The title salts, C8H13N4 +·Cl−, (I), and C8H13N4 +·NO3 −, (II), contain linked pyridinium–piperazine heterocycles. In the crystal of (I), weak N—H⋯Cl interactions lead to zigzag chains along [100] while in the crystal of (II), bifurcated N—H⋯(O,O) hydrogen bonds and weak C—H⋯O interactions collectively link the components into infinite chains along [100].
Keywords: crystal structure, pyrimidine–piperazine heterocyclic salts, chloride salt, nitrate salt, bifurcated hydrogen bonds
Abstract
The title salts, C8H13N4 +·Cl−, (I), and C8H13N4 +·NO3 −, (II), contain linked pyridinium–piperazine heterocycles. In both salts, the piperazine ring adopts a chair conformation with protonation at the N atom not linked to the other ring. In the crystal of (I), weak N—H⋯Cl interactions are observed, leading to zigzag chains along [100]. In the crystal of (II), both H atoms on the NH2 + group form bifurcated N—H⋯(O,O) hydrogen bonds. Weak C—H⋯O interactions are also observed. These bonds collectively link the components into infinite chains along [100].
Chemical context
Pyrimidine-containing compounds exhibit various biological activities (Goldmann & Stoltefuss, 1991 ▶) and related fused heterocycles are unique classes of heterocyclic compounds that exhibit a broad spectrum of biological activities such as anticancer (Amin et al., 2009 ▶; Pandey et al., 2004 ▶), antiviral (Ibrahim & El-Metwally, 2010 ▶), antibacterial (Kuyper et al., 1996 ▶) and anti-oxidant (Padmaja et al., 2009 ▶), antidepressant (Kim et al., 2010 ▶) and possess anti-inflammatory effects (Clark et al., 2007 ▶). In addition, several piperazine derivatives have reached the stage of clinical application; among the known drugs that are used to treat anxiety is a pyrimidinylpiperazinyl compound, buspirone (trade name BuSpar®) (Tollefson et al., 1991 ▶). Our research group has published a number of papers on incorporated heterocyclic ring structures, viz. imatinibium dipicrate (Jasinski et al., 2010 ▶), 1-(2-hydroxyethyl)-4-[3-(2-trifluoromethyl-9H-thioxanthen-9-ylidene)propyl]piperazine-1,4-diium dichloride, which is the dihydrochloride salt of flupentixol (Siddegowda et al., 2011a
▶) and opipramolium fumarate (Siddegowda et al., 2011b
▶). Other related crystal structures are 4-(pyrimidin-2-yl)piperazin-1-ium (E)-3-carboxyprop-2-enoate (Yamuna et al., 2014a
▶), flupentixol tartarate and enrofloxacinium oxalate (Yamuna et al., 2014b
▶,c
▶). As part of our ongoing studies in this area, we report herein the crystal structures of the title salts, (I) and (II).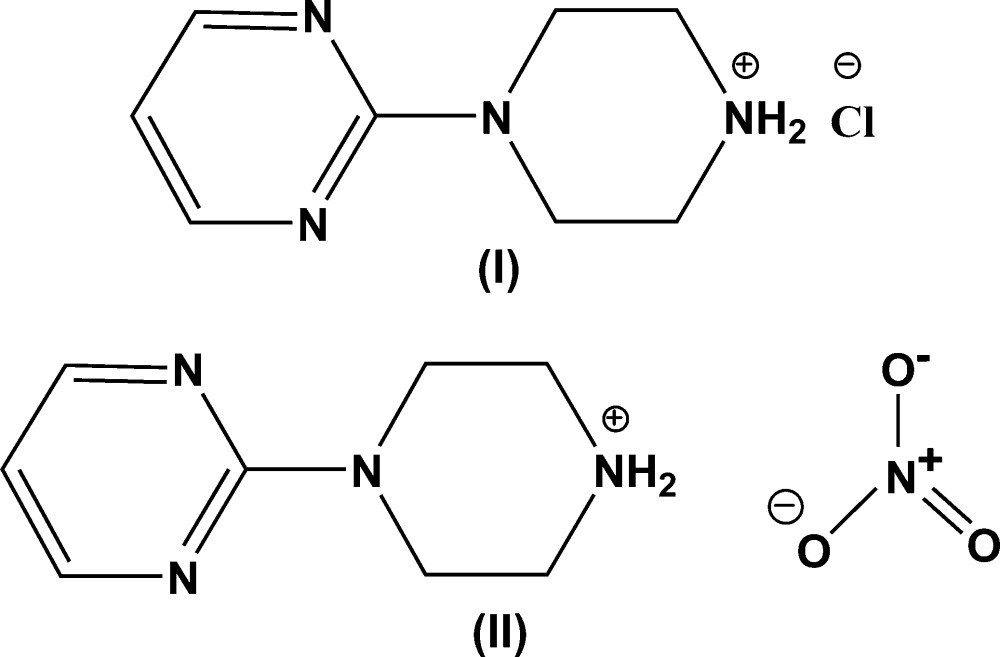
