Abstract
Follicular dendritic cell sarcoma (fdcs) is a rare entity, often presenting a diagnostic challenge for both the pathologist and the clinician. It accounts for only 0.4% of soft-tissue sarcomas, and its underlying causes are largely unknown. Most of these tumours occur in lymph nodes, and extranodal involvement is uncommon. In the gastrointestinal tract, fdcs is extremely rare. Here, we report a case of primary fdcs originating in the stomach. Upon review of the literature, we identified only 2 additional cases of fdcs presenting as a primary stomach tumour. Given the rarity of this tumour in gastrointestinal sites and the lack of consensus on treatment, evaluation of this entity must continue.
Keywords: Follicular dendritic cell sarcoma, stomach malignancy
1. INTRODUCTION
Follicular dendritic cells are a component of the humoral immune response, cooperating with dendritic cells and Langerhans cells. They form a nonlymphoid accessory component to the humoral response by providing antigen presentation and B-cell response maturation. In contrast to bone marrow hematopoiesis-derived dendritic cells, follicular dendritic cells are of mesenchymal origin1.
Follicular dendritic cell sarcoma (fdcs) is a rare entity, accounting for only 0.4% of soft-tissue sarcoma. Follicular dendritic cell sarcoma often arises in lymph nodes. It is found most commonly in the cervical, mediastinal, and axillary areas2,3. Primary gastrointestinal cases of fdcs are extremely rare, having been described in few case reports to date. Reported extranodal sites include colon, appendix, small bowel, and liver4–8. Here, we report a patient with fdcs arising in the stomach. To our knowledge, ours is the third case of gastric fdcs reported in the literature9,10. Given the relative paucity of information related to intervention in gastric fdcs, an improved description of the disease process and treatment modalities is essential.
2. CASE DESCRIPTION
Our patient, a 60-year-old woman, originally presented with crampy epigastric pain. Her associated symptoms included nausea and vomiting. Her physical exam demonstrated only a tender and irreducible mass near the umbilicus. Given her incarcerated hernia, she was taken to the operating room and underwent resection of entrapped omentum and ileum. She underwent umbilical hernia repair without complications.
Histologic examination of the hernia content demonstrated a 2×1.4×1.2 cm focus of fdcs within necrotic fat. Computed tomography imaging demonstrated a suspicious mass at the pylorus (Figure 1), with no additional evidence of metastasis on positron-emission tomography. Preoperative esophagogastroduodenoscopy demonstrated only mild gastritis near the pylorus, with submucosal bulging. Biopsies confirmed the lesion to be fdcs. The patient was taken for an exploratory laparotomy, and she underwent distal gastrectomy, with a palpable tumour discovered at the pylorus. The patient underwent a Billroth ii reconstruction. She elected not to receive adjuvant therapy. Unfortunately, she developed liver recurrence and died at 8 months after presentation.
FIGURE 1.
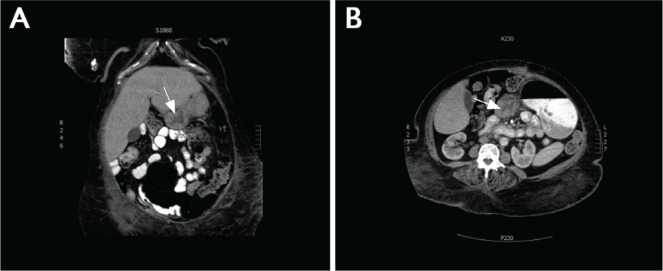
Abdominal computed tomography images showed a mass (arrow) in the pylorus of the stomach. (A) Coronal section. (B) Axial section.
Histologic examination of the resected surgical specimen showed sheets and ill-defined fascicles and whorls of oval-to-spindle cells with ill-defined cytoplasmic borders and vesicular—at times pleomorphic—nuclei, with prominent nucleoli. Interspersed among the tumour cells were numerous small non-neoplastic lymphocytes. Mitotic figures were easily seen (Ki-67 immunoproliferative index of approximately 40%). Immunoperoxidase staining showed tumour cells to be reactive with CD21 and vimentin. There was no reactivity with antibodies to CD45, CD117, CD43, CD23, CD34, CD1a, CD3, CD20, PAX5, Ki-1, CD68, Alk1, Leu-M1, myeloperoxidase, cam 5.2, S100, calretinin, melanoma cocktail, muscle actin, desmin, synaptophysin, and chromogranin.
