Abstract
The title molecule, [Fe(C5H5)(C16H11O4)], consists of a ferrocenyl moiety and a 4-methylcoumarin group linked through an ester unit to one of the cyclopentadienyl (Cp) rings. The two Cp rings are virually parallel, with an angle between the two least-squares planes of 0.74 (16)°. The distances between the FeII atom and the centroids of the two Cp rings are 1.639 (2) and 1.652 (2) Å. The conformation of the ferrocenyl moiety is slightly away from eclipsed. The dihedral angle between the coumarin ring system and the ferrocenyl ester moiety is 69.17 (19)°. π–π stacking interactions involving the benzene rings of neighbouring coumarin moieties, with centroid–centroid distances of 3.739 (2) Å, consolidate the crystal packing.
Keywords: crystal structure, ferrocene, coumarin, pharmacological activity 4-methyl-2-oxo-2H-chromene-7-yl ferrocenecarboxylate
Related literature
For background to ferrocene and its derivatives, see: Štěpnička (2002 ▶). For coumarin and its pharmacological activities, see: Peng et al. (2013 ▶). For the crystal structures of related ferrocenyl derivatives, see: Chen & Lu (2004 ▶); Imrie et al. (2002 ▶, 2005 ▶).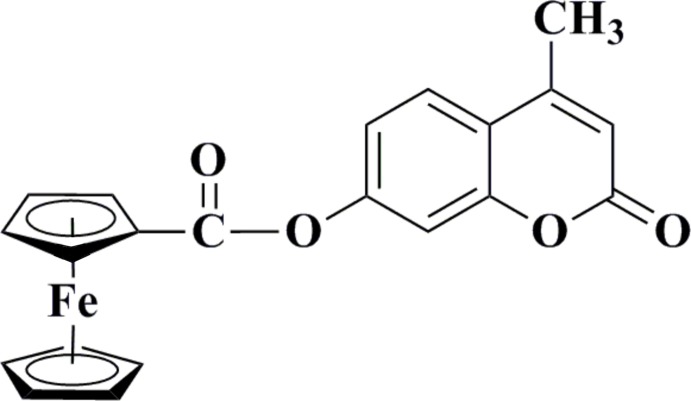
Experimental
Crystal data
[Fe(C5H5)(C16H11O4)]
M r = 388.19
Monoclinic,
a = 7.8678 (11) Å
b = 20.294 (4) Å
c = 11.1455 (18) Å
β = 108.243 (14)°
V = 1690.1 (5) Å3
Z = 4
Mo Kα radiation
μ = 0.92 mm−1
T = 293 K
0.20 × 0.10 × 0.10 mm
Data collection
Bruker APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2007 ▶) T min = 0.838, T max = 0.914
11857 measured reflections
2979 independent reflections
2398 reflections with I > 2σ(I)
R int = 0.031
Refinement
R[F 2 > 2σ(F 2)] = 0.031
wR(F 2) = 0.087
S = 1.16
2979 reflections
236 parameters
H-atom parameters constrained
Δρmax = 0.23 e Å−3
Δρmin = −0.18 e Å−3
Data collection: APEX2 (Bruker, 2007 ▶); cell refinement: SAINT (Bruker, 2007 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablock(s) I, Global. DOI: 10.1107/S1600536814022120/wm5068sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536814022120/wm5068Isup2.hkl
. DOI: 10.1107/S1600536814022120/wm5068fig1.tif
The molecular structure of the title compound showing atoms as ellipsoids at the 30% probability level.
