Abstract
Objective:
To identify and classify tools for assessing the influence of spasticity on quality of life (QOL) after spinal cord injury (SCI).
Methods:
Electronic databases (MEDLINE/PubMed CINAHL and PsycInfo) were searched for studies published between 1975 and 2012. Dijkers’s theoretical framework on QOL was used to classify tools as either objective or subjective measures of QOL.
Results:
Sixteen studies met the inclusion criteria. Identified objective measures that were used to assess the influence of spasticity on QOL included the Short Form-36 (SF-36) the Sickness Impact Profile (SIP) and the Health Utilities Index-III (HUI-III). Subjective measures included the Quality of Life Index–SCI Version III (QLI-SCI) Life Situation Questionnaire–Revised (LSQ-R) Reciprocal Support Scale (RSS) Profile of Mood States (POMS) Spinal Cord Injury Spasticity Evaluation Tool (SCI-SET) and the Patient Reported Impact of Spasticity Measure (PRISM). A number of tools proved either to be insensitive to the presence of spasticity (QLI-SCI) or yielded mixed (SF-36) or weak (RSS LSQ-R) results. Tools that were sensitive to spasticity had limited psychometric data for use in the SCI population (HUI-III SIP POMS) although 2 were developed specifically for assessing spasticity on daily life post SCI (SCI-SET PRISM).
Conclusions:
Two condition-specific subjective measures the SCI-SET and PRISM emerged as the most promising tools for the assessment of spasticity impact on QOL after SCI. Further research should focus on establishing the psychometric properties of these measures for use in the SCI population.Key words: outcome measurement quality of life spasticity spinal cord injury
Key words: outcome, measurement, quality of life, spasticity, spinal cord injury
Spasticity of the upper limbs, trunk, or lower limbs is typically experienced by individuals with an upper motor neuron spinal cord injury (SCI) following spinal shock, and the resulting spasms often negatively impact quality of life (QOL).1 Although there is great variability in definitions of spasticity, the most commonly cited definition is by Lance2(p485): “Spasticity is a motor disorder characterized by a velocity-dependent increase in tonic stretch reflexes (muscle tone) with exaggerated tendon jerks, resulting from hyperexcitability of the stretch reflex, as one component of the upper motor neuron syndrome.” A wider definition of spasticity includes increased exteroceptive reflexes, as well as loss of motor function (ie, muscle power and coordination).3 The notion is that muscle weakness and impaired coordination are not part of the spasticity syndrome itself, but rather are associated with spasticity.3–6 Spasticity following SCI is prevalent, with 65% to 78% of persons more than 1 year post injury reporting its occurence.7,8
The decision to treat spasticity largely depends on the frequency, severity, and impact of the spasms on a person’s daily life.9,10 Treatment may include conservative physical therapy,11 with a possible combination of other modalities,1 including pharmacological treatments (eg, diazepam,12,13 baclofen,14 clonidine,14,15 tazanidine,12,13,16 dantrolene sodium,12,17 cyproheptadine,14,18 and cannabis17). Persons who do not respond to oral administration of medications may be surgically implanted with a pump for intrathecal administration of baclofen19–21 or receive injections of chemodenervation agents (phenol and ethanol22 or botulinum toxin16,23). Severe recalcitrant cases require surgical intervention, including dorsal rhizotomy and cordotomy.24 Continued improvements in the definition and management of spasticity are hampered by the development of valid and reliable tools for assessing spasticity impact.1
Relatedly, the valid and reliable assessment of QOL post SCI, which is an important outcome for understanding the additional burden of specific secondary health conditions that emerge post SCI and for gauging the success of rehabilitation interventions in minimizing their frequency and severity, is a challenge.25 Symptoms of spasticity may have a profound influence on an individual’s QOL,7,26 including lifestyle and sense of well-being,27 by limiting workplace participation, adding to the cost of medication, and increasing attendant care requirements.8,21,28 Despite these findings, there are problems with assessing the influence of spasticity on QOL that are related to the multidimensionality and breadth of spasticity definitions, the fluctuating nature of associated symptoms, and their clinical impact. It is therefore essential that health care professionals be made aware of available tools that are designed to assess the influence of spasticity on QOL after SCI.
Furthermore, investigators should possess a broader understanding of the different conceptualizations of QOL and which tools correspond to each of these. This will help to ensure that the objectives of a study are well aligned with the selected QOL tool. Gaining prominence in the field is the notion that QOL can be measured from an objective or subjective perspective.29 Objective measures are based on the assumption that there is widespread agreement about what constitutes QOL.25 Such measures focus on external conditions and contain items that can be defined and quantified to reflect societal standards. Conversely, subjective measures are designed with the assumption that QOL can only be judged by the individuals experiencing it.30 Although there are advantages and disadvantages inherent in each measurement type,29 subjective measures give patients a means of providing health professionals with a greater understanding of QOL and its connection to their health and well-being following SCI, whereas objective measures can be used to inform decision makers on how to allocate funds and resources for various interventions.
To date, no systematic reviews on the influence of spasticity on QOL, or on the appropriateness of QOL measures for assessing spasticity, have been conducted. Given the substantial influence spasticity has on QOL, there is a need to improve conceptual understandings of QOL to ensure that investigators employ appropriate research designs as well as suitable outcome measures to assess this prevalent secondary health condition. Hence, the purpose of this systematic literature review was to classify and evaluate outcome measures that are used to assess the influence of spasticity on QOL following SCI.
Methods
A systematic review of the literature was conducted using multiple databases (MEDLINE/ PubMed, CINAHL, and PsycInfo). The key term “spinal cord injuries” and its variants, along with spasticity (or muscle spasms, muscle tone, or muscle spasticity), quality of life, participation, personal or life satisfaction, and activities of daily living, were used to identify relevant articles published between January 1975 and December 2012. Reference lists of identified articles were also reviewed. To be included, studies had to be in English and have adult (>18 years) participants with SCI comprising at least 50% of the sample. Abstracts were verified by 2 independent reviewers. Related constructs reflecting well-being were also incorporated into the review (eg, participation, social support, affect). Studies that examined global constructs of QOL post SCI and/or those that contained pediatric populations or pediatriconset SCI were excluded.
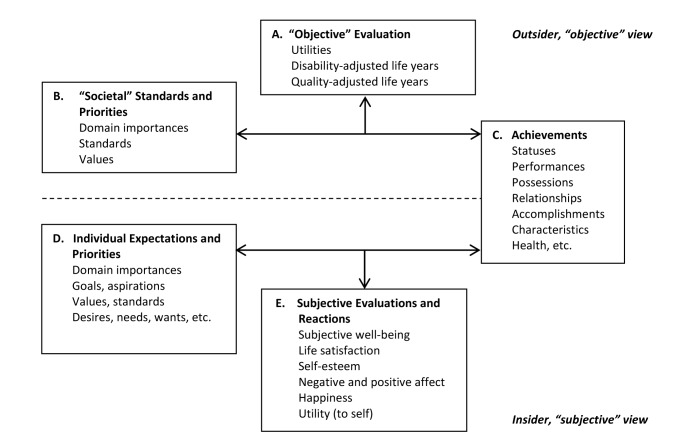
Dijkers’s theoretical model25 was used to categorize measures as objective or subjective and to provide a deeper understanding of the specific QOL constructs that are underlying the measures (see Figure 1). QOL as “utility” (Box A) reflects the desirability and preferences of life valuations and is obtained by judging one’s tradition, achievements, and status in terms of societal norms and standards (Box B). It is expressed as an outsider’s viewpoint (objective) and typically provides a metric framing of QOL that is used in health economic analyses.25 QOL as “achievements” (Box C), another objective QOL perspective, takes into account the individual’s possessions, accomplishments, characteristics, and so on.25 Therefore, QOL is predicted by what a person has obtained in his or her life (ie, good health, marriage), which society generally views as indicative of what is needed to have a good life. QOL as “subjective well-being” recognizes that humans develop individual priorities and wants (Box E), which may range in relative importance (Box D). When these needs and wants are compared to reality (Box C), the resulting reactions can range from positive to negative and are likely to affect morale, life satisfaction, and other expectations (Box E).25 QOL as “subjective well-being” can therefore be defined as “feelings of wellbeing as influenced by the good things in life.”31(pS21)
Figure 1. Three conceptualizations of quality of life and their interrelations. Adapted, with permission, from Dijkers MP. Individualization in quality of life measurement: Instruments and approaches. Arch Phys Med Rehabil. 2003;84(4 Suppl 2):S3-14. Copyright © 2003 by Elsevier.
