Abstract
Background
In the phase iii palette trial of pazopanib compared with placebo in patients with advanced or metastatic soft-tissue sarcoma (sts) who had received prior chemotherapy, pazopanib treatment was associated with improved progression-free survival (pfs). We used an economic model and data from palette and other sources to evaluate the cost-effectiveness of pazopanib in patients with advanced sts who had already received chemotherapy.
Methods
We developed a multistate model to estimate expected pfs, overall survival (os), lifetime sts treatment costs, and quality-adjusted life-years (qalys) for patients receiving pazopanib or placebo as second-line therapy for advanced sts. Cost-effectiveness was calculated alternatively from the health care system and societal perspectives for the province of Quebec. Estimated pfs, os, incidence of adverse events, and utilities values for pazopanib and placebo were derived from the palette trial. Costs were obtained from published sources.
Results
Compared with placebo, pazopanib is estimated to increase qalys by 0.128. The incremental cost of pazopanib compared with placebo is CA$20,840 from the health care system perspective and CA$15,821 from the societal perspective. The cost per qaly gained with pazopanib in that comparison is CA$163,336 from the health care system perspective and CA$124,001 from the societal perspective.
Conclusions
Compared with placebo, pazopanib might be cost-effective from the Canadian health care system and societal perspectives depending on the threshold value used by reimbursement authorities to assess novel cancer therapies. Given the unmet need for effective treatments for advanced sts, pazopanib might nevertheless be an appropriate alternative to currently used treatments.
Keywords: Cost-effectiveness, palliative care, partitioned-survival analysis, pazopanib, post-progression survival, quality-adjusted life-years, soft-tissue sarcoma
1. INTRODUCTION
Soft-tissue sarcomas (stss) are rare solid tumours that comprise more than 50 histologic subtypes originating from mesenchymal cells and their precursors and that affect extraskeletal connective tissue including muscle, fatty tissue, nerves, fibrous tissue, blood vessels, and cartilage1,2. According to the Canadian Cancer Society, approximately 1116 individuals in Canada were diagnosed with these cancers in 2007, and 430 died in 2008; however, the disease burden could be higher, because sarcomas are known often to be both misdiagnosed and underreported3,4.
Primary initial treatment for localized sts is surgery, often combined with radiotherapy. Most patients diagnosed with sts eventually develop local recurrence or metastases after initial treatment5,6. Advanced (that is, unresectable or metastatic) sts (asts) is usually treated with palliative chemotherapy, and median overall survival (os) from time of diagnosis of metastatic disease is 12–18 months7. The standard of care for first-line systemic treatment of most asts subtypes is an anthracycline, typically doxorubicin, alone or in combination with ifosfamide6,8,9. In Canada, however, adding ifosfamide to a first-line doxorubicin-containing regimen is not recommended over single-agent doxorubicin, except in cases in which a better tumour response might reduce symptoms or render tumours resectable10. There is no standard of care after first-line chemotherapy11. However, results from a retrospective chart review of asts patients in North America and Europe found that the most frequently used second-line therapy was gemcitabine plus docetaxel, followed by ifosfamide monotherapy12. The most frequently used third-line therapy was trabectedin, followed by investigational drugs.
Pazopanib (Votrient: GlaxoSmithKline, Research Triangle Park, NC, U.S.A.), a multi-target tyrosine kinase inhibitor, was approved in July 2012 by Health Canada for the treatment of adult patients with selected subtypes of asts who have received prior chemotherapy for metastatic disease or who have progressed within 12 months after adjuvant or neoadjuvant therapy13–15. The phase iii palette trial (search for NCT00753688 at http://clinicaltrials.gov/) compared pazopanib with placebo in 369 asts patients for whom standard chemotherapy had failed16. In palette, pazopanib significantly improved progression-free survival (pfs) (4.6 months vs. 1.6 months with placebo; hazard ratio: 0.35; 95% confidence interval: 0.26 to 0.48; p < 0.0001). Median os was 12.6 months and 10.7 months respectively (hazard ratio: 0.87; 95% confidence interval: 0.67 to 1.12; p = 0.256)13,16–17. Patients in the placebo arm were more likely than those in the pazopanib arm to have received post-treatment anticancer therapy (ptact), which could have attenuated differences in os between the groups. Compared with patients in the placebo arm, those in the pazopanib arm were more likely to experience at least 1 on-therapy adverse event (ae) (99% vs. 89%) and at least 1 serious ae (41% vs. 24%). The most frequent on-therapy aes in the pazopanib arm were fatigue, diarrhea, nausea, weight loss, hypertension, and decreased appetite.
The objective of the present study was to evaluate, from both the health care system perspective and the societal perspective in Quebec, the cost-effectiveness of pazopanib compared with placebo in patients with asts who had received prior anthracycline-based chemotherapy.
2. METHODS
2.1. Approach
We used a multistate model to estimate expected pfs, os, lifetime asts treatment costs, and quality-adjusted life-years (qalys) for asts patients who had received prior anthracycline-based chemotherapy and who were assumed to receive pazopanib or placebo. Although patients with asts can, in clinical practice in Canada, receive a variety of systemic therapies after anthracycline-based chemotherapy10,18,19 (most notably ifosfamide or gemcitabine with or without docetaxel), no randomized controlled trials have compared pazopanib or placebo with such therapies17, and a robust comparison of pazopanib with those agents was therefore infeasible.
The study model was developed in Microsoft Excel (Microsoft Corporation, Redmond, WA, U.S.A.). Consistent with the approach used in numerous other treatment evaluations for advanced oncology indications20,21, patients in the model were assumed to be in one of three mutually exclusive heath states at any given time:
Alive with no progression (pfs)
Alive with disease progression [post-progression survival (pps)]
Dead
The model was designed to permit two alternative approaches for estimating the proportion of patients in each health state over time. In the partitioned-survival analysis, survival distributions for pfs and os were entered into the model, and the proportion of patients in the pps state was calculated as the difference between os and pfs. In the Markov cohort analysis, survival distributions for pfs and pps were entered into the model, together with the estimated proportion of pfs events that were deaths. Transition probabilities were then derived from those inputs and combined to calculate the survival distribution for os. In both approaches, expected costs and qalys for each strategy were calculated as the product of the expected pfs and pps and the corresponding cost and utility value estimates for pre- and post-progression survival time, adjusted for “one-off” decrements in costs and quality of life associated with treatment initiation, aes, progression, and death. Expected lifetime outcomes and costs were evaluated over a 10-year timeframe, approximating a lifetime projection for patients with asts (that is, almost all patients were projected to be dead after 10 years). The model periodicity (that is, the minimum period of time that a patient might remain in any disease state) was 1 week. Effectiveness measures were calculated on a discounted and undiscounted basis; costs were calculated on a discounted basis only. A 5% annual discount rate was used, beginning at the end of the first year of the model22.