Structural commentary
The structure of (I) and its atom numbering are shown in Fig. 1 ▶. It consists of a pyrimidylpiperazine cation joined by the C1/N3 atoms of each unit and a chloride anion. The C1—N3 bond is 1.373 (3) Å long, which compares favorably with similar ionic structures containing this cation [1.369 (3) (Yamuna et al., 2014a ▶), and 1.36 (6) and 1.37 (1) Å (Ding et al., 2014 ▶)]. The N3/C5/C6/N4/C7/C8 piperazine unit adopts a slightly distorted chair conformation with protonation at the N4 nitrogen atom. The structure of (II) and its atom numbering are shown in Fig. 2 ▶. Similarly, it consists of a pyrimidylpiperazine cation joined by the C1/N3 atoms of each unit and a nitrate anion. The C1—N3 bond is 1.369 (3) Å, also in the range of the related structures described above. The N3/C5/C6/N4/C7/C8 piperazine unit also adopts a slightly distorted chair conformation with protonation at the N4 atom.
Figure 1.

ORTEP drawing of C8H13N4 +·Cl−, (I), showing 30% probability displacement ellipsoids.
Figure 2.

ORTEP drawing of C8H13N4 +·NO3 −, (II), showing 30% probability displacement ellipsoids.
Supramolecular features
In the crystal of (I), N4—H4A⋯Cl1 and N4—H4B⋯Cl1 interactions are observed between pyrimidylpiperazine cations and chloride anions, forming zigzag chains along [100] (Fig. 3 ▶ and Table 1 ▶). In the crystal of (II), both of the H atoms on the N4 atom of the pyrimidylpiperazine cation are bifurcated, forming N—H⋯(O,O) hydrogen bonds (Fig. 4 ▶ and Table 2 ▶). Additional C—H⋯O interactions between the pyrimidyl unit and the nitrate anion are present which, in concert with the N—H⋯O hydrogen bonds between the piperazine group and nitrate anions, form infinite chains along [100].
Figure 3.
Molecular packing for C8H13N4 +·Cl−, (I), viewed along the b axis. Dashed lines indicate N—H⋯Cl interactions forming zigzag chains along the a axis (see Table 1 ▶ for details). H atoms not involved in hydrogen bonding have been omitted for clarity.
Table 1. Hydrogen-bond geometry (Å, °) for (I) .
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N4—H4A⋯Cl1 | 0.91 | 2.21 | 3.102 (2) | 167 |
| N4—H4B⋯Cl1i | 0.91 | 2.21 | 3.114 (2) | 175 |
Symmetry code: (i)  .
.
Figure 4.
Molecular packing for C8H13N4 +·NO3 −, (II), viewed along the c axis. Dashed lines indicate N—H⋯O hydrogen bonds and additional C—H⋯O interactions forming infinite chains along [100] (see Table 2 ▶ for details). H atoms not involved in hydrogen bonding have been omitted for clarity.
Table 2. Hydrogen-bond geometry (Å, °) for (II) .