CCDC reference: 1027955
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China (No. 81403318) and the Natural Science Foundation of Education Committee of Anhui Province (No. KJ2014A134).
supplementary crystallographic information
S1. Experimental
To a CH2Cl2 solution (30 ml) of ferrocenylcarboxylic acid (1.0 g, 4.3 mmol), 7-hydroxy-4-methylcoumarin (0.76 g, 4.3 mmol), 1-ethyl-3-(dimethylaminopropyl)carbodimide hydrochloride (EDCI) (0.89 g, 4.5 mmol) and N,N-dimethylaminopyridine (DMAP) (0.26 g, 2.2 mmol) were added into a 50 ml round bottom flask. Then the reaction mixture was stirred at room temperature. The reaction process was monitored by thin layer chromatography (TLC). After completion of the reaction, the product was extracted with ethyl acetate. The extracts were combined, washed with water and dried over anhydrous Na2SO4. After removing ethyl acetate under reduced pressure, the crude product was purified by column chromatography using petroleum ether/EtOAc (8:1) as eluent. Yellow crystals were obtained by slow evaporation of an ether/EtOAc (8:1) solution to give 1.03 g (62% yield) of the title compound, 1H-NMR (CDCl3, δ p.p.m.), 2.48 (s, 2H, CH3), 4.34 (s, 5H, C5H5), 4.57 (s, 2H, C5H2), 5.00 (s, 2H, C5H2), 7.18–7.22 (m, 2H, ArH), 7.68 (d, 1H, ArH).
S2. Refinement
All hydrogen atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms, with C—H = 0.93 Å and Uiso(H) = 1.2 Ueq.
Figures
Fig. 1.
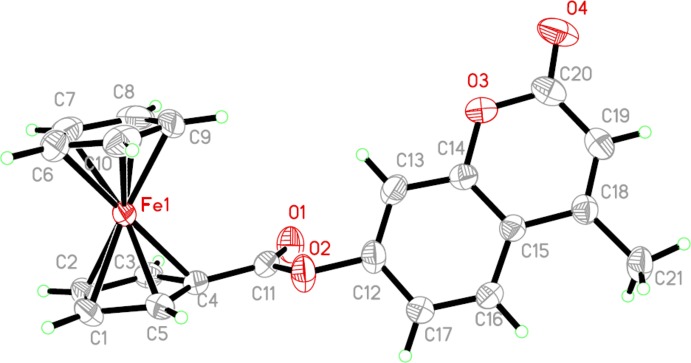
The molecular structure of the title compound showing atoms as ellipsoids at the 30% probability level.
Crystal data
| [Fe(C5H5)(C16H11O4)] | F(000) = 800 |
| Mr = 388.19 | Dx = 1.526 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 3613 reflections |
| a = 7.8678 (11) Å | θ = 2.7–23.4° |
| b = 20.294 (4) Å | µ = 0.92 mm−1 |
| c = 11.1455 (18) Å | T = 293 K |
| β = 108.243 (14)° | Block, yellow |
| V = 1690.1 (5) Å3 | 0.20 × 0.10 × 0.10 mm |
| Z = 4 |
Data collection
| Bruker APEXII CCD diffractometer | 2979 independent reflections |
| Radiation source: fine-focus sealed tube | 2398 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.031 |
| φ and ω scans | θmax = 25.0°, θmin = 2.0° |
| Absorption correction: multi-scan (SADABS; Bruker, 2007) | h = −9→9 |