Two sets of analyses were conducted. In the first set, data on pfs and os for placebo patients from palette were used without any adjustment in os for the differential receipt of ptact in the two groups. The utilization (and therefore the cost) of ptact was assumed to differ between the groups as observed in palette. This analysis used the partitioned-survival modelling approach (that is, the model took the distribution of os rather than the distribution of pps for inputs). Although this analysis is internally consistent with respect to pps and os and the utilization of ptact in palette, a strategy of placebo followed by ptact is not likely to be used in any setting outside of a placebo-controlled trial. Also, the distribution of ptacts received in palette are probably not generalizable to Canada. For example, the most frequently used ptact in palette was trabectedin, which is approved for sts, but is not marketed in Canada. A second analysis was therefore conducted in which the pps and the ptact utilization and cost were assumed to be the same for placebo and for pazopanib and the difference in mean os between the two treatment strategies would be equal to the difference in pfs (that is, the benefit in pfs for pazopanib compared with placebo was assumed to translate directly into an os benefit of equal magnitude). The second analysis used the Markov cohort modelling approach (that is, the model took the distributions of pps rather than of os as inputs).
For each of the two sets of analyses, cost-effectiveness was calculated alternatively using the health care system and societal perspectives for the province of Quebec. The health care system perspective considered only the direct costs of medical care related to the treatment of sts. The societal perspective considered nonmedical direct costs (for example, patient travel and parking to receive treatment) and indirect costs (that is, costs of work lost to absenteeism and early departure from the work-force because of sts). All costs were adjusted to 2012 dollars, as necessary.
2.2. Model Estimation
Table i summarizes the model inputs. Accelerated failure-time regression was used to estimate pfs, os, and pps for pazopanib and placebo by fitting parametric survival functions to patient-level failure time data from palette23. Investigator-assessed pfs (including clinically determined progression) was used because it was considered most likely to reflect pfs in clinical practice. Overall survival was based on intention-to-treat analyses, and survival distributions for pazopanib and placebo were estimated independently. Exponential, Weibull, and log-logistic models were considered. Based on visual inspection and comparison of the restricted mean (that is, the area under the curve) for the empirical versus the fitted distributions, Weibull distribution provided the best fit for all distributions, and it was used in base-case analyses. The parameters of the pps Weibull distribution were adjusted by calibrating the parameters of the distribution to minimize the differences between the model projections of expected os and those obtained from the Weibull distribution directly fit to os, because the os distribution for pazopanib derived from the pfs and pps distributions in the pps-based analysis approach did not match the tail end of the empirical os distribution well.
TABLE I.
Model inputs
| Input | Estimate for | |
|---|---|---|
|
| ||
| Pazopanib | Placebo | |
| Weibull survival function parameters | ||
| Progression-free survival (pfs), months | ||
| Lambda | 0.1279 | 0.3714 |
| Gamma | 1.1252 | 1.0809 |
| Overall survival, months | ||
| Lambda | 0.0282 | 0.0469 |
| Gamma | 1.2341 | 1.1027 |
| Post-progression survival (pps), months | ||
| Lambda | 0.118 | 0.104 |
| Gamma | 0.898 | 0.902 |
| Utility values [mean (standard error)] | ||
| Pre-progression | 0.674 (0.015) | 0.678 (0.024) |
| Post-progression vs. pre-progression | 0.239 (0.025) | 0.253 (0.024) |
| Costs (CA$) | ||
| Pazopanib | ||
| Medication, per 200 mg tablet | 37.00 | |
| Administration, per 28-day cycle | 20.50 | |
| Post-treatment anticancer therapy (ptact), per patient | ||
| Cyclophosphamide | 138 | 137 |
| Dacarbazine | 373 | 684 |
| Doxorubicin | 97 | 96 |
| Etoposide | 454 | 601 |
| Etoposide + ifosfamide (+ mesna) | 866 | 861 |
| Gemcitabine | 1,110 | 840 |
| Gemcitabine + docetaxel (+ filgrastim) | 2,527 | 5,021 |
| Ifosfamide (+ mesna) | 1,027 | 1,569 |
| Sorafenib | 902 | 2,048 |
| Sunitinib | 1,371 | 1,167 |
| Temozolomide | 461 | 262 |
| Total cost of ptact | 9,327 | 13,286 |
| Other direct medical costs | ||
| During pfs, per month | 213.24 | 213.24 |
| During pps, per month | 426.48 | 426.48 |
| Direct nonmedical costs, per month of pfs | 24.55 | 24.55 |
| Indirect costs | ||
| Absenteeism, per month of pfs | 29.49 | 29.49 |
| Early retirement, per months of pfs lost | 1,508 | 1,508 |
In the palette trial, the EuroQol Group’s EQ-5D (Rotterdam, Netherlands) was assessed only at baseline and week 4; the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (qlq-C30) was also assessed at weeks 8 and 12. A mapping algorithm was therefore developed using data from the EQ-5D and qlq-C30 at baseline and week 4 to predict EQ-5D utility values at weeks 8 and 12 from the qlq-C30 at those later assessments24. The observed and mapped utility values were then combined to calculate mean utility values for each group for all pre- and post-progression assessments. The mean time from progression to post-progression utility assessment in palette was limited to approximately 1 week in both groups. For that reason, the mean differences in utility for post- compared with pre-progression in palette reflect only declines in utility values immediately after progression; they do not reflect the declines in utility that would be expected over the entire post-progression period25–28. As a consequence, post-progression utility values for pazopanib and placebo were calculated by combining treatment group–specific estimates of the mean decrement in utility post-progression in palette (reflecting the period immediately after progression) with an estimate of utility in the terminal phase of the disease. The latter estimate was based on the estimated utility value for progressive disease from a vignettes study (mean ± standard error: 0.263 ± 0.0231)29.
In the model, patients who remained alive and progression-free and who were receiving pazopanib were assumed to incur the cost of a 28-day supply of pazopanib each 28-day cycle. Any medication supplied but not taken was assumed to be discarded. Drug utilization was adjusted for early discontinuation, dose adjustments, and dose interruptions.
