Abstract
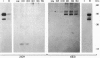
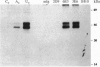
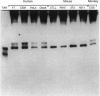
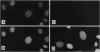
We have determined the structure, intracellular localization, and tissue distribution of TIAR, a TIA-1-related RNA-binding protein. Two related isoforms of TIAR, migrating at 42 and 50 kDa, are expressed in primate cells. Unlike TIA-1, which is found in the granules of cytotoxic lymphocytes, TIAR is concentrated in the nucleus of hematopoietic and nonhematopoietic cells. Because TIAR can trigger DNA fragmentation in permeabilized thymocytes, it is a candidate effector of apoptotic cell death. Consistent with this possibility, we have found that the expression and intracellular localization of TIAR change dramatically during Fas-mediated apoptosis. TIAR moves from the nucleus to the cytoplasm within 30 min of Fas ligation. Redistribution of TIAR precedes the onset of DNA fragmentation and is not a nonspecific consequence of nuclear disintegration. Cytoplasmic redistribution of TIAR is not observed during cellular activation triggered by mitogens such as concanavalin A or phytohemagglutinin. Our results suggest that cytoplasmic redistribution of TIAR may be a general feature of the apoptotic program.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Nagler-Anderson C., O'Brien C., Levine H., Watkins S., Slayter H. S., Blue M. L., Schlossman S. F. A monoclonal antibody reactive with a 15-kDa cytoplasmic granule-associated protein defines a subpopulation of CD8+ T lymphocytes. J Immunol. 1990 Jan 15;144(2):574–582. [PubMed] [Google Scholar]
- Anderson P. TIA-1: structural and functional studies on a new class of cytolytic effector molecule. Curr Top Microbiol Immunol. 1995;198:131–143. doi: 10.1007/978-3-642-79414-8_8. [DOI] [PubMed] [Google Scholar]
- Bandziulis R. J., Swanson M. S., Dreyfuss G. RNA-binding proteins as developmental regulators. Genes Dev. 1989 Apr;3(4):431–437. doi: 10.1101/gad.3.4.431. [DOI] [PubMed] [Google Scholar]
- Fukui Y., Yumura S., Yumura T. K. Agar-overlay immunofluorescence: high-resolution studies of cytoskeletal components and their changes during chemotaxis. Methods Cell Biol. 1987;28:347–356. doi: 10.1016/s0091-679x(08)61655-6. [DOI] [PubMed] [Google Scholar]
- Gaido M. L., Cidlowski J. A. Identification, purification, and characterization of a calcium-dependent endonuclease (NUC18) from apoptotic rat thymocytes. NUC18 is not histone H2B. J Biol Chem. 1991 Oct 5;266(28):18580–18585. [PubMed] [Google Scholar]
- Gottlieb E., Steitz J. A. Function of the mammalian La protein: evidence for its action in transcription termination by RNA polymerase III. EMBO J. 1989 Mar;8(3):851–861. doi: 10.1002/j.1460-2075.1989.tb03446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami A., Tian Q., Duan X., Streuli M., Schlossman S. F., Anderson P. Identification and functional characterization of a TIA-1-related nucleolysin. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8681–8685. doi: 10.1073/pnas.89.18.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenan D. J., Query C. C., Keene J. D. RNA recognition: towards identifying determinants of specificity. Trends Biochem Sci. 1991 Jun;16(6):214–220. doi: 10.1016/0968-0004(91)90088-d. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Francoeur A. M. La antigen recognizes and binds to the 3'-oligouridylate tail of a small RNA. Mol Cell Biol. 1984 Jun;4(6):1134–1140. doi: 10.1128/mcb.4.6.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerovitch K., Svitkin Y. V., Lee H. S., Lejbkowicz F., Kenan D. J., Chan E. K., Agol V. I., Keene J. D., Sonenberg N. La autoantigen enhances and corrects aberrant translation of poliovirus RNA in reticulocyte lysate. J Virol. 1993 Jul;67(7):3798–3807. doi: 10.1128/jvi.67.7.3798-3807.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue K., Fujio Y., Kanda K. Tumor promoter induces reorganization of actin filaments and calspectin (fodrin or nonerythroid spectrin) in 3T3 cells. Proc Natl Acad Sci U S A. 1988 Jan;85(2):482–486. doi: 10.1073/pnas.85.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q., Streuli M., Saito H., Schlossman S. F., Anderson P. A polyadenylate binding protein localized to the granules of cytolytic lymphocytes induces DNA fragmentation in target cells. Cell. 1991 Nov 1;67(3):629–639. doi: 10.1016/0092-8674(91)90536-8. [DOI] [PubMed] [Google Scholar]
- Vivier E., Sorrell J. M., Ackerly M., Robertson M. J., Rasmussen R. A., Levine H., Anderson P. Developmental regulation of a mucinlike glycoprotein selectively expressed on natural killer cells. J Exp Med. 1993 Dec 1;178(6):2023–2033. doi: 10.1084/jem.178.6.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]