| Tmin = 0.838, Tmax = 0.914 | k = −24→24 |
| 11857 measured reflections | l = −13→12 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.031 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.087 | H-atom parameters constrained |
| S = 1.16 | w = 1/[σ2(Fo2) + (0.0432P)2] where P = (Fo2 + 2Fc2)/3 |
| 2979 reflections | (Δ/σ)max = 0.001 |
| 236 parameters | Δρmax = 0.23 e Å−3 |
| 0 restraints | Δρmin = −0.18 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Fe1 | 0.74731 (4) | 0.157566 (14) | 0.42513 (3) | 0.04477 (13) | |
| O1 | 0.4801 (2) | 0.07407 (8) | 0.61150 (16) | 0.0717 (5) | |
| O2 | 0.7574 (2) | 0.09846 (8) | 0.74074 (15) | 0.0695 (5) | |
| O3 | 0.7829 (2) | −0.13167 (8) | 0.81894 (15) | 0.0654 (5) | |
| O4 | 0.7893 (3) | −0.23952 (9) | 0.8434 (2) | 0.0910 (6) | |
| C1 | 0.7620 (3) | 0.25205 (11) | 0.4903 (2) | 0.0620 (7) | |
| H1 | 0.8464 | 0.2857 | 0.4811 | 0.074* | |
| C2 | 0.5913 (3) | 0.24019 (11) | 0.4035 (2) | 0.0616 (6) | |
| H2 | 0.5372 | 0.2640 | 0.3242 | 0.074* | |
| C3 | 0.5129 (3) | 0.18719 (11) | 0.4494 (2) | 0.0549 (6) | |
| H3 | 0.3943 | 0.1683 | 0.4083 | 0.066* | |
| C4 | 0.6368 (3) | 0.16608 (10) | 0.5653 (2) | 0.0490 (5) | |
| C5 | 0.7925 (3) | 0.20659 (10) | 0.5906 (2) | 0.0560 (6) | |
| H5 | 0.9001 | 0.2037 | 0.6644 | 0.067* | |
| C6 | 0.9160 (4) | 0.15047 (13) | 0.3194 (3) | 0.0734 (8) | |
| H6 | 0.9804 | 0.1869 | 0.2955 | 0.088* | |
| C7 | 0.7462 (4) | 0.12856 (15) | 0.2511 (2) | 0.0771 (8) | |
| H7 | 0.6698 | 0.1464 | 0.1707 | 0.093* | |
| C8 | 0.7034 (4) | 0.07557 (13) | 0.3160 (3) | 0.0764 (8) | |
| H8 | 0.5928 | 0.0497 | 0.2897 | 0.092* | |
| C9 | 0.8462 (4) | 0.06495 (12) | 0.4251 (3) | 0.0758 (9) | |
| H9 | 0.8544 | 0.0310 | 0.4892 | 0.091* | |
| C10 | 0.9783 (4) | 0.11104 (14) | 0.4274 (3) | 0.0737 (8) | |
| H10 | 1.0946 | 0.1155 | 0.4929 | 0.088* | |
| C11 | 0.6091 (3) | 0.10858 (11) | 0.6366 (2) | 0.0533 (6) | |
| C12 | 0.7538 (3) | 0.04467 (11) | 0.8189 (2) | 0.0573 (6) | |
| C13 | 0.7677 (3) | −0.01842 (12) | 0.7792 (2) | 0.0586 (6) | |
| H13 | 0.7736 | −0.0268 | 0.6986 | 0.070* | |
| C14 | 0.7727 (3) | −0.06931 (11) | 0.8629 (2) | 0.0497 (5) | |
| C15 | 0.7663 (3) | −0.05849 (10) | 0.9842 (2) | 0.0478 (5) | |
| C16 | 0.7540 (3) | 0.00656 (11) | 1.0201 (2) | 0.0609 (6) | |