Kaplan–Meier sample average estimates30 of the mean number of lines of ptact received and treatment group–specific estimates of the distribution of ptacts in palette were combined with corresponding estimates of the cost per course of each ptact to calculate the expected costs of ptact for each treatment group. Consistent with the mean time between lines of ptact for patients who received more than 1 ptact in palette, the mean duration of ptact was assumed to be 4 months. Dosages for ptacts were based on published studies and prescribing information.
All medication costs were obtained from the IMS Brogan database [IMS Health, Danbury, CT, U.S.A. (http://www.imshealth.com)]. Patients receiving pazopanib were assumed to require a single visit per cycle for administration of oral systemic therapy. The cost per visit was based on the Ontario Schedule of Physician Services fee code “G388–Management of special oral chemotherapy, for malignant disease.” Administration costs for ptacts were estimated using the Ontario Schedule of Physician Services and published sources31.
The treatment costs for aes were calculated by multiplying estimates of the incidences of aes from palette with estimates of the treatment costs for each event. Adverse events were considered if they were grades 3–5 aes for which the difference in incidence between pazopanib and placebo was 2% or greater, or if the aes were considered by clinical experts to be of special interest. Serious aes were assumed to require hospitalization. Costs of inpatient treatment of aes were derived from the Ontario Case Costing Initiative online database32. Costs of outpatient treatment of aes were obtained from published sources31,33.
Other asts-related direct medical costs were estimated based on a retrospective study by Judson and colleagues (Judson I, Al-Muderis O, Scott D, Lloyd A, Alonso F, Garcia B. Cost of management of metastatic soft tissue sarcoma. Poster presentation at the U.K. National Cancer Research Institute Cancer Conference; Birmingham, UK; 30 September–2 October, 2007) of the management costs of metastatic sts in the United Kingdom, with health care purchasing power parities being used to adjust costs to Canadian values34.
Direct nonmedical costs for the societal analysis included the costs of travelling and parking associated with drug administration, follow-up office visits, and office visits and hospitalizations for the treatment of aes. It was assumed that patients receiving oral or home infusion therapies would not incur travel costs for drug administration. For aes, it was assumed that 1 round trip would be required for non-serious aes and 2 round trips would be required for serious aes. Estimates for direct nonmedical costs were determined using estimated travel distances from home to the treatment centre35 multiplied by the 2011 Canada Revenue Agency vehicle expense rate for Quebec36 and the average parking rate based on 7 hospitals in Ontario37.
Indirect costs included costs of work loss for patients as a consequence of absenteeism for medication administration, follow-up visits, and treatment of aes, and the costs of early departure from the workforce (early retirement), which was assumed to occur upon disease progression. Work loss because of reduced productivity and caregiver work loss were not considered. Indirect costs were calculated by multiplying the estimated number of missed workdays by the employment rate and the average daily earnings of age-matched people in the general population of Quebec38. Each outpatient visit was assumed to result in 3 hours of work loss. The number of work-days lost because of hospitalizations for serious aes was estimated by the average length of stay from the Ontario Case Costing Initiative database32. The percentage of employed asts patients was estimated by multiplying the proportion of patients in palette who responded “not at all” or “a little” to the qlq-C30 question “Were you limited in doing either work or other daily activities during the past week?” by an estimate of the age-matched employment rate in the general population of Quebec39.
2.3. Sensitivity Analyses
To explore the effect on model results of changing assumptions about key model parameter values, deterministic sensitivity analyses were conducted. Probabilistic sensitivity analyses were conducted40, and cost-effectiveness acceptability curves were generated41.
2.4. Role of the Funding Source
GlaxoSmithKline authors had a role in the interpretation of the data and the right to approve or disapprove publication of the final manuscript.
3. RESULTS
3.1. Analysis Using the Partitioned-Survival Model and Assuming Unequal PPS and PTACT Costs for Pazopanib and Placebo
3.1.1. Base Case
In analyses based on the partitioned-survival analysis model, and in which pps and ptact costs for placebo were based on the results observed in palette (that is, not set equal to values for pazopanib), pazopanib was estimated, compared with placebo, to result in gains of 0.294 progression-free life-years and 0.115 life-years and in a loss of 0.179 post-progression life-years (not discounted, Table ii). The qalys gained with pazopanib were estimated to be 0.128 (discounted). Pazopanib was projected to increase medication costs by CA$24,262, but to reduce post-progression costs by CA$4,877 because of lower costs for ptact and a shorter expected pps. Pazopanib was estimated to result in a savings of CA$5,117 in indirect costs, largely because of increased pfs and time to retirement. Total incremental costs for pazopanib compared with placebo were estimated to be CA$20,840 from the health care system perspective and CA$15,821 from the societal perspective. The cost per qaly gained with pazopanib in that comparison was estimated to be CA$163,336 from the health care system perspective and CA$124,001 from the societal perspective.
TABLE II.
Base-case results for cost-effectiveness of pazopanib versus placebo
| Input | Model | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Partitioned survival (unequal pps and costs of ptact) | Markov cohort (equal pps and costs of ptact) | |||||
|
|
|
|||||
| Pazopanib | Placebo | Pazopanib vs. placebo | Pazopanib | Placebo | Pazopanib vs. placebo | |
| Effectiveness | ||||||
| Life-years (lys) | 1.409 | 1.294 | 0.115 | 1.409 | 1.166 | 0.243 |
| Progression-free life-years (pflys) | 0.505 | 0.211 | 0.294 | 0.505 | 0.211 | 0.294 |
| Post-progression life-years (pplys) | 0.904 | 1.082 | −0.179 | 0.904 | 0.954 | −0.051 |
| Quality-adjusted life-years (qalys) | 0.734 | 0.604 | 0.130 | 0.734 | 0.549 | 0.184 |
| Effectiveness (discounted) | ||||||
| lys | 1.362 | 1.250 | 0.112 | 1.362 | 1.131 | 0.231 |
| pflys | 0.502 | 0.211 | 0.291 | 0.502 | 0.211 | 0.291 |
| pplys | 0.859 | 1.039 | −0.179 | 0.860 | 0.920 | −0.06 |
| qalys | 0.713 | 0.585 | 0.128 | 0.713 | 0.535 | 0.178 |
| Costs, discounted (CA$) | ||||||
| Direct medical costs (health care system perspective) | ||||||
| Study medication | 24,262 | 0 | 24,262 | 24,262 | 0 | 24,262 |
| Administration | 138 | 0 | 138 | 138 | 0 | 138 |
| Adverse events | 766 | 194 | 572 | 766 | 194 | 572 |
| Other costs, pfs | 1,286 | 541 | 745 | 1,286 | 541 | 745 |
| Other costs, pps | 13,725 | 18,603 | −4,877 | 13,727 | 14,036 | −308 |
| Total direct medical costs (health care system perspective) | 40,177 | 19,337 | 20,840 | 40,179 | 14,770 | 25,409 |
| Direct nonmedical and indirect costs (societal perspective) | −4,862 | 157 | −5,019 | −4,862 | 157 | −5,018 |
| Total direct and indirect costs (societal perspective) | 35,315 | 19,494 | 15,821 | 35,317 | 14,927 | 20,391 |
| Cost-effectiveness (CA$) | ||||||
| Health care system perspective | ||||||
| Cost/pflys | 71,591 | 87,288 | ||||
| Cost/lys | 186,502 | 110,043 | ||||
| Cost/qalys | 163,336 | 142,511 | ||||
| Societal perspective | ||||||
| Cost/pflys | 54,350 | 70,047 | ||||
| Cost/lys | 141,588 | 88,308 | ||||
| Cost/qalys | 124,001 | 114,363 | ||||
pps = post-progression survival; ptact = post-treatment anticancer therapy; pfs = progression-free survival.