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N4—H4A⋯O2i | 0.91 | 1.92 | 2.829 (3) | 177 |
| N4—H4A⋯O3i | 0.91 | 2.52 | 3.138 (3) | 126 |
| N4—H4B⋯O1 | 0.91 | 2.35 | 3.197 (3) | 155 |
| N4—H4B⋯O2 | 0.91 | 2.10 | 2.900 (3) | 146 |
| C3—H3⋯O1ii | 0.95 | 2.46 | 3.240 (3) | 140 |
| C4—H4⋯O2iii | 0.95 | 2.51 | 3.291 (3) | 139 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  .
.
Database survey
A search of the Cambridge Structural Database (Version 5.35, last update May 2014: Allen 2002 ▶) revealed only three structures containing the 4-(pyrimidin-2-yl)piperazin-1-ium cation similar to the structures reported here. These include the salts of 4-(pyrimidin-2-yl)piperazin-1-ium 3-carboxyprop-2-enoate (Yamuna et al. 2014a ▶), 4-(pyrimidin-2-yl)piperazin-1-ium hydrogen d-tartrate monohydrate (Ding et al., 2014 ▶) and 4-(pyrimidin-2-yl)piperazin-1-ium hydrogen l-tartrate monohydrate (Ding et al. 2014 ▶). The 3-carboxyprop-2-enoate complex crystallizes in space group P21/c while the two hydrogen (D and L)-tartrate monohydrate salts both crystallize in P212121. In comparison, title salt (I) crystallizes in P212121 while (II) crystallizes in space group P21/c. In addition, as a related observation, 109 structures containing the pyrimidine–piperazine unit were also identified in this search. Some of these include, uniquely, the 4-(pyrimidin-2-yl)piperazin-1-yl unit itself. These include 1-[4-(pyrimidin-2-yl)piperazin-1-yl]ethanone, (1-methyl-1H-pyrrol-2-yl)[4-(pyrimidin-2-yl)piperazin-1-yl]methanone, [4-(pyrimidin-2-yl)piperazin-1-yl](2-thienyl)methanone, (4-fluorophenyl)[4-(pyrimidin-2-yl)piperazin-1-yl]methanone (Spencer et al., 2011 ▶), (E)-1-phenyl-3-[4-(pyrimidin-2-yl)piperazin-1-yl]propan-1-one oxime (Kolasa et al., 2006 ▶), N-(4-chlorophenyl)-4-(pyrimidin-2-yl)piperazine-1-carboxamide (Li, 2011 ▶) and 6-{3-[4-(pyrimidin-2-yl)piperazin-1-yl]propyl}-2,3-dihydro-5H-[1,4]dithiino[2,3-c]pyrrole-5,7(6H)-dione (Bielenica et al., 2011 ▶).
Synthesis and crystallization
For the preparation of title salt (I), a mixture of 1-(pyrimidin-2-yl)piperazine (0.2 g) and concentrated hydrochloric acid (5 ml) was stirred well over a magnetic stirrer at room temperature for 10 min and then warmed at 313 K for another 10 min. A white precipitate was obtained, which was dried in the open air overnight and then dissolved in hot dimethyl sulfoxide solvent. After few days, colourless blocks were obtained on slow evaporation (m.p. above 563 K).
For the preparation of title salt (II), a mixture of 1-(pyrimidin-2-yl)piperazine, from Sigma–Aldrich (0.2 g), and concentrated nitric acid (5 ml) was stirred well over a magnetic stirrer at room temperature for 10 min. A white precipitate was obtained immediately, which was dried in the open air overnight and then dissolved in water. After a few days, colourless blocks were obtained on slow evaporation (m.p. 463–470 K).
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 3 ▶. In both (I) and (II), all of the H atoms were placed in their calculated positions and then refined using a riding model with C—H bond lengths of 0.93 (CH) or 0.97 Å (CH2) and N—H bond lengths of 0.97 Å. Isotropic displacement parameters for these atoms were set at 1.2U eq(CH,CH2,NH).