| H16 | 0.7509 | 0.0155 | 1.1012 | 0.073* | |
| C17 | 0.7463 (3) | 0.05785 (12) | 0.9382 (2) | 0.0633 (7) | |
| H17 | 0.7361 | 0.1010 | 0.9631 | 0.076* | |
| C18 | 0.7724 (3) | −0.11504 (12) | 1.0658 (2) | 0.0528 (6) | |
| C19 | 0.7797 (3) | −0.17514 (12) | 1.0191 (3) | 0.0624 (7) | |
| H19 | 0.7826 | −0.2113 | 1.0709 | 0.075* | |
| C20 | 0.7833 (3) | −0.18689 (13) | 0.8926 (3) | 0.0674 (7) | |
| C21 | 0.7700 (4) | −0.10441 (13) | 1.1988 (2) | 0.0731 (8) | |
| H21A | 0.7715 | −0.1463 | 1.2392 | 0.110* | |
| H21B | 0.6636 | −0.0808 | 1.1972 | 0.110* | |
| H21C | 0.8733 | −0.0794 | 1.2449 | 0.110* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Fe1 | 0.0529 (2) | 0.03700 (19) | 0.0492 (2) | 0.00541 (13) | 0.02285 (16) | 0.00617 (13) |
| O1 | 0.0629 (11) | 0.0796 (12) | 0.0749 (12) | −0.0128 (10) | 0.0250 (10) | 0.0156 (10) |
| O2 | 0.0814 (12) | 0.0671 (11) | 0.0539 (10) | −0.0207 (9) | 0.0125 (9) | 0.0164 (8) |
| O3 | 0.0824 (13) | 0.0564 (10) | 0.0612 (10) | 0.0045 (9) | 0.0279 (9) | −0.0112 (8) |
| O4 | 0.1009 (15) | 0.0562 (11) | 0.1085 (16) | 0.0149 (10) | 0.0219 (13) | −0.0211 (11) |
| C1 | 0.0792 (19) | 0.0351 (11) | 0.0761 (18) | 0.0034 (11) | 0.0306 (16) | 0.0030 (12) |
| C2 | 0.0711 (17) | 0.0467 (13) | 0.0711 (17) | 0.0193 (12) | 0.0282 (14) | 0.0145 (12) |
| C3 | 0.0530 (14) | 0.0545 (13) | 0.0617 (15) | 0.0134 (11) | 0.0244 (12) | 0.0002 (12) |
| C4 | 0.0602 (15) | 0.0440 (12) | 0.0487 (13) | 0.0060 (10) | 0.0253 (12) | −0.0004 (10) |
| C5 | 0.0695 (16) | 0.0426 (12) | 0.0558 (14) | −0.0003 (11) | 0.0196 (12) | −0.0036 (11) |
| C6 | 0.089 (2) | 0.0639 (16) | 0.090 (2) | 0.0025 (15) | 0.0609 (19) | 0.0054 (15) |
| C7 | 0.100 (2) | 0.0832 (19) | 0.0534 (16) | 0.0245 (17) | 0.0307 (16) | −0.0026 (15) |
| C8 | 0.082 (2) | 0.0568 (16) | 0.106 (2) | −0.0087 (14) | 0.052 (2) | −0.0307 (16) |
| C9 | 0.106 (2) | 0.0499 (14) | 0.096 (2) | 0.0350 (16) | 0.067 (2) | 0.0242 (14) |
| C10 | 0.0569 (16) | 0.089 (2) | 0.0793 (19) | 0.0225 (15) | 0.0268 (15) | −0.0039 (16) |
| C11 | 0.0627 (16) | 0.0550 (14) | 0.0498 (14) | 0.0015 (12) | 0.0285 (13) | 0.0003 (11) |
| C12 | 0.0632 (16) | 0.0573 (14) | 0.0481 (14) | −0.0121 (11) | 0.0128 (12) | 0.0100 (11) |
| C13 | 0.0683 (16) | 0.0676 (16) | 0.0429 (13) | −0.0056 (13) | 0.0218 (12) | 0.0003 (12) |