3.1.2. Sensitivity Analyses
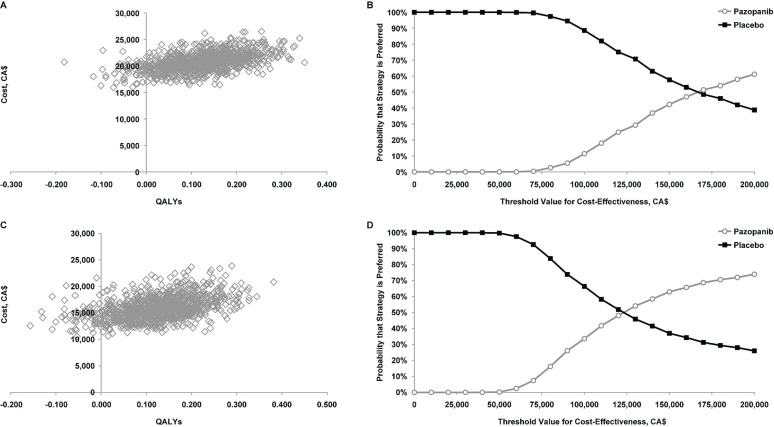
In probabilistic sensitivity analyses for both perspectives, most of the simulations were in the northeast quadrant of the cost-effectiveness plane, implying that, compared with placebo, pazopanib is likely to increase both costs and qalys (Figure 1). Given a threshold value of CA$100,000 per qaly gained, the estimated probability that pazopanib would be preferred to placebo was 11.4% from the health care system perspective and 33.6% from the societal perspective. Given a threshold value of CA$200,000 per qaly gained, the corresponding values were 61.2% and 74.0%.
FIGURE 1.
Probabilistic sensitivity analyses of cost-effectiveness and acceptability curves for pazopanib versus placebo (A,B) from the health care system perspective and (C,D) from the societal perspective, using the partitioned-survival model and assuming unequal post-progression survival and post-treatment anticancer therapy costs for pazopanib and placebo. qalys = quality-adjusted life-years.
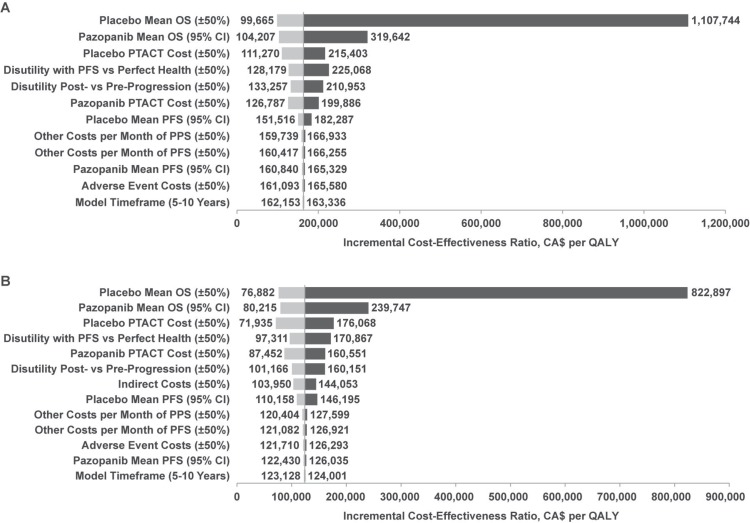
Figure 2 shows tornado plots for the deterministic sensitivity analyses. Cost-effectiveness was most sensitive to the os estimates for placebo and pazopanib; it was moderately sensitive to the ptact costs for pazopanib and placebo, the disutilities of pfs compared with perfect health, and post- compared with pre-progression. For the analysis using the societal perspective, the model was also moderately sensitive to indirect cost estimates.
FIGURE 2.
Tornado plots for pairwise comparisons of pazopanib and placebo (A) from the health care system perspective and (B) from the societal perspective for the analysis using the partitioned-survival model and assuming unequal post-progression survival (pps) and post-treatment anticancer therapy (ptact) costs for pazopanib and placebo. os = overall survival; ci = confidence interval; pfs = progression-free survival; qaly = quality-adjusted life-year.
3.2. Analysis Using Markov Cohort Model and Assuming Equal PPS and PTACT Costs for Pazopanib and Placebo
3.2.1. Base Case
In the analyses using the Markov cohort model, and assuming that the pps and ptact costs for placebo were the same as for pazopanib, pazopanib was estimated, compared with placebo, to increase life-years (not discounted) by 0.243 and qalys (discounted) by 0.178 (Table ii). Although the distribution of pps was assumed to be the same for pazopanib and placebo, post-progression life-years were lower with pazopanib because of the estimated higher probability that pfs events were deaths. Other costs during pps were reduced in this analysis by only CA$308 with pazopanib. Total incremental costs for pazopanib compared with placebo were estimated to be CA$25,409 from the health care system perspective and CA$20,391 from the societal perspective. The cost per qaly gained with pazopanib in that comparison was estimated to be CA$142,511 from the health care system perspective and CA$114,363 from the societal perspective.