Table 3. Experimental details.
| (I) | (II) | |
|---|---|---|
| Crystal data | ||
| Chemical formula | C8H13N4 +·Cl− | C8H13N4 +·NO3 − |
| M r | 200.67 | 227.23 |
| Crystal system, space group | Orthorhombic, P212121 | Monoclinic, P21/c |
| Temperature (K) | 173 | 173 |
| a, b, c (Å) | 6.84764 (17), 7.27667 (18), 19.1751 (5) | 10.5272 (6), 7.2230 (3), 14.1575 (7) |
| α, β, γ (°) | 90, 90, 90 | 90, 107.341 (6), 90 |
| V (Å3) | 955.46 (4) | 1027.58 (9) |
| Z | 4 | 4 |
| Radiation type | Cu Kα | Cu Kα |
| μ (mm−1) | 3.21 | 0.98 |
| Crystal size (mm) | 0.26 × 0.14 × 0.08 | 0.22 × 0.16 × 0.06 |
| Data collection | ||
| Diffractometer | Agilent Agilent Eos Gemini | Agilent Agilent Eos Gemini |
| Absorption correction | Multi-scan (CrysAlis RED; Agilent, 2012 ▶) | Multi-scan (CrysAlis RED; Agilent, 2012 ▶) |
| T min, T max | 0.417, 1.000 | 0.727, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 5514, 1841, 1761 | 6218, 1960, 1752 |
| R int | 0.045 | 0.040 |
| (sin θ/λ)max (Å−1) | 0.615 | 0.613 |
| Refinement | ||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.035, 0.091, 1.08 | 0.058, 0.163, 1.10 |
| No. of reflections | 1841 | 1960 |
| No. of parameters | 119 | 146 |
| H-atom treatment | H-atom parameters constrained | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.23, −0.20 | 0.42, −0.25 |
| Absolute structure | Flack x determined using 687 quotients [(I +)−(I −)]/[(I +)+(I −)] (Parsons et al. (2013 ▶) | – |
| Absolute structure parameter | 0.056 (15) | – |
Supplementary Material
Crystal structure: contains datablock(s) global, I, II. DOI: 10.1107/S1600536814020169/hb7279sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536814020169/hb7279Isup2.hkl
Structure factors: contains datablock(s) II. DOI: 10.1107/S1600536814020169/hb7279IIsup3.hkl
Supporting information file. DOI: 10.1107/S1600536814020169/hb7279Isup4.cml
Supporting information file. DOI: 10.1107/S1600536814020169/hb7279IIsup5.cml
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
TSY thanks the University of Mysore for research facilities and is also grateful to the Principal, Maharani’s Science College for Women, Mysore, for giving permission to undertake research. JPJ acknowledges the NSF–MRI program (grant No. CHE-1039027) for funds to purchase the X-ray diffractometer.
supplementary crystallographic information
Crystal data
| C8H13N4+·NO3− | F(000) = 480 |
| Mr = 227.23 | Dx = 1.469 Mg m−3 |
| Monoclinic, P21/c | Cu Kα radiation, λ = 1.54184 Å |