Results
Fifty-six studies (34 from MEDLINE/PubMed, 22 from CINAHL, and 0 from PsycInfo) were identified. Only 16 met the inclusion criteria: 7 pre-post intervention studies,27,32–37 8 crosssectional observational studies,9,38–44 and 1 crosssectional and longitudinal study45 (see Table 1). For each measure, we identified whether it assesses QOL from an objective or subjective perspective by linking it to the category of QOL (ie, QOL as a utility, achievements, or subjective well-being) as described by Dijkers’s theoretical model.25 To help illustrate these classifications, each identified measure is mapped onto the box(es) in Figure 1.
Table 1. Spasticity results.
| Author(s) Study Design | Methods | Outcome |
| Gianino et al27 Pre-post intervention and qualitative study |
Population: 25 (40% males) subjects diagnosed with intractable spasticity of the spine, including subjects with MS (15), SCI (7), and neurological diseases (3); mean age 39.4 yrs (range, 21-70 yrs) | There were no significant changes in the QLI-SCI total or subscale scores for the different time points. However, there was a trend toward improvement for the health and function subscales. |
| Objective: To explore the effect from intrathecal baclofen on spasticity and QOL | There were significant differences between MS and SCI participants with respect to the SIP total score (P = .0358) as well as the physical (P = .0026) and psychological (P = .0037) SIP subscores. | |
| QOL outcome measures:QLI-SCI, SIP, Ashworth Scale | After 3 months treatment with intrathecal baclofen, subjects reported less spasticity, improved ability to perform daily activities, greater mobility, and reduced pain. | |
| Westgren & Levi42 Cross-sectional survey |
Population: 320 persons (261 men) with SCI; mean age 42 yrs (range, 17-78 yrs); YPI ≤ 4 yrs and ≥ 4 yrs | Spasticity was associated with lower QOL. Problematic spasticity yielded significant medium effect sizes in all of the SF-36 subscales. |
| Objective: To determine associations between major outcome variables after SCI and QOL | ||
| QOL outcome measures: SF-36 | ||
| Noonan et al39 Cross-sectional survey |
Population: 70 persons (81% male) with SCI; mean age at injury, 45 ± 18 (13-91 yrs); mean age at follow-up, 51 ± 18 (19-95 yrs) | Spasticity was significantly related to the physical functioning subscore of the SF-36 in the first 2 stages of analysis, but became nonsignificant once all confounding variables were controlled for. |
| Objective: To determine the effect of associated SCI conditions on health status and QOL | ||
| QOL outcome measures: SF-36; Numeric QOL Rating | ||
| Loubser et al34 Pre-post intervention study |
Population: 9 males with SCI and spasticity; mean age, 45.6 ± 12.35 (22-63 yrs) | All but one patient benefited markedly in terms of gaining independence, of reducing time and effort for care required by caregivers, or both. |
| Objective: To explore the effect from intrathecal baclofen on spasticity | Persons with paraplegia and lower level tetraplegia gained independence in mobility and self-care. | |
| QOL outcome measures: Occupational Therapy Evaluation of Personal Independence | Persons with paraplegia with severe spasticity gained independence in driving. Although persons with high tetraplegia remained dependent in ADLs, caregiver time and effort for daily care decreased markedly. | |
| Fleuren et al9 Cross-sectional survey |
Population: 26 patients 920 males) with SCI; mean age 41.0 ± 10.6 yrs; mean mos post injury 100.9 ± 76.5 mos. | 57.7% of patients found the perceived spasticity problematic, mostly due to decreasing function (73.3%) rather than pain. Spasticity impacted ability to change position (22.0%), make a transfer (20.7%), be active (17.1%), maintain a stable body position (12.2%), as well as ADLs (17.1%). |
| Objective: To study the manifestation of spasticity in daily life of patients with SCI, their perception of spasticity, and spasticity-related discomfort | ||
| QOL outcome measures: Nonstandardized study-specific questionnaire | Findings confirm that the impact of spasticity on daily life is related to the context in which it occurs. Positive effects include helping to get dressed and prevention of pressure sores and muscle atrophy. Negative effects include initial shame regarding spasms and initial time taken to understand the new body signals. | |
| Adams et al 38 Cross-sectional survey |
Population: Study 1: 9 persons (8 males) with SCI; mean age 37.6 ± 11.5 yrs; mean YPI 13.6 ± 11.5 yrs; Study 2: 19 persons (15 males) with SCI; mean age 45.7 ± 13.9 yrs; mean YPI 13.6 ± 11.5 yrs; Study 3: 61 persons (45 males) with SCI; mean age 41.9 ± 12.6 yrs, mean YPI 10.2 ± 8.6 yrs | SCI-SET showed significant (P <.001) moderate to strong associations with self-assessed spasticity severity, self-assessed spasticity impact, QLI-SCI, and the PSFS. With regard to test-retest reliability, the intra-class correlation coefficients for SCI-SET was .91, and above the recommended minimum reliability values. |
| Objective: To develop and assess the reliability and validity of a new scale designed to measure the impact of spasticity on daily life in people with SCI | The mean SCI-SET score indicated an overall negative impact of spasticity on daily life despite perceptions of some benefits. | |
| QOL outcome measures: SCI-SET; QLI-SCI health and functioning subscale (satisfaction) | ||
| Adams & Hicks36 Random cross-over, pre-post intervention study |
Population: 7 persons (6 males) with chronic SCI; mean age 37.1 ± 7.7 yrs; mean YPI 5.0 ± 4.4 yrs. | Participation in 12 sessions of BWSTT or TTS did not result in group changes in scores on the SCI-SET. The 12 sessions of BWSTT resulted in a medium effect size (0.5) on QOL. |
| Objective: To determine the effects of body-weight-supported treadmill training (BWSTT) and tilt-table standing (TTS) on spasticity, motor neuron excitability, and related constructs in individuals with chronic SCI | ||
| QOL outcome measures: SCI-SET; QLI-SCI | ||
| Boutilier et al35 Pilot prospective pre- and post-intervention study |
Population: 8 persons (7 males) with SCI, mean age 44.12 yrs, YPI range 4 to 29 yrs, who could stand with or without the assistance of bracing or supports | Although self-evaluations of spasticity using the SCI-SET showed improvement from the initial visit (-0.91) to midway (-0.63) to the final visit (-0.57), differences were not statistically significant. |
| Objective: To determine whether a dynamic standing program using the Segway Personal Transporter results in any measurable physiological effects in individuals with SCI | ||
| QOL outcome measures: SCI-SET | ||
| Anson et al45 Cross-sectional and longitudinal survey |
Population: 125 persons with SCI; ≥18 yrs; and YPI > 1 yr | Spasticity was weakly and negatively associated with giving advice and social support (P < .05). |
| Objective: (1) To investigate relationships among social support, adjustment, and secondary complications in persons with SCI; (2) to explore potential effects of persons’ perceived contribution to the social support of others and reception of social support from others | ||
| QOL outcome measures: QLI-SCI, RSS | ||
| Lundqvist et al41 | Population: 98 (81 males) subjects with SCI; mean age 33.5 yrs (16-72 yrs); 2.3 YPI (.1-22.7 yrs) | Patients with spasticity problems scored significantly worse on the ambulation and feeding issues. |
| Cross-sectional survey | Objective: To define the QOL of SCI patients – their physical, psychological, and social functioning | |
| QOL outcome measures: SIP | ||
| Jagatsinh32 | Population: 98 (81 males) subjects with SCI; mean age 33.5 yrs (16-72 yrs), 2.3 YPI (.1-22.7 yrs) | Patients demonstrated improvements in ADLs such as feeding ability (10), self-care (10), indoor and outdoor mobility (19), and driving (4). |
| Pre-post intervention study | Objective: To assess the effects of intrathecal baclofen on symptoms of spasticity and ADLs | |
| QOL outcome measures: Modified scale comprised of items from the Barthel Index, FIM, and SCIM | ||
| Kogel et al33 Pre-post intervention study – case approach |
Population: 5 male subjects with tetraplegia | Emotionally, all participants showed decreases in vigor. Test data also showed slight to significant increases in at least one dysphoric mood for each patient, although results were extremely variable. Consequently, dronabinol seems to produce a heightened emotionality, but in an extremely individualized manner. |
| Objective: To assess the effects of dronabinol, a THC derivative, for the treatment of spasticity | ||
| QOL outcome measures: POMS | ||
| Jones et al37 Pre-post intervention study with a qualitative component |
Population: 7 males with SCI (AIS A or B); mean age 36.7 yrs (25-45 yrs); with positive bulbocavernous reflex | Participants reported perceived improvement in spasticity severity and overall healthrelated QOL. |
| Objective: To document changes in perceived sexual function and spasticity after implant of a baclofen pump | ||
| QOL outcome measures: SF- 36; Schwartz et al’s performance scale items for perception of spasticity severity | ||