3.2.2. Sensitivity Analyses
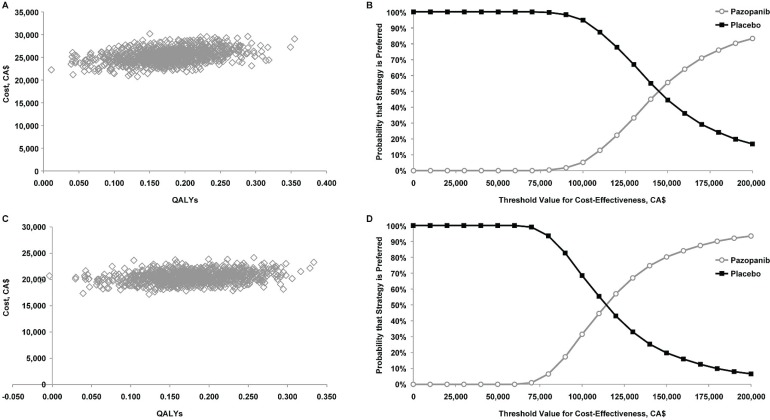
In probabilistic sensitivity analyses, all of the simulations for both perspectives were in the northeast quadrant of the cost-effectiveness plane, implying that, compared with placebo, pazopanib is likely to increase both costs and qalys (Figure 3). Given a threshold value of CA$100,000 per qaly gained, the estimated probability that pazopanib would be preferred to placebo was 5.2% from the health care system perspective and 31.5% from the societal perspective. Given a threshold value of CA$200,000 per qaly gained, the corresponding values were 83.2% and 93.4%.
FIGURE 3.
Probabilistic sensitivity analyses of cost-effectiveness and acceptability curves for pazopanib versus placebo (A,B) from the health care system perspective and (C,D) from the societal perspective, using the Markov cohort model and assuming equal post-progression survival and post-treatment anticancer therapy costs for pazopanib and placebo. qalys = quality-adjusted life-years.
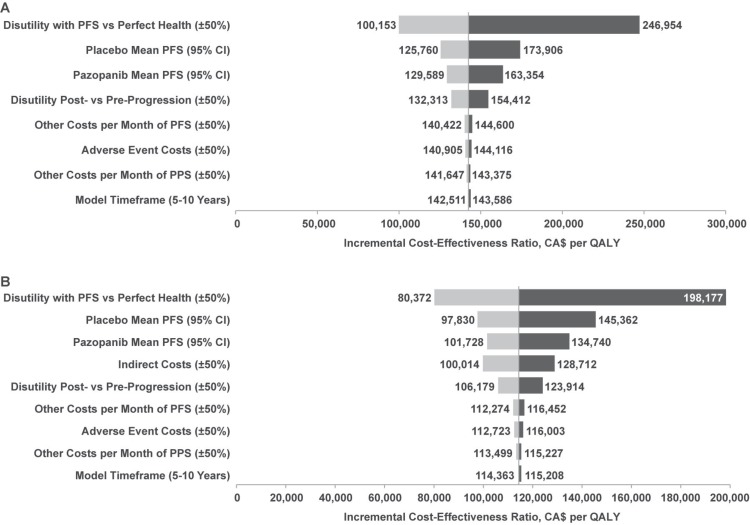
In deterministic sensitivity analyses, cost-effectiveness was most sensitive to the disutilities of pfs compared with perfect health and post- compared with pre-progression; it was moderately sensitive to the pfs of pazopanib and placebo and the disutilities of post- compared with pre-progression (Figure 4). For the analysis using the societal perspective, the model was also moderately sensitive to indirect cost estimates. Results were virtually identical to the base case when a 5-year time horizon was applied.
FIGURE 4.
Tornado plots for pairwise comparison of pazopanib versus placebo (A) from the health care system perspective and (B) from the societal perspective for the analysis using the Markov cohort model and assuming equal post-progression survival (pps) and post-treatment anticancer therapy (ptact) costs for pazopanib and placebo. pfs = progression-free survival; ci = confidence interval; qaly = quality-adjusted life-year.
4. DISCUSSION
In the present study, we evaluated the cost-effectiveness of pazopanib compared with placebo in patients with asts in Canada who had received prior chemo-therapy. In analyses in which pps and the costs of ptact were assumed to differ for pazopanib and placebo, the estimated cost per qaly gained with pazopanib compared with placebo was approximately CA$163,000 from a health care system perspective and CA$124,000 from a societal perspective. In analyses in which pps and the costs of ptact were assumed to be the same for pazopanib and placebo, the corresponding cost-effectiveness ratios were approximately CA$142,000 and CA$114,000 per qaly gained. In a similar economic analysis using a pan-Canadian health care system perspective that was submitted to the pan-Canadian Oncology Drug Review42, the cost per qaly gained with pazopanib compared with placebo, assuming unequal pps and costs of ptact, was estimated to be CA$165,246. The findings of the Quebec-specific analysis reported here can therefore be considered broadly applicable to all of Canada.
Neither Canadian federal nor provincial health authorities have explicit guidelines specifying a threshold value of cost per qaly gained. Assessments are based on several considerations, including clinical effectiveness, direct and indirect medication costs, toxicity profile of the treatment, and health-related quality of life. Therefore, pazopanib might or might not be a cost-effective treatment alternative compared with placebo in Canada depending on the threshold value of cost-effectiveness used by reimbursement authorities to assess novel cancer therapies.
Results of the analysis in which pps and costs of ptact were assumed to differ for pazopanib and placebo were less favourable than those in which pps and costs of ptact were assumed to be the same. Which of those two approaches is most appropriate is uncertain. Although the assumption of different pps and costs of ptact is internally consistent with the palette trial, a strategy of placebo followed by systemic therapy is unlikely to be used in a real-world clinical setting. Additionally, many of the ptacts used in patients in the palette trial are unavailable or not widely used in Canada, limiting the generalizability of those results. Also, if pazopanib affects disease progression only while patients are receiving treatment, estimates of treatment effects based on observed os are less precise than those based on observed pfs, because variability in pps adds additional statistical “noise” without adding “signal” to the estimation of treatment effects43.
On the other hand, the assumption that pps and costs of ptact are equal for pazopanib and placebo disregards the empirical data relating to os for placebo patients in palette and requires an assumption that the effects of pazopanib on clinical and economic outcomes do not extend beyond the end of treatment with pazopanib. Results from numerous analyses in a variety of solid tumours demonstrate that the effects of treatment on pfs are strongly associated with the effects of treatment on os44. Similar analyses have demonstrated that, among patients with metastatic breast or colorectal cancer, gains in time to disease progression are generally associated with no gains or with very slight gains or losses in pps45,46. The assumption that pps is no worse with pazopanib than with placebo might therefore not be unreasonable.