| a = 10.5272 (6) Å | Cell parameters from 2763 reflections |
| b = 7.2230 (3) Å | θ = 6.2–71.4° |
| c = 14.1575 (7) Å | µ = 0.98 mm−1 |
| β = 107.341 (6)° | T = 173 K |
| V = 1027.58 (9) Å3 | Irregular, colourless |
| Z = 4 | 0.22 × 0.16 × 0.06 mm |
Data collection
| Agilent Agilent Eos Gemini diffractometer | 1960 independent reflections |
| Radiation source: Cu Kα | 1752 reflections with I > 2σ(I) |
| Detector resolution: 16.0416 pixels mm-1 | Rint = 0.040 |
| ω scans | θmax = 71.0°, θmin = 4.4° |
| Absorption correction: multi-scan (CrysAlis RED; Agilent, 2012) | h = −9→12 |
| Tmin = 0.727, Tmax = 1.000 | k = −8→8 |
| 6218 measured reflections | l = −17→16 |
Refinement
| Refinement on F2 | Hydrogen site location: inferred from neighbouring sites |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.058 | w = 1/[σ2(Fo2) + (0.0789P)2 + 0.9595P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.163 | (Δ/σ)max < 0.001 |
| S = 1.10 | Δρmax = 0.42 e Å−3 |
| 1960 reflections | Δρmin = −0.25 e Å−3 |
| 146 parameters | Extinction correction: SHELXL2012 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 0 restraints | Extinction coefficient: 0.0099 (14) |
| Primary atom site location: structure-invariant direct methods |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.4119 (2) | 0.6964 (4) | 0.41222 (17) | 0.0615 (7) | |
| O2 | 0.50951 (18) | 0.6257 (2) | 0.30424 (14) | 0.0381 (5) | |
| O3 | 0.55020 (17) | 0.8884 (2) | 0.37996 (13) | 0.0323 (5) | |
| N5 | 0.49103 (17) | 0.7390 (3) | 0.36677 (13) | 0.0238 (4) | |
| N1 | 0.00592 (19) | 0.2396 (3) | 0.48106 (14) | 0.0291 (5) | |
| N2 | −0.11846 (18) | 0.3821 (3) | 0.32856 (15) | 0.0273 (5) | |
| N3 | 0.10930 (18) | 0.3372 (3) | 0.36702 (14) | 0.0268 (5) | |
| N4 | 0.33344 (18) | 0.3134 (3) | 0.29632 (15) | 0.0278 (5) | |
| H4A | 0.3814 | 0.2536 | 0.2617 | 0.033* | |
| H4B | 0.3777 | 0.4191 | 0.3216 | 0.033* | |
| C1 | −0.0049 (2) | 0.3204 (3) | 0.39365 (16) | 0.0220 (5) | |
| C2 | −0.1085 (3) | 0.2126 (3) | 0.50188 (19) | 0.0346 (6) | |
| H2 | −0.1054 | 0.1544 | 0.5627 | 0.042* | |
| C3 | −0.2307 (2) | 0.2647 (4) | 0.4398 (2) | 0.0362 (6) | |
| H3 | −0.3111 | 0.2420 | 0.4553 | 0.043* | |
| C4 | −0.2290 (2) | 0.3519 (3) | 0.3537 (2) | 0.0329 (6) | |
| H4 | −0.3113 | 0.3927 | 0.3097 | 0.039* | |
| C5 | 0.2387 (2) | 0.2876 (4) | 0.43489 (16) | 0.0282 (5) | |
| H5A | 0.2266 | 0.2035 | 0.4867 | 0.034* | |
| H5B | 0.2848 | 0.4005 | 0.4676 | 0.034* | |
| C6 | 0.3222 (2) | 0.1932 (3) | 0.37877 (17) | 0.0270 (5) | |