| Westerkam et al43 Cross-sectional survey |
Population: 1,549 participants with SCI; mean age 45.1 yrs; YPI <1. | Three aspects of spasticity (daily activities, positive impact, and spasticity at its worst) were negatively correlated with home life satisfaction, global satisfaction, and overall QOL. The daily activities scale and the spasticity at its worst rating were negatively correlated with vocational satisfaction. Spasticity is negatively associated with QOL after SCI, and this should be an important consideration throughout patient treatment and rehabilitation. |
| Objective: To identify the relationship between spasticity and life satisfaction among participants with SCI | ||
| QOL outcome measures: Home life satisfaction, global satisfaction, vocational satisfaction, overall QOL, 3 subscales of the PRISM | ||
| Craven et al44 Cross-sectional telephone survey |
Population: 357 (218 males) community-dwelling persons with chronic traumatic and non-traumatic SCI; mean age 54 yrs; mean YPI 19.3 (onset range, 2-65 yrs) | HUI-Mark III scores were lower (P < .001) in high impact groups for spasms compared to low/no impact groups. |
| Objective: To describe the relationships between secondary health conditions and health preference in a cohort of adults with chronic SCI | ||
| QOL Outcome Measures: HUI-Mark III; SCS-M | ||
| Cook et al40 Cross-sectional survey |
Population: 180 persons (162 males) with SCI; mean age 52.12 ± 11 yrs; YPI range, 2 mos to 56 yrs | The intraclass correlation coefficient values for the PRISM’s 7 subscales were all high (.82 to .91), and internal consistency was the lowest for the need for intervention scale (α =.74) and highest for social avoidance/anxiety scale (α = .96). |
| Objective: To develop an instrument that measures the impact of spasticity on QOL | The a priori hypotheses regarding abnormal and involuntary muscle movement were partially confirmed, and thus provided some content validity for the PRISM. | |
| QOL outcome measures: PRISM |
Note: ADLs = activities of daily living; AIS = American Spinal Injury Association Impairment Scale; BWSTT = body-weight-supported treadmill training; HUI-Mark III = Health Utilities Index-Mark III; mos = months: MS = multiple sclerosis; POMS = Profile of Mood States; PRISM = Patient Reported Impact of Spasticity Measure; PSFS = Penn Spasm Frequency Scale; QLI-SCI = Ferrans and Powers Quality of Life Index-SCI Version III; QOL = quality of life; RSS = Reciprocal Social Support; SCI = spinal cord injury; SCIM = Spinal Cord Independence Measure; SCI-SET = Spinal Cord Injury Spasticity Evaluation Tool; SCS-M = SCI Secondary Conditions Scale-Modified; SIP = Sickness Impact Profile; TTS = tilt-table standing; YPI = years post injury.
Applicability of objective QOL measures on assessing spasticity
Five objective measures were identified: (1) the Short Form-36 (SF-36)46; (2) the Sickness Impact Profile (SIP); (3) the Health Utilities Index-III (HUI-III)47; (4) the Evaluation of Personal Independence (EPI); and (5) a survey comprised of questions from the Spinal Cord Independence Measure (SCIM),48 Functional Independence Measure (FIM),49 and the Barthel Index50(see Tables 1 and 2). The SF-36 (Boxes B and C, Figure 1) is the most commonly cited measure of objective QOL, with well-established psychometric properties for multiple health conditions.51 The SF-36 is suitable for use in SCI,52 with reliability being moderate to high (α = 0.72-0.98), with the exception of the general health item (see Table 2).
Table 2. Summary of outcome measures.
| Measure | QOLa | QOL construct | SCI psychometrics | Format/scoring | Administrative burden | Sensitive to spasticity impactb |
| Evaluation of Personal Independence (EPI) | O | SWB | NA | Assesses 4 dimensions of activities of daily living: communications, hygiene, eating, and dressing. Scores are assigned through a grading key ranging from normal performance to activity impossible. | ~5 min | +Loubser et al34 |
| Ferrans and Powers Quality of Life Index– (SCI Version III) | S | SWB | Cronbach’s α = .73-.99 Validity established for SCI (see Hill et al57for further details) |
74 items divided into 2 parts: satisfaction (37 items) and importance (37 items). Each item is ranked on a 6-pt scale ranging from 1 (very dissatisfied or unimportant) to 6 (very satisfied or important). The scale assesses the following domains: health and functioning, socioeconomic status, psychological and spiritual well-being, and family relationships. Scores range from 0 to 30, with higher scores indicating better QOL. |
~ 10 min | –Gianino et al27 |
| Health Utilities Index-III (HUI-II I) | O | SWB | Lacks psychometric validation | Assesses 8 attributes (vision, hearing, speech, ambulation, dexterity, emotion, cognition, and pain) in respondents over the 4 weeks prior to the administration date. Each attribute is assigned to 1 of the 5 or 6 levels of ability/disability. Scores for each attribute are combined using a multiplicative utility function and are reported as a single metric ranging from 0 (death) to 1 (perfect health). There is a 15- and a 40-item version of the HUI-III. | ~3-10 min | +Craven et al44 |
| Life Situation Questionnaire–Revised (LSQ-R) | S | SWB | High levels of reliability (Cronbach’s α = 0.76-0.86) | Assesses current level of satisfaction with various domains of life (ie, employment, medical treatments, social activities, and subjective well-being). | ~10 min | +Westerkam et al43 |
| It is embedded into a larger survey, the LSQ, which measures a broad range of long-term SCI outcomes. It consists of 50 items divided into 2 parts. Part 1 consists of 20 items that measure satisfaction on scale ranging from 1 (very dissatisfied) to 5 (very satisfied). Part 2 consists of 30 items measuring problems on a scale ranging from 1 (no problem) to 5 (major problem). Scoring is achieved through 8 embedded subjective well-being scales.) | ||||||
| Patient Reported Impact of Spasticity Measure (PRISM) | S | SWB | Good internal consistency (Cronbach’s α = .74 to .96) and reproducibility (ICC range from .82 to .91). | 41 items covering 7 subscales. Items are ranked on a 5-point scale ranging from 0 (never true for me) to 4 (very often true for me), with higher scores indicating great impact. | ~ 10 min | +Cook et al40 +Westerkam et al43 |
| Validity still needs to be established. | The tool covers 7 subscales: Social Avoidance/Anxiety, Psychological Agitation, Daily Activities, Need for Assistance/ Positioning, Positive Impact, Need for Intervention, and Social Embarrassment. | |||||
| Profile of Mood States (POMS) | S | SWB | N/A | 65 items measuring 6 mood states on a 5-point scale ranging from 0 (not at all) to 4 (extremely). Total score ranges from 0 to 200, with higher scores indicating greater mood disturbance. | ~ 5-10 min | +Kogel et al33 |
| The POMS yields score on the following dimensions: tension- anxiety, depression-dejection, anger-hostility, vigor-activity, fatigue-inertia, and confusion-bewilderment | ||||||
| Reciprocal Social Support (RSS) | S | SWB | Alpha coefficients ranged from .70 to .76 for the 4 types of support, with an average of .73. The alpha for the subsets was .55, but is expected as it sums 3 types of support. | Eight questions rating types of support received from families, friends, and the community. Responses are ranked on a 7-point scale ranging from 1 (never) to 7 (always). Higher scores indicate greater levels of support. | ~ 10-15 min | +Anson et al45 |
| Short-Form Health Survey (SF-36) | O | A; HRQO | Reliability and validity established for SCI (see Noonan et al39for further details) | 36 items covering 8 domains related to functioning and health. Scoring is norm-based, with a general population mean score of 50 and a SD of 10. Higher scores indicate higher levels of health. | ~ 5-10 min | –Noonan et al39 +Westgren & Levi42 |
| Sickness Impact Profile (SIP) | O | SWB | Limited. Data and norms for the SCI population do exist for the SIP 68. | 136 severity-weighted negative health impact statements to which a respondent answers only if the statement is true based on the day the SIP is completed. | ~ 20-30 min for SIP ~ 15-20 min for SIP 68 |
+Gianino et al27 +Lundqvist et al41 |
| Twelve domains of functioning assessed: sleep and rest, eating, work, home management, recreation and pastimes, ambulation, mobility, body care and movement, social interaction, alertness behavior, emotional behavior, and communication. | ||||||
| Spinal Cord Injury Spaticity Evaluation Tool (SCI-SET) | S | SWB | High internal consistency (Cronbach’s α = .90) and test-retest reliability (ICC = .91) | 35 items ranked on a 7-point scale ranging from -3 (extremely problematic) to 3 (extremely helpful). The total score is the sum of all applicable responses divided by the number of applicable items. | ~ 10 min | +Adams et al38 |
Note: A = Achievement; HRQOL = health-related quality of life; ICC = intercorrelation coefficient; N/A = not available; SCI = spinal cord injury; SWB = subjective well-being.