To the best of our knowledge, our study is the first to use both a partitioned-survival analysis model and a Markov cohort model for the same evaluation. Markov models have long been used in cost-effectiveness analyses, but partitioned-survival analyses are increasingly being used in the evaluation of targeted therapies for advanced cancers. Although the two approaches are similar in many respects, and potentially interchangeable, one advantage of the partitioned-survival analysis approach is that it generates estimates of os that closely match the source data. Markov models can be calibrated to yield os distributions similar to those obtained with the partitioned-survival analysis approach, but the goodness of fit can be limited unless additional model states are created for pps, defined by the time to progression. Because our objective in the first analysis was to match the os results from the palette trial as closely as possible, the partitioned-survival analysis approach was considered most appropriate. In our second analysis, our objective was to model the counterfactual scenario in which expected pps and costs of ptact were the same for pazopanib and placebo. The Markov modelling approach was ideally suited to modelling such a scenario. Further research is required to assess the relative strengths and weaknesses of these two modelling approaches in cost-effectiveness analyses of oncology therapies.
Limitations of the present study should be noted. First, it focused on a comparison of pazopanib with placebo only and did not consider other systemic therapies such as gemcitabine with or without docetaxel and ifosfamide that are frequently used as second-line therapy in Canada18. Because controlled clinical trials comparing the efficacy of pazopanib or placebo with those treatments are unavailable17, it was infeasible to perform a robust comparison of the cost-effectiveness of pazopanib against those active therapies. In the economic evaluation submitted to the pan-Canadian Oncology Drug Review that was based on the model reported here and on an unadjusted (“naïve”) indirect comparison, pazopanib was found to dominate (that is, be less costly and yield more qalys than) gemcitabine monotherapy and gemcitabine plus docetaxel. Compared with ifosfamide, pazopanib also had a cost-effectiveness ratio of CA$115,568 per qaly gained (results that were not disclosed in the final Economic Guidance Report)42. However, results of those analyses were based on nonrandomized comparisons and, being subject to confounding, should thus be interpreted cautiously.
Data on utilization and costs were not collected in the palette trial, and our model data were therefore based on U.K. resource utilization adjusted to Canadian dollars using health care pps. Although the U.K. study represents a relatively rich source of data on the treatment costs of asts, the generalizability of resource utilization for sts patients in the U.K. to patients seen in the Canadian setting is uncertain. Because the information from palette on utility values after progression was limited, data from the trial were combined with data from a vignettes study to estimate utility values for the pps state. Although utility values based on vignettes studies have been used in earlier cost-effectiveness analyses of oncology therapies25–28, the validity of this approach has not been formally assessed29.
In analyses using a societal perspective, the indirect costs of early retirement were calculated as the difference in mean pfs for placebo compared with pazopanib (discounted), multiplied by the annual wage rate and the employment rate. That approach corresponds to the human capital method. Although some might argue that the friction method is more appropriate for estimating the costs of work loss, the friction method is more difficult to apply. Because the estimated percentage of patients with asts who are employed was low (43%), the estimated difference between treatments in mean time to progression was small (15 weeks) and similar to the likely duration of the friction period47, and thus the use of the friction method instead of the human capital method would not have materially affected the results.
Although cost-effectiveness can be an important factor in reimbursement decisions, other considerations support the use of pazopanib for asts in Canada. Advanced or metastatic sts is a rare, incurable disease with a short life expectancy. Pazopanib is the only agent approved in Canada for asts after failure of first-line chemotherapy14. For asts patients, it is also the only available treatment that demonstrated efficacy in a phase iii randomized controlled trial13,16. Results of naïve indirect treatment comparisons suggest that pazopanib probably has an efficacy that is at least comparable to that of ifosfamide and gemcitabine plus docetaxel17,48.
Pazopanib represents an entirely new treatment class that has a unique mechanism of action and, compared with cytotoxic chemotherapy, a relatively favourable safety profile. Unlike ifosfamide and gemcitabine plus docetaxel, which are administered intravenously every 3–4 weeks (sometimes as multi-day infusions to reduce toxicity), pazopanib is an oral agent, which might be preferred by some patients49. In light of those factors, pazopanib could be an appropriate alternative to treatments in current use.
5. CONCLUSIONS
Compared with placebo, pazopanib might be a cost-effective treatment alternative in Canada depending on the threshold value for cost-effectiveness used by reimbursement authorities in Canada to assess novel cancer therapies. Given the unmet need for effective treatments for asts, pazopanib might nevertheless be an appropriate alternative to treatments currently in use.
6. ACKNOWLEDGMENTS
The authors thank Nicole Mittman phd and Soo Jin Seung of e6 Research Organization Ltd. for their assistance in preparing the cost analysis for this manuscript. Funding for this study was provided to Policy Analysis Inc. (pai) by GlaxoSmithKline (gsk). Editorial support (assembling tables and figures, collating author comments, copyediting, fact-checking, referencing) and graphic services were provided by Nate Connors phd, Prasad Kulkarni phd, and Nancy Price phd of aoi Communications, lp, and were funded by gsk.
7. CONFLICT OF INTEREST DISCLOSURES
TED, JA, AW, and NF are employees of pai, which has received research funding and consulting fees from gsk for activities related to this study and support for travel to meetings to present the study results. TED’s institution also received consulting fees and research funding from gsk for activities unrelated to this study. SCM is an employee of gsk and holds stock in gsk. AC was an employee of gsk during the conduct of this study and holds stock in gsk. HRN is an employee of gsk.