| H6A | 0.4121 | 0.1674 | 0.4242 | 0.032* | |
| H6B | 0.2808 | 0.0738 | 0.3519 | 0.032* | |
| C7 | 0.1993 (2) | 0.3620 (3) | 0.22801 (17) | 0.0277 (5) | |
| H7A | 0.1537 | 0.2483 | 0.1960 | 0.033* | |
| H7B | 0.2095 | 0.4461 | 0.1755 | 0.033* | |
| C8 | 0.1166 (2) | 0.4552 (3) | 0.28517 (17) | 0.0276 (5) | |
| H8A | 0.1571 | 0.5756 | 0.3112 | 0.033* | |
| H8B | 0.0258 | 0.4789 | 0.2407 | 0.033* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0614 (14) | 0.0836 (17) | 0.0562 (13) | −0.0362 (13) | 0.0431 (11) | −0.0184 (12) |
| O2 | 0.0436 (10) | 0.0314 (10) | 0.0460 (11) | −0.0072 (8) | 0.0238 (8) | −0.0153 (8) |
| O3 | 0.0379 (9) | 0.0249 (9) | 0.0353 (9) | −0.0045 (7) | 0.0129 (7) | −0.0021 (7) |
| N5 | 0.0175 (9) | 0.0301 (10) | 0.0240 (9) | −0.0015 (7) | 0.0064 (7) | 0.0012 (8) |
| N1 | 0.0292 (10) | 0.0327 (11) | 0.0282 (10) | 0.0020 (8) | 0.0126 (8) | 0.0038 (8) |
| N2 | 0.0210 (9) | 0.0270 (10) | 0.0331 (11) | 0.0037 (7) | 0.0071 (8) | 0.0001 (8) |
| N3 | 0.0189 (9) | 0.0380 (11) | 0.0243 (9) | 0.0068 (8) | 0.0075 (7) | 0.0087 (8) |
| N4 | 0.0231 (9) | 0.0278 (10) | 0.0368 (11) | −0.0042 (8) | 0.0153 (8) | −0.0045 (8) |
| C1 | 0.0207 (10) | 0.0220 (10) | 0.0246 (11) | 0.0023 (8) | 0.0087 (8) | −0.0035 (8) |
| C2 | 0.0416 (14) | 0.0328 (13) | 0.0372 (13) | −0.0021 (11) | 0.0235 (11) | −0.0014 (10) |
| C3 | 0.0300 (13) | 0.0340 (13) | 0.0525 (15) | −0.0049 (10) | 0.0247 (11) | −0.0130 (12) |
| C4 | 0.0224 (11) | 0.0304 (12) | 0.0456 (15) | 0.0020 (9) | 0.0098 (10) | −0.0063 (11) |
| C5 | 0.0208 (11) | 0.0395 (13) | 0.0234 (11) | 0.0087 (9) | 0.0054 (9) | 0.0023 (9) |
| C6 | 0.0219 (10) | 0.0296 (12) | 0.0293 (11) | 0.0038 (9) | 0.0074 (9) | 0.0014 (9) |
| C7 | 0.0291 (11) | 0.0305 (12) | 0.0255 (11) | −0.0013 (9) | 0.0111 (9) | 0.0039 (9) |
| C8 | 0.0267 (11) | 0.0290 (12) | 0.0283 (11) | 0.0033 (9) | 0.0098 (9) | 0.0080 (9) |
Geometric parameters (Å, º)
| O1—N5 | 1.233 (3) | C2—C3 | 1.376 (4) |
| O2—N5 | 1.263 (2) | C3—H3 | 0.9500 |
| O3—N5 | 1.232 (2) | C3—C4 | 1.377 (4) |
| N1—C1 | 1.342 (3) | C4—H4 | 0.9500 |
| N1—C2 | 1.337 (3) | C5—H5A | 0.9900 |
| N2—C1 | 1.349 (3) | C5—H5B | 0.9900 |
| N2—C4 | 1.333 (3) | C5—C6 | 1.512 (3) |
| N3—C1 | 1.369 (3) | C6—H6A | 0.9900 |
| N3—C5 | 1.459 (3) | C6—H6B | 0.9900 |
| N3—C8 | 1.459 (3) | C7—H7A | 0.9900 |
| N4—H4A | 0.9100 | C7—H7B | 0.9900 |
| N4—H4B | 0.9100 | C7—C8 | 1.512 (3) |
| N4—C6 | 1.487 (3) | C8—H8A | 0.9900 |
| N4—C7 | 1.496 (3) | C8—H8B | 0.9900 |
| C2—H2 | 0.9500 | ||