O = objective; S = subjective.
+ = sensitive to spasticity impact; – = not sensitive to spasticity impact
With regard to assessing the influence of spasticity on QOL, findings for the SF-36 have been inconsistent. The study by Noonan et al39 found the SF-36 to be insensitive to the presence of spasticity once influencing factors (ie, demographics, years post injury) were controlled for. Conversely, Westgren and Levi42 found it sensitive to problematic spasticity. Similarly, Jones et al37 found that the scores of persons with SCI who were implanted with a baclofen pump improved pre to post implantation, with the exception of scores on the SF-36 physical subscale. In the studies by Westgren and Levi42 and Noonan et al,39 the severity of spasticity was not assessed. Jones and colleagues37 used a spasticity self-perception scale developed by Schwartz et al53 rather than a validated clinical measure of spasticity (ie, Ashworth Scale).
In addition to the mixed findings, there are some controversies associated with the SF-36. Some reports by wheelchair users indicate that certain questions are offensive54,55 because 5 of 10 items refer to climbing or walking. Some people with disabilities also perceive that the SF-36 (including other health-related QOL measures) has flawed underlying assumptions regarding their QOL (ie, disability equates poor health).52,55 These limitations could potentially be minimized by pairing the SF-36 with a condition-specific tool.55 The development of the SF-36V has addressed some of the noted problems of the SF-36, but the psychometric properties of this scale have not yet been fully established for the SCI population.56
The SIP57,58 (Boxes B and C, Figure 1) is a generic health status measure of change in behavior as a consequence of illness. The SIP has been used in 2 studies,27,41 and both found it to be sensitive to spasticity (see Table 1). Like the aforementioned studies,39,42 no details were provided by Lundqvist et al41 on the impact or severity of spasticity. However, the study by Gianino et al27 did quantify spasticity with the Ashworth Scale, among other spasm scales, which is considered the current standard for assessing spasticity post SCI.59 The SIP has been used in several SCI studies,41,60,61 but psychometric data for use in the SCI population are lacking.62 The SIP faces some of the same controversies as the SF-36 regarding its underlying assumptions.55
The HUI-III (Boxes A, B, and C, Figure 1) is a comprehensive system for describing the health status of individuals and for assigning a preference (or utility) score to them.47 A preference score is a societal representation of well-being, which is typically reported as a single metric anchored at 0 (death) and 1 (perfect health), and represents a preference for a health state.63 These health state morbidities are measured across a group of individuals and combined into a utility score, which can then be used as a quality weight for calculating the number of quality-adjusted life-years gained in cost utility analyses.64
Craven et al44 used the HUI-III to ascribe the burden of various secondary health conditions, including spasticity, post SCI and found it to be sensitive to the presence of spasms. Although this study provided some evidence with regard to QOL as “utility” for spasticity, the HUI-III lacks psychometric validation for SCI. As well, presence and impact of spasticity were based on self-report via the Secondary Conditions Scale,65 a validated measure that provides standardized definitions of secondary health conditions and records their presence and perceived impact without assessing signs and symptoms.
Similar findings were obtained by Jones et al37 in their study assessing the impact of an implantable intrathecal baclofen pump. They used a single item to ask respondents to assign a general utility rating (0 = worst health to 100 = perfect health) to their spasticity’s impact on health and well-being. A significant improvement was detected on this item from pre to post implantation. However, the item lacks validation for SCI and the vigor typically associated with other direct utility measures (ie, standard gamble or time trade-off scenarios).
Jagatsinh32 examined the effects of intrathecal baclofen pump implantation on activities of daily living in a pre-post intervention study using a survey derived from items in the Barthel Index,50 FIM,49 and SCIM48 (Boxes B and C, Figure 1). The scores for each measure were not reported separately, but rather were amalgamated to identify whether improvements were achieved in a particular domain (eg, improved, no change, worsened). Results showed that the intrathecal baclofen pump improved symptoms; Jagatsinh32 concluded that this indicated an improved QOL by allowing participants to live more independently. Similarly, Loubser et al34 examined 9 patients who received an intrathecal baclofen infusion for treatment of spasticity using the EPI (Boxes B and C, Figure 1). The EPI assesses 4 dimensions of activities of daily living, including communications, hygiene, eating, and dressing, and was administered by occupational therapists from the clinical program.34 Although the assessments revealed improvements in function and activities of daily living from pre to post intervention, the EPI is a study-specific measure and is therefore limited in scope as an outcome measure of QOL, much like the measure used by Jagatsinh.32 Subsequent searches for the EPI in the literature yielded negative results. As such, there are no psychometric data supporting the use of the EPI for this population.
Study-specific measures, such as the one used by Jagatsinh32 and Loubser et al,34 are informative but tend not to be generalizable to the larger population. Moreover, effect sizes are not calculated, information on cross-cultural applications is not available, and the instruments typically cover a number of different dimensions.66 Although the SCIM,48,67,68 FIM,69,70 and Barthel Index50,71 all have data supporting their use in SCI, the amalgamation of these tools by Jagatsinh32 negates their validity. Further, the SCIM and FIM could be classified under Dijkers’s model (Boxes B and C, Figure 1), but they should not be viewed as QOL measures, given that their scope is too narrow to qualify as measures of health-related QOL and that their developers did not intend for these measures to be used in this fashion.25 Rather, they are indexes of disability and should not be confounded with QOL. The description of the EPI in Loubser et al34 might have strong clinical utility for SCI because it was administered by occupational therapists at a rehabilitation hospital for people with SCI, but there remain a number of questions regarding its psychometric properties. As well, the domains covered are similar to the SCIM and FIM. As such, use of the EPI is not strongly recommended as a measure of spasticity impact on QOL.
In summary, the objective measures of QOL (SF-36, SIP, HUI-III) appear to be sensitive to the presence of spasticity, but the psychometric properties of these scales are limited for use in SCI. Only 3 of the studies27,34,44 adequately captured the severity of spasticity in their samples, but only 227,34 of those used the Ashworth Scale to do so. Although the Ashworth Scale is a clinical measure currently held as the standard in spasticity assessment, its use is considered problematic by researchers and clinicians due to its lack of psychometric rigor.59 It is essential that future studies use a test battery approach to assess spasticity or refine current spasticity measures for use in the SCI population. Two study-specific outcome measures, the EPI and one derived from items of the SCIM, FIM, and Barthel Index,32 were identified, but they are problematic on several fronts. Both the SF-36 and SIP assess QOL under the “achievements” category, whereas the HUI-III is classified as a measure of QOL as “utility.” Further work is warranted to examine the applicability of the SIP. It is recommended that the SF-36 be used in conjunction with the HUI-III; the SF-36 could be used to provide some psychometric validation of the HUI-III, and it could further the understanding of the influence of spasticity on QOL as “utility.” Specifically, scores on the SF-36 can be converted into SF-6D scores, which is a preference-based single-index measure for health from data using general population values. As with the HUI-III, the SF-6D can be used to obtain quality-adjusted life-years (QALYs) from the SF-36 for use in cost utility analysis.72 Further, use of the SF-36V for investigating the influence of spasticity might be warranted, because it appears to have qualities that are more appropriate to studying SCI-related outcomes than does its predecessor.