8. REFERENCES
- 1.American Cancer Society. Sarcoma: Adult Soft Tissue Cancer. Atlanta, GA: American Cancer Society; 2012. [Available online at: http://www.cancer.org/acs/groups/cid/documents/webcontent/003138-pdf.pdf; cited February 13, 2013] [Google Scholar]
- 2.Fletcher CDM, Unni KK, Mertens F, editors. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France; IARC Press; 2002. [Google Scholar]
- 3.Canadian Cancer Society. Home > Cancer information > Cancer type > Soft tissue sarcoma > Statistics > Soft tissue sarcoma statistics [Web page] Toronto, ON: Canadian Cancer Society; 2013. [Available at: http://www.cancer.ca/en/cancer-information/cancer-type/soft-tissue-sarcoma/statistics/?region=on; cited April 24, 2013] [Google Scholar]
- 4.Cancer Care Ontario (cco) Adult Sarcoma Management in Ontario: Expert Panel Report 2009. Toronto, ON: CCO; 2009. [Available online at: https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=121319; cited July 2, 2013] [Google Scholar]
- 5.Coindre JM, Terrier P, Guillou L, et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer. 2001;91:1914–26. doi: 10.1002/1097-0142(20010515)91:10<1914::AID-CNCR1214>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Grimer R, Judson I, Peake D, Seddon B. Guidelines for the management of soft tissue sarcomas. Sarcoma. 2010;2010:506182. doi: 10.1155/2010/506182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Italiano A, Mathoulin–Pelissier S, Cesne AL, et al. Trends in survival for patients with metastatic soft-tissue sarcoma. Cancer. 2011;117:1049–54. doi: 10.1002/cncr.25538. [DOI] [PubMed] [Google Scholar]
- 8.Casali PG, Jost L, Sleijfer S, Verweij J, Blay JY, on behalf of the esmo Guidelines Working Group Soft tissue sarcomas: esmo clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(suppl 4):132–6. doi: 10.1093/annonc/mdp153. [DOI] [PubMed] [Google Scholar]
- 9.esmo/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(suppl 7):vii92–9. doi: 10.1093/annonc/mds310. [DOI] [PubMed] [Google Scholar]
- 10.Verma S, Younus J, Stys–Norman D, Haynes AE, Blackstein M. Ifosfamide-based combination chemotherapy in advanced soft-tissue sarcoma: a practice guideline. Curr Oncol. 2007;14:144–8. doi: 10.3747/co.2007.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kopp HG, Patel S, Brucher B, Hartmann JT. Potential combination chemotherapy approaches for advanced adult-type soft-tissue sarcoma. Am J Clin Dermatol. 2008;9:207–17. doi: 10.2165/00128071-200809040-00001. [DOI] [PubMed] [Google Scholar]
- 12.Leahy M, Garcia Del Muro X, Reichardt P, et al. Chemotherapy treatment patterns and clinical outcomes in patients with metastatic soft tissue sarcoma. The Sarcoma Treatment and Burden of Illness in North America and Europe (sabine) study. Ann Oncol. 2012;23:2763–70. doi: 10.1093/annonc/mds070. [DOI] [PubMed] [Google Scholar]
- 13.United States, Department of Health and Human Services, Food and Drug Administration (fda), Oncologic Drugs Advisory Committee. Votrient (Pazopanib) Tablets: For Treatment of Patients with Soft Tissue Sarcoma. Silver Spring, MD: FDA; 2012. Briefing Document NDA 22–465. [Available online at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM296303.pdf; cited December 19, 2013] [Google Scholar]
- 14.GlaxoSmithKline. Votrient: Pazopanib (as Pazopanib Hydrochloride) Mississauga, ON: Glaxo-SmithKline; 2013. [product monograph] [Google Scholar]
- 15.GlaxoSmithKline. Notice of compliance information: Votrient. In: Health Canada, editor. Drugs and Health Products. Ottawa, ON: Therapeutic Products Directorate; 2012. [Google Scholar]
- 16.van der Graaf WT, Blay JY, Chawla SP, et al. on behalf of the eortc Soft Tissue and Bone Sarcoma Group and the palette study group Pazopanib for metastatic soft-tissue sarcoma (palette): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379:1879–86. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 17.Sharma S, Takyar S, Manson SC, Powell S, Penel N. Efficacy and safety of pharmacological interventions in second- or later-line treatment of patients with advanced soft tissue sarcoma: a systematic review. BMC Cancer. 2013;13:385. doi: 10.1186/1471-2407-13-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cancer Care Ontario (cco), Sarcoma Disease Site Group. Doxorubicin-Based Chemotherapy for the Palliative Treatment of Adult Patients with Locally Advanced or Metastatic Soft Tissue Sarcoma. Toronto, ON: CCO; 2011. (Evidence-based series 11-1. Ver. 2). [In review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta A, Yao X, Verma S, Mackay H, Hopkins L, the Sarcoma Disease Site Group, and the Gynecology Cancer Disease Site Group . Chemotherapy (i.e., Gemcitabine, Docetaxel plus Gemcitabine, Doxorubicin, or Trabectedin) for Inoperable, Locally Advanced, Recurrent, or Metastatic Uterine Leiomyosarcoma. Toronto, ON: Cancer Care Ontario; 2012. (Evidence-based series 11-11). [Google Scholar]
- 20.Delea TE, Amdahl J, Chit A, Amonkar MM. Cost-effectiveness of lapatinib plus letrozole in her2-positive, hormone receptor-positive metastatic breast cancer in Canada. Curr Oncol. 2013;20:e371–87. doi: 10.3747/co.20.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delea TE, Tappenden P, Sofrygin O, et al. Cost-effectiveness of lapatinib plus capecitabine in women with her2+ metastatic breast cancer who have received prior therapy with trastuzumab. Eur J Health Econ. 2012;13:589–603. doi: 10.1007/s10198-011-0323-1. [DOI] [PubMed] [Google Scholar]
- 22.Canadian Agency for Drugs and Technologies in Health (cadth) Guidelines for the Economic Evaluation of Health Technologies: Canada. 3rd ed. Ottawa, ON: CADTH; 2006. [Available online at: http://www.cadth.ca/media/pdf/186_EconomicGuidelines_e.pdf; cited December 19, 2013] [Google Scholar]
- 23.Kalbfleisch J, Prentice R. Rank regression and the accelerated failure time model. In: Balding DJ, Bloomfield P, Cressie NAC, et al., editors. The Statistical Analysis of Failure Time Data. 2nd ed. New York, NY: John Wiley and Sons; 1980. pp. 218–46. [Google Scholar]
- 24.Amdahl J, Manson SC, Isbell R, Chit A, Delea TE. Utility mapping of the eortc qlq-C30 onto eq-5D in patients with soft tissue sarcoma [abstract] Value Health. 2013;16:A149. doi: 10.1016/j.jval.2013.08.554. [DOI] [Google Scholar]