3. LITERATURE REVIEW
A search of the literature at PubMed, using the term “follicular dendritic cell tumor” combined with “extranodal,” “stomach,” and “gastrointestinal,” identified relevant articles for review. Articles were limited to English. References of the collected articles were also used to identify other publications of relevance. Primary nodal tumours were disregarded.
4. DISCUSSION
Follicular dendritic cell sarcoma is an uncommon tumour, and extranodal disease accounts for only one third of fdcs cases11. Most of the extranodal disease shows a predilection for head and neck locations11. Examining all extranodal fdcs disease together, background characteristics have been described. The age of patients with extranodal fdcs is variable, with the mean falling in the 5th decade of life, and the disease is slightly more common in women (1.2:1)3. The causes of fdcs are largely unknown. Associations with Castleman disease12 and Epstein–Barr viral infection13 have been reported. The Epstein–Barr virus is suspected to carry a viral oncogene—latent membrane protein 1—that might encourage transformation. In addition, relationships have been found with autoimmune diseases such as paraneoplastic pemphigus14 and myasthenia gravis15. It is suggested that fdcs encourages aberrant immune system activation, given that patients often demonstrate immature T cells.
Gastrointestinal fdcs tumours often present as slow-growing and painless masses16. However, their location is variable, and the abdominal pain can be nonspecific. For the abdominal component of this disease, common imaging findings include a well-defined mass with regional lymphadenopathy and homogenous enhancement with internal necrosis and often with internal calcifications17. The primary differential diagnosis includes gastrointestinal stromal tumour and primary gastrointestinal lymphomas. The inflammatory pseudotumour-like fdcs variant must also be considered. The latter entity shows greater prevalence in liver and spleen and presents primarily in women. Histology shows atypical spindle cells scattered by prominent lymphoplasmacytic infiltrate, with expression of CD21, CD23, CD35, and D2-4018.
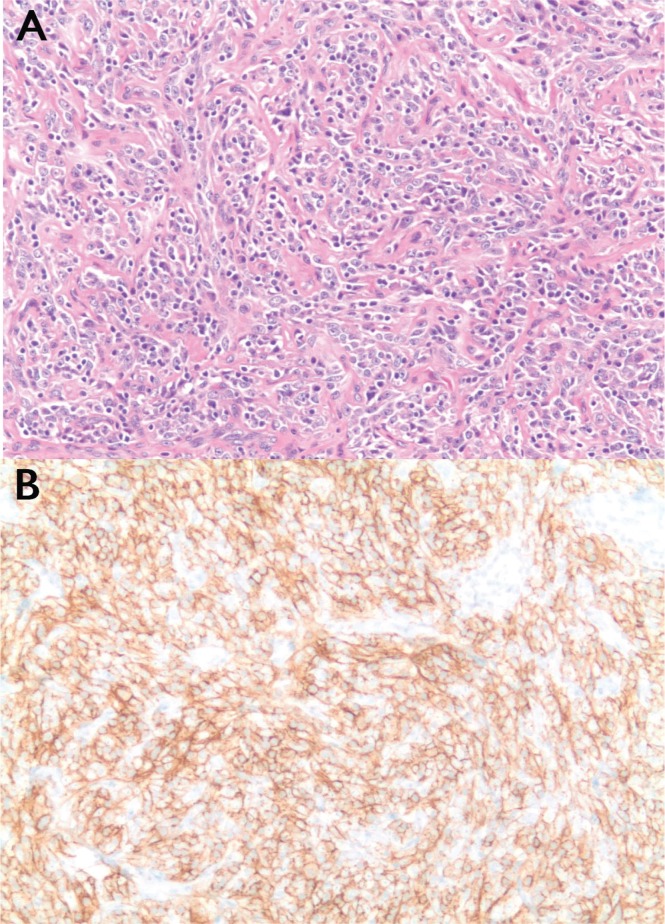
One third of fdcs tumours are initially misdiagnosed3. The most common reason for misdiagnosis is a failure to consider fdcs at the initial pathology evaluation. A routine immunohistochemistry panel will often not test the required markers, making the diagnosis difficult. In addition, fdcs can express markers typical for T-cell lymphoma, which can delay a correct diagnosis6. Tissue diagnosis is essential for fdcs; biopsy samples classically demonstrate fusiform ovoid cells in whirling pattern [Figure 2(A)]. Multiple markers, including CD21, CD35, and CD23, can be used for confirmation19 [Figure 2(B)]. Other markers that can stain positive include CD23, vimentin, CD68, S100 protein, fascin, and Ki-M4p, although they lack specificity20. One study noted that, fdcs is positive for CD21 83% of the time, CD23 90% of the time, and CD35 44% of the time, with wide variability compared with other studies21,22.