Applicability of subjective QOL measures on assessing spasticity
Six subjective measures were identified: the Ferrans and Powers Quality of Life Index–SCI Version III (QLI-SCI),59,73 the Reciprocal Support Scale (RSS),45 the Profile of Mood States (POMS),74 the Life Situation Questionnaire–Revised (LSQ-R),75,76and 2 condition-specific measures: the Spinal Cord Injury Spasticity Evaluation Tool (SCI-SET)38 and the Patient Reported Impact of Spasticity Measure (PRISM).40
The QLI-SCI59,73 (Boxes C, D, and E, Figure 1) measures satisfaction in and the relative importance of several life domains. The QLI-SCI has been used in other SCI-related studies examining QOL,24,77,78 and it has good underlying psychometric properties.78,79 The homogeneity reliability of the scale is good, with Cronbach’s alpha scores of 0.73 to 0.99.77,80 Factorial analysis of the scale, however, revealed that the domain structure did not fit with subject interpretations.77 As well, the correlation of the importance of scores was very low (r = 0.47). With regard to sensitivity to spasticity, the QLI-SCI did not detect improvements over time in persons with SCI in a study by Gianino et al.27 The lack of effects on the QLI-SCI was partly attributed to the fact that for some patients, the benefits of spasticity may have taken longer to achieve than the duration of the study.27 Also, the alleviation of one symptom of a complex injury may not affect non-health-related domains.27 These interpretations are reasonable. A longer follow-up may have provided a clearer picture of the impact of reduced spasticity. It has also been noted that the structure of the importance scale scoring on the QLI-SCI might require alteration and the scale may require domain rearrangement.56 It is therefore possible that this may have influenced the findings in the study by Gianino et al.27
Conversely, Adams and Hicks36 found that the QLI-SCI is sensitive to changes in spasticity over time. They conducted a body-weight-supported treadmill training (BWSTT) and tilt-table standing (TTS) study on spasticity and obtained a moderate effect size for the QLI-SCI.36 Although patients’ scores on the QLI-SCI improved after undergoing BWSTT, it is difficult to attribute the changes in QOL to reduced spasticity. The authors acknowledge that changes in QOL may have resulted in increases in function due to the BWSTT.36 Overall, further work is needed to conclusively show this relationship between the QLI-SCI and spasticity.
The LSQ-R (Boxes C–E, Figure 1) measures a broad range of long-term SCI outcomes81,82 and has undergone major revisions to increase its core content coverage, particularly for items relating to subjective well-being.45,83 Factor analysis of the scale by Krause and Reed84 yielded 3 satisfaction factors, which were identified as home life satisfaction, vocational satisfaction, and global satisfaction. Each satisfaction factor indicates a subscale of the measure. Westerkam and colleagues43 examined the influence of spasticity on QOL using the 3 satisfaction factors of the LSQ-R and found that spasticity was negatively associated with life satisfaction. Although significant, the models for these satisfaction scales accounted for a small proportion of the variance (10% or less).
Hallin et al66 noted that the LSQ or its revised form have not been used consistently; there are discrepancies in the number of items used and the scales that were developed. The LSQ uses a mix of both objective and subjective items, although later versions emphasize the items that affect subjective well-being.66 In their meta-analysis of QOL measures used in SCI research, Hallin et al66 found that the LSQ-R has a high level of reliability (Cronbach’s α = 0.76–0.86), despite the variety in its application.
The RSS45 (Boxes C and E, Figure 1) rates the frequency with which respondents receive 4 types of support (social, material, emotional, and nonpaid personal) and the frequency with which upsetting things happen between respondents and members of their family, their friends, or the community. Some reliability for the scale was obtained in a sample of persons who were American Indians with SCI,85 which yielded alpha coefficients that ranged from 0.70 to 0.76 for the 4 types of support, with an average of 0.73. The alpha for the upsets scale was 0.55; however, low internal consistency is expected given that the scale sums the evaluations that were made by 3 groups: family, friends, and community.85
With regard to spasticity, Anson et al45 provided evidence that spasticity was negatively, though weakly, associated with giving advice and social support as measured by the RSS. One possibility for the weak associations detected by Anson et al45 might be due to the fact that the health status of the sample was obtained through a section of the QLI-SCI.73 Additionally, the focus was on a multitude of health conditions and not on spasticity per se. Despite the limitations of this study, the RSS appears to be a viable measure of social support for the SCI population, but further work is warranted to establish its psychometric properties and clinical utility for assessing spasticity impact.
The POMS74 (Box E, Figure 1) is a measure of mood states. Kogel et al33 evaluated the effects of dronabinol, a derivative of tetrahydrocannabinol (THC), on memory and spasticity in 5 males with tetraplegia. Results were mixed with respect to the effects of dronabinol on spasticity. The POMS indicated that participants experienced decreased vigor and emotional changes related to variations in drug dosage. Although the POMS has been used in other SCI studies,86 Kogel et al33 used it to assess treatment effects and not spasticity per se.
The demand for subjective measures of the impact of spasticity on QOL is evident, because new measures have been developed that ensure that the individual’s experience is taken into consideration, specifically, the SCI-SET and the PRISM. The SCI-SET (Boxes C and E, Figure 1) was developed by Adams et al38 and assesses the impact of spasticity on various activities of daily living and social participation after SCI. The SCI-SET was validated in 61 participants with chronic SCI against self-assessed spasticity severity and impact scores on the Penn Spasm Frequency Scale (PSFS), the FIM motor subscale, and the health and functioning subscale from the QLI-SCI. The SCI-SET was found to be significantly moderately to strongly associated with self-assessed spasticity impact (r = -.61) and spasticity severity (r = -.48), as well as the QLI-SCI health and functioning subscale (r = .68) and the PSFS (r = -.66). The SCI-SET also showed high internal consistency (α = .90) and test-retest reliability (intercorrelation coefficient [ICC] = .91).
The SCI-SET takes into account both the positive and negative effects of spasticity. Given its recent development, more testing is required to thoroughly assess its clinical utility. This is particularly important given the number of items, the need for posttest analysis, and the fact that it contains a 7-day recall component, which is too long of a period for accurate symptom recall.87 Although the tool shows promise as a reputable measure of spasticity impact on QOL, the use of the QLI-SCI58,87 for validation in the study by Adams et al38 is potentially problematic given the issues noted above (ie, QLI potentially requiring domain rearrangement). The use of a noncontentious measure for validation would strengthen the argument for the use of the SCI-SET in QOL measurement within the context of spasticity impact. In a study using BWSTT and TTS to improve spasticity in a sample of persons with SCI, Adams and Hicks36 found that the scores on the SCI-SET did not change over time, which might be partly attributable to the intervention not being intensive or long enough. An intervention study by Boutilier et al also found no change in SCI-SET scores.35
In a similar vein, Cook et al40 developed an instrument for assessing the impact of spasticity on QOL from the patient’s perspective, called the PRISM (Boxes C and E, Figure 1). They administered a developmental form of the instrument to 180 participants, with a subsample of 36 participants recruited to provide validation for the completed form. The intraclass correlation coefficient (ICC) values for its 7 subscales were high, ranging from .82 to .91, and internal consistency was the lowest for the need for intervention scale and highest for the social avoidance/anxiety scale. The a priori hypotheses regarding abnormal and involuntary muscle movement were partially confirmed and thus provided some content validity for the PRISM. Despite this, the inclusion of a more established measure as a comparator in this study would have provided greater insight into the tool’s psychometric properties and clinical utility. In addition, the PRISM’s scoring system does not take into account the ordinal nature of the scores, which is a considerable limitation of this tool.40 The PRISM does show promise, however, as an effective measure of spasticity impact.
Further support for the PRISM comes from a study by Westerkam et al.43 As described above, these authors used scales of the LSQ-R (home life satisfaction, global satisfaction, vocational satisfaction), as well as 3 subscales of the PRISM (daily activities, positive impact, and spasticity at its worst) to determine the relationship between spasticity and life satisfaction post SCI. Daily activities, positive impact, and spasticity at its worst were all negatively correlated with home life satisfaction, global satisfaction, and overall QOL. Daily activities and spasticity at its worst were also negatively correlated with vocational satisfaction. Again, Westerkam et al’s43 findings indicate that spasticity is negatively related with QOL after SCI, but the models only accounted for a small proportion of the variance.
Fleuren et al9 used a study-specific and nonstandardized measure to assess activities of daily living, participation, and functional domains (Boxes C and E, Figure 1) in 26 participants with complete SCI. This measure was informed by the World Health Organization’s International Classification of Functioning, Disability and Health.88 Given that the scale was designed to assess the influence of spasticity on life activities and perceived degree of spasticity and resulting discomfort, it holds some good clinical utility for providing an understanding of patients’ perceptions related to this health condition. Based on its theoretical underpinnings, the scale assesses the construct of participation. Further work is needed, however, to establish its psychometric properties for persons with SCI.
In summary, there are a number of potential subjective measures for assessing spasticity. In terms of more global domains of well-being, further work with the QLI-SCI and LSQ-R would be beneficial for refinement and to determine their viability as measures of spasticity impact on QOL.80 Further work with the RSS is also needed to investigate how social support is affected by spasticity. No strong recommendations can be made regarding the POMS given the focus of the study in which it was used. Although their psychometric properties require further investigation, the more promising subjective measures are the SCI-SET and PRISM because they assess both positive and negative effects of spasticity and provide a more complete picture of the overall effect of spasticity on an individual.