| C14 | 0.0499 (13) | 0.0500 (12) | 0.0511 (14) | −0.0007 (10) | 0.0187 (11) | −0.0044 (11) |
| C15 | 0.0503 (13) | 0.0486 (12) | 0.0468 (13) | −0.0011 (10) | 0.0184 (11) | −0.0001 (10) |
| C16 | 0.0886 (18) | 0.0535 (14) | 0.0466 (14) | −0.0041 (13) | 0.0298 (13) | −0.0036 (11) |
| C17 | 0.092 (2) | 0.0468 (13) | 0.0555 (16) | −0.0043 (12) | 0.0292 (14) | −0.0028 (11) |
| C18 | 0.0517 (14) | 0.0540 (14) | 0.0550 (14) | 0.0031 (10) | 0.0199 (12) | 0.0082 (11) |
| C19 | 0.0591 (16) | 0.0518 (14) | 0.0778 (18) | 0.0053 (12) | 0.0234 (14) | 0.0106 (13) |
| C20 | 0.0589 (16) | 0.0546 (15) | 0.085 (2) | 0.0083 (12) | 0.0177 (14) | −0.0042 (15) |
| C21 | 0.092 (2) | 0.0718 (17) | 0.0631 (17) | 0.0049 (15) | 0.0353 (15) | 0.0209 (13) |
Geometric parameters (Å, º)
| Fe1—C4 | 2.019 (2) | C6—C10 | 1.400 (4) |
| Fe1—C7 | 2.024 (2) | C6—H6 | 0.9793 |
| Fe1—C8 | 2.026 (2) | C7—C8 | 1.395 (4) |
| Fe1—C5 | 2.027 (2) | C7—H7 | 0.9791 |
| Fe1—C9 | 2.034 (2) | C8—C9 | 1.390 (4) |
| Fe1—C6 | 2.037 (3) | C8—H8 | 0.9793 |
| Fe1—C3 | 2.037 (2) | C9—C10 | 1.393 (4) |
| Fe1—C1 | 2.041 (2) | C9—H9 | 0.9794 |
| Fe1—C10 | 2.042 (2) | C10—H10 | 0.9796 |
| Fe1—C2 | 2.047 (2) | C12—C13 | 1.370 (3) |
| O1—C11 | 1.192 (3) | C12—C17 | 1.376 (3) |
| O2—C11 | 1.379 (3) | C13—C14 | 1.384 (3) |
| O2—C12 | 1.402 (3) | C13—H13 | 0.9300 |
| O3—C14 | 1.368 (3) | C14—C15 | 1.387 (3) |
| O3—C20 | 1.388 (3) | C15—C16 | 1.391 (3) |
| O4—C20 | 1.208 (3) | C15—C18 | 1.456 (3) |
| C1—C2 | 1.408 (3) | C16—C17 | 1.373 (3) |
| C1—C5 | 1.411 (3) | C16—H16 | 0.9300 |
| C1—H1 | 0.9799 | C17—H17 | 0.9300 |
| C2—C3 | 1.413 (3) | C18—C19 | 1.335 (3) |
| C2—H2 | 0.9799 | C18—C21 | 1.503 (3) |
| C3—C4 | 1.419 (3) | C19—C20 | 1.439 (4) |
| C3—H3 | 0.9800 | C19—H19 | 0.9300 |
| C4—C5 | 1.428 (3) | C21—H21A | 0.9600 |
| C4—C11 | 1.466 (3) | C21—H21B | 0.9600 |
| C5—H5 | 0.9800 | C21—H21C | 0.9600 |
| C6—C7 | 1.387 (4) | ||
| C4—Fe1—C7 | 153.19 (12) | C1—C5—Fe1 | 70.25 (13) |
| C4—Fe1—C8 | 120.07 (10) | C4—C5—Fe1 | 69.05 (13) |
| C7—Fe1—C8 | 40.29 (11) | C1—C5—H5 | 126.3 |
| C4—Fe1—C5 | 41.35 (9) | C4—C5—H5 | 126.4 |
| C7—Fe1—C5 | 164.06 (12) | Fe1—C5—H5 | 126.4 |
| C8—Fe1—C5 | 153.89 (12) | C7—C6—C10 | 107.9 (3) |
| C4—Fe1—C9 | 109.67 (9) | C7—C6—Fe1 | 69.54 (16) |