FIGURE 2.
Tumour histology. (A) Section demonstrates fusiform cells in whirling patterns (hematoxylin and eosin stain; 20× original magnification). (B) Positive staining for CD21 is a characteristic feature of follicular dendritic cell sarcoma (20× magnification).
This tumour is treated as an intermediate-grade soft-tissue sarcoma. Surgical resection is preferred, and there is no consensus on optimal chemotherapy and adjuvant therapy22. The chop (cyclophosphamide–hydroxydaunorubicin–vincristine–prednisolone) chemotherapy regimen is perhaps the most widely used, producing some degree of response as defined by increased tumour necrosis and decreased uptake on positron-emission tomography23. Other centres are using chemotherapy regimens consistent with sarcoma treatment (doxorubicin, ifosfamide, gemcitabine, and vinorelbine)24. An alternative to doxorubicin, polyethylene glycol liposomal doxorubicin, has also been used, with a favourable response and a favourable side effect and pharmacokinetic profile in fdcs of the neck25. Response to chemotherapy has been modest at best.
Overall, the prognosis for fdcs is concerning, with one study reporting recurrence, metastasis, and mortality rates to be 35%, 35%, and 18% respectively in its population12. However, those data must be interpreted cautiously, because follow-up in the latter dataset ranged from 2 months to 9 years. Other series suggested that the 2- and 5-year disease-free survival rates were only 57% and 32%22. Compared with patients having nodal fdcs, those with extranodal fdcs have a worse prognosis. Factors portending poorly for patients have included a tumour diameter greater than 6 cm, intra-abdominal involvement, coagulative necrosis, high mitotic count (>5 of 10 high-power fields), and significant cellular atypia12. Spontaneous regression has been reported, perhaps suggesting an incomplete understanding of the pathophysiology26.
A number of factors could be responsible for the worse prognosis in intraabdominal, extranodal fdcs. In one study that demonstrated worse prognosis with intraabdominal fdcs, the authors also demonstrated a trend toward higher recurrence12. Interestingly, hepatic lesions recurred far less frequently (3 of 12 patients) than did extrahepatic lesions (15 of 25 patients). In addition, the intraabdominal tumours were larger on average (9.5 cm) than those at other sites (5.3 cm). It is possible that the delay in diagnosis, with the resulting increase in lesion size, is more responsible than a true difference in fdcs biology for the increased recurrence. Other studies have not demonstrated worse prognosis with intraabdominal involvement27. It is plausible that other factors, such as size or delay in diagnosis, could be confounding factors in the outcome studies. Our review noted that abdominal pain was the most common manifestation of intraabdominal fdcs, but that such pain was present in only 78 of 236 previously described cases. Of those cases, 11 were diagnosed incidentally. Table i summarizes our review of demographics, treatment, and prognosis in gastrointestinal fdcs.
TABLE I.
Clinical features and outcomes in representative reports of gastrointestinal follicular dendritic cell sarcoma
| Reference | Pt age (years) | Cancer location | Treatment modality | Follow-up (months) | Recurrence |
|---|---|---|---|---|---|
| Han et al., 200010 | 45 | Stomach | Surgery | 10 | None |
| Geerts et al., 20049 | 40 | Stomach | Surgery | 1.15 | None |
| Khalid et al., 200524 | 19 | Liver | Chemo | 24 | None |
| Shia et al., 20063 | 30 | Rectum | Surgery | 15 | Disease recurrence |
| Soriano et al., 200721 | 25 | Pancreas | Surgery and radiation therapy | 14 | Distant metastasis |
| Granados et al., 20087 | 57 | Liver | Surgery | 24 | None |
| De Pas et al., 200828 | 53 | Liver | Surgery | 22 | Distant metastasis |
| Kang et al., 201017 | 52 | Descending colon | Surgery | 8 | Distant metastasis |
| Shen et al., 20095 | 43 | Appendix | Surgery | 8 | Distant metastasis |
| Yamada et al., 20096 | 82 | Small intestine | Surgery and chemotherapy | 12 | None |
| Li et al., 201022 | 45 | Liver | Surgery | 27 | Distant metastasis |
| 36 | Mesentery | Surgery | 36s | Distant metastasis |
Pt = patient.