| O1—N5—O2 | 118.2 (2) | C3—C4—H4 | 118.2 |
| O3—N5—O1 | 121.9 (2) | N3—C5—H5A | 109.7 |
| O3—N5—O2 | 119.82 (18) | N3—C5—H5B | 109.7 |
| C2—N1—C1 | 115.6 (2) | N3—C5—C6 | 109.86 (18) |
| C4—N2—C1 | 115.5 (2) | H5A—C5—H5B | 108.2 |
| C1—N3—C5 | 121.45 (19) | C6—C5—H5A | 109.7 |
| C1—N3—C8 | 121.92 (18) | C6—C5—H5B | 109.7 |
| C5—N3—C8 | 114.01 (18) | N4—C6—C5 | 110.12 (18) |
| H4A—N4—H4B | 108.0 | N4—C6—H6A | 109.6 |
| C6—N4—H4A | 109.4 | N4—C6—H6B | 109.6 |
| C6—N4—H4B | 109.4 | C5—C6—H6A | 109.6 |
| C6—N4—C7 | 111.33 (17) | C5—C6—H6B | 109.6 |
| C7—N4—H4A | 109.4 | H6A—C6—H6B | 108.2 |
| C7—N4—H4B | 109.4 | N4—C7—H7A | 109.7 |
| N1—C1—N2 | 126.0 (2) | N4—C7—H7B | 109.7 |
| N1—C1—N3 | 116.88 (19) | N4—C7—C8 | 109.99 (18) |
| N2—C1—N3 | 117.06 (19) | H7A—C7—H7B | 108.2 |
| N1—C2—H2 | 118.3 | C8—C7—H7A | 109.7 |
| N1—C2—C3 | 123.4 (2) | C8—C7—H7B | 109.7 |
| C3—C2—H2 | 118.3 | N3—C8—C7 | 109.85 (18) |
| C2—C3—H3 | 122.1 | N3—C8—H8A | 109.7 |
| C2—C3—C4 | 115.8 (2) | N3—C8—H8B | 109.7 |
| C4—C3—H3 | 122.1 | C7—C8—H8A | 109.7 |
| N2—C4—C3 | 123.6 (2) | C7—C8—H8B | 109.7 |
| N2—C4—H4 | 118.2 | H8A—C8—H8B | 108.2 |
| N1—C2—C3—C4 | −1.3 (4) | C4—N2—C1—N1 | −2.7 (3) |
| N3—C5—C6—N4 | 55.5 (3) | C4—N2—C1—N3 | 175.4 (2) |
| N4—C7—C8—N3 | −55.3 (2) | C5—N3—C1—N1 | −6.3 (3) |
| C1—N1—C2—C3 | −0.7 (4) | C5—N3—C1—N2 | 175.4 (2) |
| C1—N2—C4—C3 | 0.2 (3) | C5—N3—C8—C7 | 56.9 (3) |
| C1—N3—C5—C6 | 141.1 (2) | C6—N4—C7—C8 | 56.9 (2) |
| C1—N3—C8—C7 | −141.2 (2) | C7—N4—C6—C5 | −57.0 (2) |
| C2—N1—C1—N2 | 2.9 (3) | C8—N3—C1—N1 | −166.9 (2) |
| C2—N1—C1—N3 | −175.2 (2) | C8—N3—C1—N2 | 14.8 (3) |
| C2—C3—C4—N2 | 1.6 (4) | C8—N3—C5—C6 | −57.0 (3) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N4—H4A···O2i | 0.91 | 1.92 | 2.829 (3) | 177 |
| N4—H4A···O3i | 0.91 | 2.52 | 3.138 (3) | 126 |
| N4—H4B···O1 | 0.91 | 2.35 | 3.197 (3) | 155 |
| N4—H4B···O2 | 0.91 | 2.10 | 2.900 (3) | 146 |
| C3—H3···O1ii | 0.95 | 2.46 | 3.240 (3) | 140 |
| C4—H4···O2iii | 0.95 | 2.51 | 3.291 (3) | 139 |
Symmetry codes: (i) −x+1, y−1/2, −z+1/2; (ii) −x, −y+1, −z+1; (iii) x−1, y, z.
References
- Agilent (2012). CrysAlis PRO and CrysAlis RED Agilent Technologies, Yarnton, England.
- Allen, F. H. (2002). Acta Cryst. B58, 380–388. [DOI] [PubMed]
- Amin, K. M., Hanna, M. M., Abo-Youssef, H. E., Riham, F. & George, R. F. (2009). Eur. J. Med. Chem. 44, 4572–4584. [DOI] [PubMed]
- Bielenica, A., Kossakowski, J., Struga, M., Dybala, I., La Colla, P., Tamburini, E. & Loddo, R. (2011). Med. Chem. Res. 20, 1411–1420.