| C7—Fe1—C9 | 67.55 (11) | C10—C6—Fe1 | 70.10 (15) |
| C8—Fe1—C9 | 40.03 (12) | C7—C6—H6 | 125.7 |
| C5—Fe1—C9 | 119.71 (11) | C10—C6—H6 | 126.4 |
| C4—Fe1—C6 | 165.92 (12) | Fe1—C6—H6 | 126.2 |
| C7—Fe1—C6 | 39.94 (11) | C6—C7—C8 | 108.1 (3) |
| C8—Fe1—C6 | 67.30 (11) | C6—C7—Fe1 | 70.52 (15) |
| C5—Fe1—C6 | 126.92 (12) | C8—C7—Fe1 | 69.91 (15) |
| C9—Fe1—C6 | 67.43 (10) | C6—C7—H7 | 126.4 |
| C4—Fe1—C3 | 40.94 (9) | C8—C7—H7 | 125.5 |
| C7—Fe1—C3 | 118.74 (12) | Fe1—C7—H7 | 125.9 |
| C8—Fe1—C3 | 109.32 (11) | C9—C8—C7 | 108.2 (3) |
| C5—Fe1—C3 | 69.04 (10) | C9—C8—Fe1 | 70.30 (15) |
| C9—Fe1—C3 | 129.41 (11) | C7—C8—Fe1 | 69.80 (15) |
| C6—Fe1—C3 | 151.50 (12) | C9—C8—H8 | 125.3 |
| C4—Fe1—C1 | 68.54 (9) | C7—C8—H8 | 126.5 |
| C7—Fe1—C1 | 126.66 (11) | Fe1—C8—H8 | 126.0 |
| C8—Fe1—C1 | 164.88 (13) | C8—C9—C10 | 107.8 (2) |
| C5—Fe1—C1 | 40.59 (9) | C8—C9—Fe1 | 69.67 (14) |
| C9—Fe1—C1 | 152.77 (13) | C10—C9—Fe1 | 70.30 (14) |
| C6—Fe1—C1 | 107.33 (10) | C8—C9—H9 | 126.7 |
| C3—Fe1—C1 | 68.16 (10) | C10—C9—H9 | 125.5 |
| C4—Fe1—C10 | 128.88 (11) | Fe1—C9—H9 | 126.0 |
| C7—Fe1—C10 | 67.28 (12) | C9—C10—C6 | 108.0 (3) |
| C8—Fe1—C10 | 67.12 (12) | C9—C10—Fe1 | 69.73 (14) |
| C5—Fe1—C10 | 108.31 (11) | C6—C10—Fe1 | 69.76 (15) |
| C9—Fe1—C10 | 39.97 (11) | C9—C10—H10 | 126.3 |
| C6—Fe1—C10 | 40.14 (11) | C6—C10—H10 | 125.6 |
| C3—Fe1—C10 | 167.07 (11) | Fe1—C10—H10 | 125.9 |
| C1—Fe1—C10 | 118.65 (11) | O1—C11—O2 | 122.9 (2) |
| C4—Fe1—C2 | 68.50 (9) | O1—C11—C4 | 126.9 (2) |
| C7—Fe1—C2 | 107.62 (11) | O2—C11—C4 | 110.1 (2) |
| C8—Fe1—C2 | 128.22 (13) | C13—C12—C17 | 121.7 (2) |
| C5—Fe1—C2 | 68.47 (10) | C13—C12—O2 | 120.5 (2) |
| C9—Fe1—C2 | 166.40 (13) | C17—C12—O2 | 117.7 (2) |
| C6—Fe1—C2 | 117.71 (10) | C12—C13—C14 | 117.9 (2) |
| C3—Fe1—C2 | 40.49 (9) | C12—C13—H13 | 121.0 |
| C1—Fe1—C2 | 40.30 (9) | C14—C13—H13 | 121.0 |
| C10—Fe1—C2 | 151.52 (11) | O3—C14—C13 | 116.2 (2) |
| C11—O2—C12 | 117.52 (18) | O3—C14—C15 | 121.31 (19) |
| C14—O3—C20 | 121.64 (19) | C13—C14—C15 | 122.5 (2) |
| C2—C1—C5 | 108.8 (2) | C14—C15—C16 | 117.2 (2) |
| C2—C1—Fe1 | 70.10 (13) | C14—C15—C18 | 118.70 (19) |
| C5—C1—Fe1 | 69.16 (13) | C16—C15—C18 | 124.1 (2) |
| C2—C1—H1 | 125.3 | C17—C16—C15 | 121.4 (2) |