Given the criticality of the prognosis as described earlier, there is a need for the creation of better prognostic systems to guide optimal treatment for fdcs. Li and colleagues22 reported a scheme that stratifies tumours into low-, medium-, and high-risk groups based on size and tumour grade. Their model demonstrated good stratification of mortality rates (0%, 4%, and 45% for patients with low-, medium-, and high-risk tumours respectively) over a 120-month surveillance period. This prognostic system is probably useful for selecting patients who should receive aggressive adjuvant therapy after surgical resection. To validate the prognostic system for this rare disease, compilation of the data for each case from multiple institutions is critical.
5. CONCLUSIONS
The complex entity fdcs and its gastrointestinal variant is poorly described in the literature. Patients with gastrointestinal fdcs often present late, with vague symptoms. Pathology confirmation is challenging, thus leading to a further delay in diagnosis. Surgical resection remains essential for definitive treatment, especially in gastric disease; and as yet, no consensus on adjuvant therapy has been developed. Further research into the pathogenesis and therapy of fdcs is needed to improve outcomes in this rare disease.
6. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to declare.
7. REFERENCES
- 1.van Nierop K, de Groot C. Human follicular dendritic cells: function, origin and development. Semin Immunol. 2002;14:251–7. doi: 10.1016/S1044-5323(02)00057-X. [DOI] [PubMed] [Google Scholar]
- 2.Perez–Ordonez B, Rosai J. Follicular dendritic cell tumor: review of the entity. Semin Diagn Pathol. 1998;15:144–54. [PubMed] [Google Scholar]
- 3.Shia J, Chen W, Tang LH, et al. Extranodal follicular dendritic cell sarcoma: clinical, pathologic, and histogenetic characteristics of an underrecognized disease entity. Virchows Arch. 2006;449:148–58. doi: 10.1007/s00428-006-0231-4. [DOI] [PubMed] [Google Scholar]
- 4.Chang KC, Jin YT, Chen FF, Su IJ. Follicular dendritic cell sarcoma of the colon mimicking stromal tumor. Histopathology. 2001;38:25–9. doi: 10.1046/j.1365-2559.2001.01035.x. [DOI] [PubMed] [Google Scholar]
- 5.Shen DP, Ni XZ, Yin XL, Wu ZY. Clinical and pathological features of follicular dendritic cell sarcoma of appendix: a case report. Chin Med J (Engl) 2009;122:1595–7. [PubMed] [Google Scholar]
- 6.Yamada Y, Haga H, Hernandez M, et al. Follicular dendritic cell sarcoma of small intestine with aberrant T-cell marker expression. Pathol Int. 2009;59:809–12. doi: 10.1111/j.1440-1827.2009.02449.x. [DOI] [PubMed] [Google Scholar]
- 7.Granados R, Aramburu JA, Rodriguez JM, Nieto MA. Cytopathology of a primary follicular dendritic cell sarcoma of the liver of the inflammatory pseudotumor-like type. Diagn Cytopathol. 2008;36:42–6. doi: 10.1002/dc.20744. [DOI] [PubMed] [Google Scholar]
- 8.Hollowood K, Stamp G, Zouvani I, Fletcher CD. Extranodal follicular dendritic cell sarcoma of the gastrointestinal tract. Morphologic, immunohistochemical and ultrastructural analysis of two cases. Am J Clin Pathol. 1995;103:90–7. doi: 10.1093/ajcp/103.1.90. [DOI] [PubMed] [Google Scholar]
- 9.Geerts A, Lagae E, Dhaene K, et al. Metastatic follicular dendritic cell sarcoma of the stomach: a case report and review of the literature. Acta Gastroenterol Belg. 2004;67:223–7. [PubMed] [Google Scholar]
- 10.Han JH, Kim SH, Noh SH, Lee YC, Kim HG, Yang WI. Follicular dendritic cell sarcoma presenting as a submucosal tumor of the stomach. Arch Pathol Lab Med. 2000;124:1693–6. doi: 10.5858/2000-124-1693-FDCSPA. [DOI] [PubMed] [Google Scholar]
- 11.Youens KE, Waugh MS. Extranodal follicular dendritic cell sarcoma. Arch Pathol Lab Med. 2008;132:1683–7. doi: 10.5858/2008-132-1683-EFDCS. [DOI] [PubMed] [Google Scholar]