- Clark, M. P., George, K. M. & Bookland, R. G. (2007). Bioorg. Med. Chem. Lett. 17, 1250–1253. [DOI] [PubMed]
- Ding, X.-H., Li, Y.-H., Wang, S. & Huang, W. (2014). J. Mol. Struct. 1062, 61–67.
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Goldmann, S. & Stoltefuss, J. (1991). Angew. Chem. Int. Ed. Engl. 30, 1559–1578.
- Ibrahim, D. A. & El-Metwally, A. M. (2010). Eur. J. Med. Chem. 45, 1158–1166. [DOI] [PubMed]
- Jasinski, J. P., Butcher, R. J., Hakim Al-Arique, Q. N. M., Yathirajan, H. S. & Narayana, B. (2010). Acta Cryst. E66, o411–o412. [DOI] [PMC free article] [PubMed]
- Kim, J. Y., Kim, D. & Kang, S. Y. (2010). Bioorg. Med. Chem. Lett. 20, 6439–6442. [DOI] [PubMed]
- Kolasa, T., et al. (2006). J. Med. Chem. 49, 5093–5109. [DOI] [PubMed]
- Kuyper, L. F., Garvey, J. M., Baccanari, D. P., Champness, J. N., Stammers, D. K. & Beddell, C. R. (1996). Bioorg. Med. Chem. Lett. 4, 593–602. [DOI] [PubMed]
- Li, Y.-F. (2011). Acta Cryst. E67, o2575. [DOI] [PMC free article] [PubMed]
- Padmaja, A., Payani, T., Reddy, G. D., Dinneswara Reddy, G. & Padmavathi, V. (2009). Eur. J. Med. Chem. 44, 4557–4566. [DOI] [PubMed]
- Palatinus, L. & Chapuis, G. (2007). J. Appl. Cryst. 40, 786–790.
- Pandey, S., Suryawanshi, S. N., Gupta, S. & Srivastava, V. M. L. (2004). Eur. J. Med. Chem. 39, 969–973. [DOI] [PubMed]
- Parsons, S., Flack, H. D. & Wagner, T. (2013). Acta Cryst. B69, 249–259. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Siddegowda, M. S., Butcher, R. J., Akkurt, M., Yathirajan, H. S. & Narayana, B. (2011a). Acta Cryst. E67, o2079–o2080. [DOI] [PMC free article] [PubMed]
- Siddegowda, M. S., Jasinski, J. P., Golen, J. A., Yathirajan, H. S. & Swamy, M. T. (2011b). Acta Cryst. E67, o2296. [DOI] [PMC free article] [PubMed]
- Spencer, J., Patel, H., Callear, S. K., Coles, S. J. & Deadman, J. J. (2011). Tetrahedron Lett., 52, 5905–5909.
- Tollefson, G. D., Lancaster, S. P. & Montague-Clouse, J. (1991). Psychopharmacol. Bull. 27, 163–170. [PubMed]
- Yamuna, T. S., Kaur, M., Anderson, B. J., Jasinski, J. P. & Yathirajan, H. S. (2014b). Acta Cryst. E70, o206–o207. [DOI] [PMC free article] [PubMed]
- Yamuna, T. S., Kaur, M., Anderson, B. J., Jasinski, J. P. & Yathirajan, H. S. (2014c). Acta Cryst. E70, o200–o201. [DOI] [PMC free article] [PubMed]
- Yamuna, T. S., Kaur, M., Jasinski, J. P. & Yathirajan, H. S. (2014a). Acta Cryst. E70, o702–o703. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I, II. DOI: 10.1107/S1600536814020169/hb7279sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536814020169/hb7279Isup2.hkl
Structure factors: contains datablock(s) II. DOI: 10.1107/S1600536814020169/hb7279IIsup3.hkl
Supporting information file. DOI: 10.1107/S1600536814020169/hb7279Isup4.cml
Supporting information file. DOI: 10.1107/S1600536814020169/hb7279IIsup5.cml
Additional supporting information: crystallographic information; 3D view; checkCIF report




