicc-c study. J Clin Oncol. 2007;25:4779–86. doi: 10.1200/JCO.2007.11.3357. [DOI] [PubMed] [Google Scholar]
- 19.Souglakos J, Ziras N, Kakolyris S, et al. Randomised phase-ii trial of capiri (capecitabine, irinotecan) plus bevacizumab vs folfiri (folinic acid, 5-fluorouracil, irinotecan) plus bevacizumab as first-line treatment of patients with unresectable/metastatic colorectal cancer (mcrc) Br J Cancer. 2012;106:453–9. doi: 10.1038/bjc.2011.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 21.Kabbinavar FF, Schulz J, McCleod M, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase ii trial. J Clin Oncol. 2005;23:3697–705. doi: 10.1200/JCO.2005.05.112. [DOI] [PubMed] [Google Scholar]
- 22.Douillard JY, Siena S, Cassidy J, et al. Randomized, phase iii trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (folfox4) versus folfox4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the prime study. J Clin Oncol. 2010;28:4697–705. doi: 10.1200/JCO.2009.27.4860. [DOI] [PubMed] [Google Scholar]
- 23.Van Cutsem E, Kohne CH, Lang I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–19. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 24.Tebbutt NC, Wilson K, Gebski VJ, et al. Capecitabine, bevacizumab, and mitomycin in first-line treatment of metastatic colorectal cancer: results of the Australasian Gastrointestinal Trials Group randomized phase iii max study. J Clin Oncol. 2010;28:3191–8. doi: 10.1200/JCO.2009.27.7723. [DOI] [PubMed] [Google Scholar]