- 12.Chan JK, Fletcher CD, Nayler SJ, Cooper K. Follicular dendritic cell sarcoma. Clinicopathologic analysis of 17 cases suggesting a malignant potential higher than currently recognized. Cancer. 1997;79:294–313. doi: 10.1002/(SICI)1097-0142(19970115)79:2<294::AID-CNCR13>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 13.Arber DA, Weiss LM, Chang KL. Detection of Epstein–Barr virus in inflammatory pseudotumor. Semin Diagn Pathol. 1998;15:155–60. [PubMed] [Google Scholar]
- 14.Wang J, Bu DF, Li T, et al. Autoantibody production from a thymoma and a follicular dendritic cell sarcoma associated with paraneoplastic pemphigus. Br J Dermatol. 2005;153:558–64. doi: 10.1111/j.1365-2133.2005.06599.x. [DOI] [PubMed] [Google Scholar]
- 15.Hsu C, Vega F, Grimes LM, Hunt KK. Follicular dendritic cell sarcoma and associated myasthenia gravis: true, true, related? J Clin Oncol. 2011;29:e369–71. doi: 10.1200/JCO.2010.32.7932. [DOI] [PubMed] [Google Scholar]
- 16.Kalrouz S, Hashash J, Kabbara W, McHayleh W, Tabbara IA. Dendritic cell neoplasm: an overview. Am J Hematol. 2007;82:924–8. doi: 10.1002/ajh.20857. [DOI] [PubMed] [Google Scholar]
- 17.Kang TW, Lee SJ, Song HJ. Follicular dendritic cell sarcoma of the abdomen: the imaging findings. Korean J Radiol. 2010;11:239–43. doi: 10.3348/kjr.2010.11.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan ST, Cheng CY, Lee NS, Liang PI, Chuang SS. Follicular dendritic cell sarcoma of the inflammatory pseudotumor-like variant presenting as a colonic polyp. Korean J Pathol. 2014;48:140–5. doi: 10.4132/KoreanJPathol.2014.48.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi BS, Baek JH, Shin YM, et al. Follicular dendritic cell sarcoma: a case report and review of the literature. Cancer Res Treat. 2010;42:121–4. doi: 10.4143/crt.2010.42.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H, Su Z, Hu Z, Wen J, Liu B. Follicular dendritic cell sarcoma: a report of six cases and review of the Chinese literature. Diagn Pathol. 2010;5:67. doi: 10.1186/1746-1596-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soriano AO, Thompson MA, Admirand JH, et al. Follicular dendritic cell sarcoma: a report of 14 cases and a review of the literature. Am J Hematol. 2007;82:725–8. doi: 10.1002/ajh.20852. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Shi YH, Guo ZJ, et al. Clinicopathological features and prognosis assessment of extranodular follicular dendritic cell sarcoma. World J Gastroenterol. 2010;16:2504–19. doi: 10.3748/wjg.v16.i20.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shinagare AB, Ramaiya NH, Jagannathan JP, Hornick JL, Swanson RS. Primary follicular dendritic cell sarcoma of liver treated with cyclophosphamide, doxorubicin, vincristine, and prednisone regimen and surgery. J Clin Oncol. 2011;29:e849–51. doi: 10.1200/JCO.2011.37.1906. [DOI] [PubMed] [Google Scholar]
- 24.Khalid T, Folman R. Symptoms in cancer patients and an unusual tumor: case 3. Follicular dendritic cell sarcoma. J Clin Oncol. 2005;23:9425–6. doi: 10.1200/JCO.2004.00.9365. [DOI] [PubMed] [Google Scholar]
- 25.Pisani F, Marino M, Sentinelli S, Petti MC. Follicular dendritic cell sarcoma of the neck: report of a case treated by surgical excision and cop plus (peg)-liposomal doxorubicin. J Exp Clin Cancer Res. 2008;27:33. doi: 10.1186/1756-9966-27-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caces DB, Daniel S, Paredes–Tejada JM, Smith S. Spontaneous regression of follicular dendritic cell sarcoma. J Clin Oncol. 2012;30:e24–6. doi: 10.1200/JCO.2011.38.4164. [DOI] [PubMed] [Google Scholar]
- 27.Saygin C, Uzunasian D, Ozguroglu M, Senocak M, Tuzuner N. Dendritic cell sarcoma: a pooled analysis including 462 cases with presentation of our case series. Crit Rev Oncol Hematol. 2013;88:253–71. doi: 10.1016/j.critrevonc.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 28.De Pas T, Spitaleri G, Pruneri G, et al. Dendritic cell sarcoma: an analytic overview of the literature and presentation of original five cases. Crit Rev Oncol Hematol. 2008;65:1–7. doi: 10.1016/j.critrevonc.2007.06.003. [DOI] [PubMed] [Google Scholar]