Discussion
The purpose of this systematic review was to highlight the influence of spasticity on QOL in the SCI population and to identify and classify appropriate measures for assessing this relationship. Identified measures from this review were representative of all 3 domains of QOL as indicated by Dijkers’s model25: QOL as utility (HUI-III), as achievements (SF-36; SIP), and as subjective well-being. For QOL as subjective well-being, measures of social support (RSS), mood states (POMS), and specific life domains (QLI-SCI), including condition-specific surveys (PRISM, SCI-SET), were assessed. Although some of these measures appeared to be sensitive to spasticity, few had adequate levels of psychometric evidence for use in the SCI population (HUI-III, QLI-SCI, RSS, PRISM). There is some evidence that supports the use of the SCI-SET in the SCI population, however further work is recommended to establish the psychometric properties of this measure given its mixed face validity. Based on the findings, the PRISM might have greater sensitivity to spasticity than the SCI-SET, because it has items that take into account affective reactions to the presence of spasticity. The importance of accounting for the affective reactions of spasticity are illustrated by a qualitative study89 that examined how persons with SCI (N = 24) experienced their spasticity on a daily basis and provided some evidence on its positive aspects. As such, the PRISM contains a number of items that tap into these concepts. The SCI-SET taps into some of the issues noted above, but the PRISM also assesses the positive benefits of spasticity, such as opportunities for muscle exertion. Both the PRISM and SCI-SET are promising measures and their continued use is recommended, but there is a need to further establish their reliability and validity.
A number of the identified articles used obscure or nonstandardized study-specific outcome measures.9,32,34,89 Given the issues noted, such as psychometric adequacy, it is essential that investigators attempt to adopt valid and reliable QOL scales for SCI. If use of a widely established measure is not possible, then there needs to be a strong justification for developing a new scale. Although some of the QOL measures demonstrated sensitivity to spasticity, it is recommended that, if used, they be paired with more established tools in order to help determine their clinical utility and psychometric properties for SCI.
Only 2 of the identified studies27,34 used the Ashworth Scale of spasticity to measure spasticity in their sample. The Ashworth Scale90 and Modified Ashworth Scale91 are the leading clinical tools to quantify spasticity by measuring resistance during passive soft-tissue stretching. These measures are quick and easy to administer, are well tolerated by patients,87 and are the most common scales used in SCI studies.92 Despite these attributes, the poor interrater and intersession reliability associated with these tools has shed doubt on their applicability for SCI.59
As noted, the multitude of available definitions and conceptualizations of spasticity2–6 poses a number of challenges for its assessment. Thus, it is possible that this issue (using the Ashworth Scale versus self-report) might have affected the sensitivity and specificity of some of the identified tools used to assess spasticity’s influence on QOL. Regardless, the use of QOL measures, both global (QLI-SCI, HUI-III) and condition-specific (PRISM, SCI-SET), along with the designs of some of the studies clearly indicate how QOL improves when spasticity is better managed27,34,37 and how QOL is better in persons with no to mild spasticity compared to those with moderate to severe spasticity.38,41,42,44 Moreover, framing QOL within a theoretical framework (ie, Dijkers’s model25) contributes to a better understanding of what concepts QOL measures are purported to assess. This understanding will help to ensure that the selected measures are congruent with the intended goals and outcomes of the project and are meaningful to the target audience.93 Further work is needed to develop more appropriate assessment measures of spasticity. Future studies should ensure that a clinical measure of the presence, frequency, and severity of spasticity be added, when possible, to quantify spasticity. Along with providing an enhanced understanding of QOL measurement, this would enable a more refined understanding of the impact of spasticity in people with SCI and would facilitate comparisons of findings across studies, thus advancing the state of knowledge in the field.
Conclusions
The assessment of the impact of spasticity on QOL should be an important consideration in rehabilitation practice, as it influences the extent and nature of the treatment(s) applied. Based on this review, the SCI-SET and the PRISM emerged as promising measures for assessing the impact of spasticity on subjective QOL in persons with SCI. Further work is recommended, however, to improve their psychometric properties and clinical utility.
Although the literature includes studies that assess the influence of spasticity on QOL using both objective and subjective measures, more work using both types of measures in the same study is needed to illustrate its impact from multiple perspectives. In particular, more studies should focus on quantifying the impact of spasticity from the view of QOL as utility in tandem with subjective outcomes. This would inform recommendations for resource allocation within the health care system and would emphasize both the societal and patient perspectives on the burden of spasticity, while potentially improving clinical practices related to its treatment. Concurrent development of a psychometrically robust tool for quantification of the clinical phenomena associated with spasticity would accelerate care and advance the field.
Acknowledgments
Financial support/disclosures: This project was supported by the Ontario Neurotrauma Foundation and the Réseau provincial de recherche en adaptation-réadaptation (REPAR) (Grant nos. 2010-KM-SCI-QOL-825; 2008-ONF-REPAR-601; 2007-ONF-REPAR-518). Additional support was provided by the Toronto Rehabilitation Institute, which receives funding under the Provincial Rehabilitation Research Program from the Ministry of Health and Long-Term Care in Ontario. The views expressed do not necessarily reflect those of the Ministry.
The authors have no conflicts of interest to report.
References
- 1.Adams MM, Hicks AL. Spasticity after spinal cord injury. Spinal Cord. 2005;43:577–586. [DOI] [PubMed] [Google Scholar]
- 2.Lance JW. Symposium synopsis. In: Young RR, Koeila WE, eds. Spasticity: Disordered Motor Control. Chicago: Yearbook Medical Publishers; 1980:485–494. [Google Scholar]
- 3.Young RR, Shahani BT. Spasticity in spinal cord injured patients. In: Bloch RE, Basbaum M, eds. Management of Spinal Cord Injuries. Baltimore: Williams & Wilkins; 1986:241–283. [Google Scholar]
- 4.Katz RT, Royal GP, Brait C, Rymer Z. Objective quantification of spastic hypertonia: Correlation with clinical findings. Arch Phys Med Rehabil. 1992;73:339–347. [DOI] [PubMed] [Google Scholar]
- 5.Thilmann AF, Burke DJ, Rymer WZ. Preface. In: Thilmann AF, Burke DJ, Rymer WZ, eds. Spasticity: Mechanisms and Management. Berlin: Springer-Verlag; 1993:v–vi. [Google Scholar]
- 6.O’Dwyer NJ, Ada L, Neilson PD. Spasticity and muscle contracture following stroke. Brain. 1996;119:1737–1749. [DOI] [PubMed] [Google Scholar]
- 7.Skold C, Levi R, Seiger A. Spasticity after traumatic spinal cord injury: Nature, severity, and location. Arch Phys Med Rehabil. 1999;80:1548–1557. [DOI] [PubMed] [Google Scholar]
- 8.Maynard FM, Karunas RS, Waring WP. Epidemiology of spasticity following traumatic spinal cord injury. Arch Phys Med Rehabil. 1990;71:566–569. [PubMed] [Google Scholar]