- 25.Giesinger JM, Wintner LM, Oberguggenberger AS, et al. Quality of life trajectory in patients with advanced cancer during the last year of life. J Palliat Med. 2011;14:904–12. doi: 10.1089/jpm.2011.0086. [DOI] [PubMed] [Google Scholar]
- 26.Mayrbaurl B, Wintner LM, Giesinger JM, et al. Chemotherapy line–associated differences in quality of life in patients with advanced cancer. Support Care Cancer. 2012;20:2399–405. doi: 10.1007/s00520-011-1355-x. [DOI] [PubMed] [Google Scholar]
- 27.Elmqvist MA, Jordhoy MS, Bjordal K, Kaasa S, Jannert M. Health-related quality of life during the last three months of life in patients with advanced cancer. Support Care Cancer. 2009;17:191–8. doi: 10.1007/s00520-008-0477-2. [DOI] [PubMed] [Google Scholar]
- 28.Hwang SS, Chang VT, Fairclough DL, Cogswell J, Kasimis B. Longitudinal quality of life in advanced cancer patients: pilot study results from a VA medical cancer center. J Pain Symptom Manage. 2003;25:225–35. doi: 10.1016/S0885-3924(02)00641-3. [DOI] [PubMed] [Google Scholar]
- 29.Shingler SL, Swinburn P, Lloyd A, et al. Elicitation of health state utilities in soft tissue sarcoma. Qual Life Res. 2013;22:1697–706. doi: 10.1007/s11136-012-0301-9. [DOI] [PubMed] [Google Scholar]
- 30.Young TA. Estimating mean total costs in the presence of censoring: a comparative assessment of methods. Pharmacoeconomics. 2005;23:1229–42. doi: 10.2165/00019053-200523120-00007. [DOI] [PubMed] [Google Scholar]
- 31.Ontario Ministry of Health and Long-Term Care. Schedule of Benefits: Physician Services Under the Health Insurance Act (2013) Toronto, ON: Queen’s Printer for Ontario; 2013. [Google Scholar]
- 32.Ontario Case Costing Initiative (occi) OCCI Costing Analysis Tool [Web resource; inputs: Acute inpatient, 2009/2010, by Diagnosis] Toronto, ON: OCCI; 2013. [Choose Costing Analysis (CAT) Tool at: http://www.occp.com/mainPage.htm; cited December 19, 2013] [Google Scholar]
- 33.Ng R, Hasan B, Mittmann N, et al. on behalf of the Working Group on Economic Analysis, the Lung Disease Site Group, and the ncic Clinical Trials Group Economic analysis of ncic ctg jbr.10: a randomized trial of adjuvant vinorelbine plus cisplatin compared with observation in early stage non-small-cell lung cancer—a report of the Working Group on Economic Analysis, and the Lung Disease Site Group, ncic Clinical Trials Group. J Clin Oncol. 2007;25:2256–61. doi: 10.1200/JCO.2006.09.4342. [DOI] [PubMed] [Google Scholar]
- 34.Global Purchasing Power Parities and Real Expenditures: 2005 International Comparison Program. Washington, DC: International Bank for Reconstruction and Development/The World Bank; 2008. [Google Scholar]
- 35.Lauzier S, Maunsell E, Drolet M, et al. Wage losses in the year after breast cancer: extent and determinants among Canadian women. J Natl Cancer Inst. 2008;100:321–32. doi: 10.1093/jnci/djn028. [DOI] [PubMed] [Google Scholar]
- 36.Canadian Revenue Agency (cra) Meal and vehicle rates used to calculate travel expenses for 2011 [Web page] Ottawa, ON: CRA; n.d. [Current version available at: http://www.cra-arc.gc.ca/tx/ndvdls/tpcs/ncm-tx/rtrn/cmpltng/ddctns/lns248-260/255/rts2011-eng.html; cited July 18, 2013] [Google Scholar]
- 37.Brennan RJ. Hospital parking fees should be scrapped, journal says. The Toronto Star. 2011 Nov 28; [Available online at: http://www.thestar.com/news/canada/2011/11/28/hospital_parking_fees_should_be_scrapped_journal_says.html; cited November 5, 2014] [Google Scholar]
- 38.Statistics Canada. Average hourly wages of employees by selected characteristics and occupation, unadjusted data, by province (monthly) (Quebec) [Web page] Ottawa, ON: Statistics Canada; 2012. [Current version available at: http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/labr69f-eng.htm; cited December 6, 2013] [Google Scholar]
- 39.Statistics Canada. Labour force, employment and unemployment, levels and rates, by province (Quebec, Ontario, Manitoba) [Web page] Ottawa, ON: Statistics Canada; 2012. [Current version available at: http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/labor07b-eng.htm; cited January 4, 2013] [Google Scholar]
- 40.Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics. 2000;17:479–500. doi: 10.2165/00019053-200017050-00006. [DOI] [PubMed] [Google Scholar]
- 41.Lothgren M, Zethraeus N. Definition, interpretation and calculation of cost-effectiveness acceptability curves. Health Econ. 2000;9:623–30. doi: 10.1002/1099-1050(200010)9:7<623::AID-HEC539>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 42.Pan-Canadian Oncology Drug Review (pcodr) Pan-Canadian Oncology Drug Review Final Economic Guidance Report: Pazopanib (Votrient) for Soft Tissue Sarcoma. Toronto, ON: pCODR; 2012. [Available online at: http://www.pcodr.ca/idc/groups/pcodr/documents/pcodrdocument/pcodr-votrientstsfn-egr.pdf; cited July 1, 2013] [Google Scholar]
- 43.Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst. 2009;101:1642–9. doi: 10.1093/jnci/djp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sherrill B, Amonkar M, Wu Y, et al. Relationship between effects on time-to-disease progression and overall survival in studies of metastatic breast cancer. Br J Cancer. 2008;99:1572–8. doi: 10.1038/sj.bjc.6604759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bowater RJ, Bridge LJ, Lilford RJ. The relationship between progression-free and post-progression survival in treating four types of metastatic cancer. Cancer Lett. 2008;262:48–53. doi: 10.1016/j.canlet.2007.11.032. [DOI] [PubMed] [Google Scholar]
- 46.Bowater RJ, Lilford PE, Lilford RJ. Estimating changes in overall survival using progression-free survival in metastatic breast and colorectal cancer. Int J Technol Assess Health Care. 2011;27:207–14. doi: 10.1017/S0266462311000201. [DOI] [PubMed] [Google Scholar]
- 47.Hopkins RB, Goeree R, Longo CJ. Estimating the national wage loss from cancer in Canada. Curr Oncol. 2010;17:40–9. doi: 10.3747/co.v17i2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amdahl J, Manson S, Isbell R, et al. Cost effectiveness of pazopanib in advanced soft tissue sarcoma [abstract] Value Health. 2012;15:A423–4. doi: 10.1016/j.jval.2012.08.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Borner M, Scheithauer W, Twelves C, Maroun J, Wilke H. Answering patients’ needs: oral alternatives to intravenous therapy. Oncologist. 2001;6(suppl 4):12–16. doi: 10.1634/theoncologist.6-suppl_4-12. [DOI] [PubMed] [Google Scholar]