- 9.Fleuren JF, Voerman GE, Snoek GJ, Nene AV, Rietman JS, Hermens HJ. Perception of lower limb spasticity in patients with spinal cord injury. Spinal Cord. 2009;47:396–400. [DOI] [PubMed] [Google Scholar]
- 10.Phadke CP, Balasubramanian CK, Ismail F, Boulias C. Revisiting physiologic and psychologic triggers that increase spasticity. Am J Phys Med Rehabil. 2013;92(4):357–369. [DOI] [PubMed] [Google Scholar]
- 11.Albert T, Yelnik A. Physiotherapy for spasticity. Neurochirurgie. 2003;49:239–246. [PubMed] [Google Scholar]
- 12.Elovic E. Principles of pharmaceutical management of spastic hypertonia. Phys Med Rehabil Clin N Am. 2001;12:793–816. [PubMed] [Google Scholar]
- 13.Kita M, Goodkin DE. Drugs used to treat spasticity. Drugs. 2000;59:487–495. [DOI] [PubMed] [Google Scholar]
- 14.Norman KE, Pepin A, Barbeau H. Effects of drugs on walking after spinal cord injury. Spinal Cord. 1998;36:699–715. [DOI] [PubMed] [Google Scholar]
- 15.Stewart JE, Barbeau H, Gauthier S. Modulation of locomotor patterns and spasticity with clonidine in spinal cord injured patients. Can J Neurol Sci. 1991;18:321–332. [DOI] [PubMed] [Google Scholar]
- 16.Kirshblum S. Treatment alternatives for spinal cord injury related spasticity. J Spinal Cord Med. 1999;22:199–217. [DOI] [PubMed] [Google Scholar]
- 17.Gracies JM, Nance P, Elovic E, McGuire J, Simpson DM. Traditional pharmacological treatments for spasticity. Part II: General and regional treatments. Muscle Nerve. 1997;6:S92–S120. [PubMed] [Google Scholar]
- 18.Fung J, Stewart JE, Barbeau H. The combined effects of clonidine and cyproheptadine with interactive training on the modulation of locomotion in spinal cord injured subjects. J Neurol Sci. 1990;100:85–93. [DOI] [PubMed] [Google Scholar]
- 19.Levi R, Hultling C, Seiger A. The Stockholm Spinal Cord Injury Study: 2. Associations between clinical patient characteristics and post-acute medical problems. Paraplegia. 1995;33:585–594. [DOI] [PubMed] [Google Scholar]
- 20.Sheean G. The pathophysiology of spasticity. Eur J Neurol. 2002;9(Suppl 1):3–9. [DOI] [PubMed] [Google Scholar]
- 21.Walter JS, Sacks J, Othman R, et al. A database of self-reported secondary medical problems among VA spinal cord injury patients: Its role in clinical care and management. J Rehabil Res Dev. 2002;79:53–61. [PubMed] [Google Scholar]
- 22.Gracies JM, Elovic E, McGuire J, Simpson DM. Traditional pharmacological treatments for spasticity. Part I: Local treatments. Muscle Nerve. 1997;6:S61–S91. [PubMed] [Google Scholar]
- 23.Barnes M. Botulinum toxin - mechanisms of action and clinical use in spasticity. J Rehabil Med. 2003; (41Suppl):56–59. [DOI] [PubMed] [Google Scholar]
- 24.Chambers HG. The surgical treatment of spasticity. Muscle Nerve. 1997;6:S121–S8. [PubMed] [Google Scholar]
- 25.Dijkers MP. Quality of life of individuals with spinal cord injury: A review of conceptualization, measurement, and research findings. J Rehabil Res Dev. 2005;42 (3 Suppl 1):87–110. [DOI] [PubMed] [Google Scholar]
- 26.Anderson KD, Borisoff JF, Johnson RD, Stiens SA, Elliott SL. Spinal cord injury influences psychogenic as well as physical components of female sexual ability. Spinal Cord. 2007;45:349–359. [DOI] [PubMed] [Google Scholar]
- 27.Gianino JM, York MM, Paice JA, Shott S. Quality of life: Effect of reduced spasticity from intrathecal baclofen. J Neurosci Nurs. 1998;30:47–54. [DOI] [PubMed] [Google Scholar]
- 28.Spinal Cord Injury British Columbia. Workforce participation survey of Canadians with spinal cord injuries. Spinal Cord Injury British Columbia; 2006. http://sci-bc-database.ca/wp-content/uploads/workplace-participation-survey-of-canadians-withspinal-cord-injuries.pdf
- 29.Dijkers MP. Individualization in quality of life measurement: Instruments and approaches. Arch Phys Med Rehabil. 2003;84(4 Suppl 2):S3–14. [DOI] [PubMed] [Google Scholar]
- 30.Dijkers MPJM. Quality of life of individuals with spinal cord injury: A review of conceptualization, measurement, and research findings. J Rehabil Res Dev. 2005;42(3 Suppl 1):87–110. [DOI] [PubMed] [Google Scholar]
- 31.Dijkers MP. Quality of life after traumatic brain injury: A review of research approaches and findings. Arch Phys Med Rehabil. 2004;85(4 Suppl 2): S21–35. [DOI] [PubMed] [Google Scholar]
- 32.Jagatsinh Y. Intrathecal baclofen: Its effect on symptoms and activities of daily living in severe spasticity due to spinal cord injuries: A pilot study. Indian J Orthop. 2009;43:46–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kogel RW, Johnson PB, Chintam R, Robinson CJ, Nemchausky BA. Treatment of spasticity in spinal cord injury with Dronabinol, a tetrahydrocannabinol derivative. Am J Ther. 1995;10:799–805. [DOI] [PubMed] [Google Scholar]
- 34.Loubser PG, Narayan RK, Sandin KJ, Donovan WH, Russell KD. Continuous infusion of intrathecal baclofen: Long-term effects on spasticity in spinal cord injury. Paraplegia. 1991;29:48–64. [DOI] [PubMed] [Google Scholar]
- 35.Boutilier G, Sawatzky BJ, Grant C, Wiefelspuett S, Finlayson H. Spasticity changes in SCI following a dynamic standing program using the Segway. Spinal Cord. 2012;50(8):595–598. [DOI] [PubMed] [Google Scholar]
- 36.Adams MM, Hicks AL. Comparison of the effects of body-weight-supported treadmill training and tilt-table standing on spasticity in individuals with chronic spinal cord injury. J Spinal Cord Med. 2011;34(5):488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones ML, Leslie DP, Bilsky G, Bowman B. Effects of intrathecal baclofen on perceived sexual functioning in men with spinal cord injury. J Spinal Cord Med. 2008;31:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adams MM, Martin Ginis KA, Hicks AL. The Spinal Cord Injury Spasticity Evaluation Tool: Development and evaluation. Arch Phys Med Rehabil. 2007;88:1185–1192. [DOI] [PubMed] [Google Scholar]
- 39.Noonan VK, Kopec JA, Zhang H, Dvorak MF. Impact of associated conditions resulting from spinal cord injury on health status and quality of life in people with traumatic central cord syndrome. Arch Phys Med Rehabil. 2008;89:1074–1082. [DOI] [PubMed] [Google Scholar]
- 40.Cook KF, Teal CR, Engebretson JC, et al. Development and validity of patient reported impact of spasticity measure (PRISM). J Rehabil Res Dev. 2007;44:363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lundqvist C, Siosteen A, Sullivan L, Blomstrand C, Lind B, Sullivan M. Spinal cord injuries: Clinical, functional and emotional status. Spine. 1991;16:78–83. [PubMed] [Google Scholar]
- 42.Westgren N, Levi R. Quality of life and traumatic spinal cord injury. Arch Phys Med Rehabil. 1998;79:1433–1439. [DOI] [PubMed] [Google Scholar]
- 43.Westerkam D, Saunders LL, Krause JS. Association of spasticity and life satisfaction after spinal cord injury. Spinal Cord. 2011;49:990–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Craven BC, Hitzig SL, Mittmann N. Impact of impairment and secondary health conditions on health preference among individuals with chronic spinal cord injury. J Spinal Cord Med. 2012;35:361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anson CA, Stanwyck DJ, Krause JS. Social support and health status in spinal cord injury. Int J Paraplegia. 1993;31:632–638. [DOI] [PubMed] [Google Scholar]
- 46.Ware JEJ, Kosinski M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Lincoln, RI: QualityMetric Inc; 2001. [Google Scholar]
- 47.Feeny D, Furlong W, Torrance GW, et al. Multiattribute and single attribute utility functions for the Health Utilities Index Mark 3 System. Med Care. 2002;40(2):113–128. [DOI] [PubMed] [Google Scholar]
- 48.Catz A, Itzkovich M, Agranov E, Ring H, Tamir A. SCIM–spinal cord independence measure: A new disability scale for patients with spinal cord lesions. Spinal Cord. 1997;35(12):850–856. [DOI] [PubMed] [Google Scholar]
- 49.Wright J. The FIM. The Center for Outcome Measurement in Brain Injury; 2000. http://tbims.org/combi/FIM Accessed June3, 2013.
- 50.Mahoney FI, Barthel DW. Functional evaluation: The Barthel Index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 51.McHorney C, Ware JJ, Lu R, Sherbourne CD. The MOS.36-Item Short-Form Health Survey (SF-36) III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32(1):40–66. [DOI] [PubMed] [Google Scholar]
- 52.Hays RD, Hahn H, Marshall G. Use of the SF-36 and other health-related quality of life measures to assess persons with disabilities. Arch Phys Med Rehabil. 2002;83:S4–S9. [DOI] [PubMed] [Google Scholar]
- 53.Schwartz C, Vollmer T, Lee H. Reliability and validity of two self-report measures of impairment and disability for MS. Neurology. 1999;79:494–496. [DOI] [PubMed] [Google Scholar]
- 54.Andresen EM, Fouts BS, Romeis JC, Brownson CA. Performance of health-related quality-of-life instruments in a spinal cord injured population. Arch Phys Med Rehabil. 1999;80:877–884. [DOI] [PubMed] [Google Scholar]
- 55.Andresen EM, Meyers AR. Health-related quality of life outcomes measures. Arch Phys Med Rehabil. 2000;81:S30–S45. [DOI] [PubMed] [Google Scholar]
- 56.Hill MR, Noonan VK, Sakakibara BM, Miller WC, SCIRE Research Team. Quality of life instruments and definitions in individuals with spinal cord injury: A systematic review. Spinal Cord. 2010;48(6):438–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bergner M, Bobbitt RA, Carter WB, Gilson BS. The Sickness Impact Profile: Development and final revision of a health status measure. Med Care. 1981;19:787–805. [DOI] [PubMed] [Google Scholar]
- 58.Meyers AR, Andresen EM, Hagglund KJ. A model of outcomes research: Spinal cord injury. Arch Phys Med Rehabil. 2000;81:S81–S90. [DOI] [PubMed] [Google Scholar]
- 59.Craven BC, Morris AR. Modified Ashworth scale reliability for measurement of lower extremity spasticity among patients with SCI. Spinal Cord. 2010;48:207–213. [DOI] [PubMed] [Google Scholar]
- 60.Post MWM, deWitte LP, van Asbeck FWA, van Dijk AJ, Schrijvers AJP. Predictors of health status and life satisfaction in spinal cord injury. Arch Phys Med Rehabil. 1998;78:395–402. [DOI] [PubMed] [Google Scholar]
- 61.Post MWM, Van Asbeck FWA, van Dijk AJ, Schrijvers AJP. Services for spinal cord injured: Availability and satisfaction. Spinal Cord. 1997;35:109–115. [DOI] [PubMed] [Google Scholar]
- 62.Wood-Dauphinée S, Exner G, Group SC. Quality of life in patients with spinal cord injury-basic issues, assessment, and recommendations. Restorative Neurol Neurosci. 2002;20:135–149. [PubMed] [Google Scholar]
- 63.Robinson R. Cost-utility analysis. Br Med J. 1993;307(6908):859–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boyle M, Furlong W, Feeny D, et al. Reliability of the Health Utilities Index-Mark III used in the 1991 cycle 6 Canadian General Survey Health Questionnaire. Qual Life Res. 1995;4(3):249–257. [DOI] [PubMed] [Google Scholar]
- 65.Kalpakjian CZ, Scelza WM, Forchhemimer MB, Toussaint LL. Preliminary reliability and validity of a Spinal Cord Injury Secondary Conditions Scale. J Spinal Cord Med. 2007;30(2):131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hallin P, Sullivan M, Kreuter M. Spinal cord injury and quality of life measures: A review of instrument psychometric quality. Spinal Cord. 2000;38:509–523. [DOI] [PubMed] [Google Scholar]
- 67.Catz A, Itzkovich M, Tesio L, et al. A multicenter international study of the Spinal Cord Independence Measure, version III: Rasch psychometric validation. Spinal Cord. 2007;45(4):275–291. [DOI] [PubMed] [Google Scholar]
- 68.Rudhe C, van Hedel HJ. Upper extremity function in persons with tetraplegia: Relationships between strength, capacity, and the spinal cord independence measure. Neurorehabil Neural Repair. 2009;23(5):413–421. [DOI] [PubMed] [Google Scholar]
- 69.Graves D. The construct validity and explanatory power of the ASIA Motor Score and the FIM: Implications for theoretical models of spinal cord injury. Top Spinal Cord Inj Rehabil. 2005;10:65–74. [Google Scholar]
- 70.Hall KM, Cohen ME, Wright J, Call M, Werner P. Characteristics of the Functional Independence Measure in traumatic spinal cord injury. Arch Phys Med Rehabil. 1999;80:1471–1476. [DOI] [PubMed] [Google Scholar]
- 71.Granger CV, Albrecht GL, Hamilton BB. Outcome of comprehensive medical rehabilitation: Measurement by PULSES profile and the Barthel Index. Arch Phys Med Rehabil. 1979;60:145–154. [PubMed] [Google Scholar]
- 72.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21:271–292. [DOI] [PubMed] [Google Scholar]
- 73.Ferrans CE, Powers MJ. Psychometric assessment of the Quality of Life Index. Res Nurs Health. 1992;15:29–38 [DOI] [PubMed] [Google Scholar]
- 74.McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1992. [Google Scholar]
- 75.Krause JS. Dimensions of subjective well-being after spinal cord injury: An empirical analysis by gender and race/ethnicity. Arch Phys Med Rehabil. 1998;79(8):900–909. [DOI] [PubMed] [Google Scholar]
- 76.Krause JS. Subjective well being after spinal cord injury: Relationships to gender, race/ethnicity, and chronological age. Rehabil Psychol. 1998;43(4):282–296. [Google Scholar]
- 77.May L, Warren S. Measuring quality of life of persons with spinal cord injury: Substantive and structural validation. Qual Life Res. 2001;10:503–515. [DOI] [PubMed] [Google Scholar]
- 78.Lin KH, Chuang CC, Kao MJ, Lien IN, Tsauo JY. Quality of life of spinal cord injured patients in Taiwan: A subgroup study. Spinal Cord. 1997;35(12):841–849. [DOI] [PubMed] [Google Scholar]
- 79.Lyons S, Sorenson M. Quality of life in spinal cord injury patients with pressure ulcers. SCI Nurs. 2009;26:13–18. [Google Scholar]
- 80.May LA, Warren S. Measuring quality of life of persons with spinal cord injury: External and structural validity. Spinal Cord. 2002;40(7):341–350. [DOI] [PubMed] [Google Scholar]
- 81.Crewe NM, Athelstan GT, Krumberger J. Spinal cord injury: A comparison of pre-injury and post-injury marriages. Arch Phys Med Rehabil. 1979;60(6):252–256. [PubMed] [Google Scholar]
- 82.Crewe NM, Krause JS. An eleven year follow-up of adjustment to spinal cord injury. Rehabil Psychol. 1990;35(4):205–210. [Google Scholar]
- 83.Krause JS, Crewe NM. Long-term prediction of self-reported problems following spinal cord injury. Paraplegia. 1990;28(3):186–202. [Google Scholar]
- 84.Krause JS, Reed KS. Life satisfaction and self-reported problems after spinal cord injury: Measurement of underlying dimensions. Rehabil Psychol. 2009;54(3):343–350. [DOI] [PubMed] [Google Scholar]
- 85.Krause JS, Coker JL, Charlifue S, Whiteneck GG. Health outcomes among American Indians with spinal cord injury. Arch Phys Med Rehabil. 2000;81(7):924–931. [DOI] [PubMed] [Google Scholar]
- 86.Craig A, Tran Y, Lovas J, Middleton J. Spinal cord injury and its association with negative psychological states. Int J Psychosocial Rehabil. 2008;12:115–121. [Google Scholar]
- 87.Hsieh JTC, Wolfe DL, Miller WC, Curt A, and the SCIRE Research Team. Spasticity outcome measures in spinal cord injury: Psychometric properties and clinical utility. Spinal Cord. 2007;46:86–95. [DOI] [PubMed] [Google Scholar]
- 88.World Health Organization. International Classification of Functioning, Disability and Health (ICF). Geneva: World Health Organization; 2001:18–19. [Google Scholar]
- 89.Mahoney JS, Engebretson JC, Cook KF, Hart KA, Robinson-Whelen S, Sherwood AM. Spasticity experience domains in persons with spinal cord injury. Arch Phys Med Rehabil. 2007;88:287–294. [DOI] [PubMed] [Google Scholar]
- 90.Ashworth B. Preliminary trial of carisoprodal in multiple sclerosis. Practitioner. 1964;192:540–542. [PubMed] [Google Scholar]
- 91.Bohannon R, Smith M. Interrater reliability of a Modified Ashworth Scale of muscle spasticity. Phys Ther. 1987;67(2):206. [DOI] [PubMed] [Google Scholar]
- 92.Haas BM, Bergstrom E, Jamous A, Bennie A. The inter rater reliability of the original and Modified Ashworth Scale for the assessment of spasticity in patients with spinal cord injury. Spinal Cord. 1996;34:560–564. [DOI] [PubMed] [Google Scholar]
- 93.Hitzig SL, Noreau L, Balioussis C, Routhier F, Kairy D, Craven BC. The development of the spinal cord injury participation and quality of life (PAR-QoL) tool-kit. Disabil Rehabil. 2013;35:1408–1414. [DOI] [PubMed] [Google Scholar]