| C5—C1—H1 | 125.9 | C17—C16—H16 | 119.3 |
| Fe1—C1—H1 | 125.6 | C15—C16—H16 | 119.3 |
| C1—C2—C3 | 108.1 (2) | C16—C17—C12 | 119.2 (2) |
| C1—C2—Fe1 | 69.60 (13) | C16—C17—H17 | 120.4 |
| C3—C2—Fe1 | 69.36 (12) | C12—C17—H17 | 120.4 |
| C1—C2—H2 | 125.9 | C19—C18—C15 | 118.3 (2) |
| C3—C2—H2 | 125.9 | C19—C18—C21 | 122.1 (2) |
| Fe1—C2—H2 | 125.8 | C15—C18—C21 | 119.6 (2) |
| C2—C3—C4 | 107.8 (2) | C18—C19—C20 | 123.4 (2) |
| C2—C3—Fe1 | 70.14 (13) | C18—C19—H19 | 118.3 |
| C4—C3—Fe1 | 68.86 (13) | C20—C19—H19 | 118.3 |
| C2—C3—H3 | 126.0 | O4—C20—O3 | 116.0 (3) |
| C4—C3—H3 | 126.1 | O4—C20—C19 | 127.3 (3) |
| Fe1—C3—H3 | 126.3 | O3—C20—C19 | 116.6 (2) |
| C3—C4—C5 | 108.0 (2) | C18—C21—H21A | 109.5 |
| C3—C4—C11 | 123.9 (2) | C18—C21—H21B | 109.5 |
| C5—C4—C11 | 127.9 (2) | H21A—C21—H21B | 109.5 |
| C3—C4—Fe1 | 70.20 (12) | C18—C21—H21C | 109.5 |
| C5—C4—Fe1 | 69.60 (13) | H21A—C21—H21C | 109.5 |
| C11—C4—Fe1 | 121.63 (15) | H21B—C21—H21C | 109.5 |
| C1—C5—C4 | 107.3 (2) | ||
| C4—Fe1—C1—C2 | −81.64 (15) | C5—Fe1—C6—C10 | 73.7 (2) |
| C7—Fe1—C1—C2 | 72.90 (19) | C9—Fe1—C6—C10 | −37.39 (17) |
| C8—Fe1—C1—C2 | 45.9 (5) | C3—Fe1—C6—C10 | −169.9 (2) |
| C5—Fe1—C1—C2 | −120.3 (2) | C1—Fe1—C6—C10 | 114.14 (18) |
| C9—Fe1—C1—C2 | −173.15 (19) | C2—Fe1—C6—C10 | 156.54 (17) |
| C6—Fe1—C1—C2 | 112.64 (16) | C10—C6—C7—C8 | −0.2 (3) |
| C3—Fe1—C1—C2 | −37.43 (14) | Fe1—C6—C7—C8 | −60.10 (18) |
| C10—Fe1—C1—C2 | 154.74 (15) | C10—C6—C7—Fe1 | 59.88 (19) |
| C4—Fe1—C1—C5 | 38.64 (14) | C4—Fe1—C7—C6 | −170.58 (19) |
| C7—Fe1—C1—C5 | −166.82 (16) | C8—Fe1—C7—C6 | −118.6 (2) |
| C8—Fe1—C1—C5 | 166.2 (4) | C5—Fe1—C7—C6 | 39.2 (5) |
| C9—Fe1—C1—C5 | −52.9 (3) | C9—Fe1—C7—C6 | −81.18 (18) |
| C6—Fe1—C1—C5 | −127.08 (17) | C3—Fe1—C7—C6 | 154.99 (15) |
| C3—Fe1—C1—C5 | 82.85 (16) | C1—Fe1—C7—C6 | 71.9 (2) |
| C10—Fe1—C1—C5 | −84.98 (17) | C10—Fe1—C7—C6 | −37.73 (16) |
| C2—Fe1—C1—C5 | 120.3 (2) | C2—Fe1—C7—C6 | 112.36 (17) |
| C5—C1—C2—C3 | 0.3 (3) | C4—Fe1—C7—C8 | −51.9 (3) |
| Fe1—C1—C2—C3 | 58.83 (16) | C5—Fe1—C7—C8 | 157.9 (3) |
| C5—C1—C2—Fe1 | −58.48 (16) | C9—Fe1—C7—C8 | 37.47 (18) |
| C4—Fe1—C2—C1 | 81.76 (15) | C6—Fe1—C7—C8 | 118.6 (2) |
| C7—Fe1—C2—C1 | −126.44 (17) | C3—Fe1—C7—C8 | −86.37 (19) |
| C8—Fe1—C2—C1 | −166.21 (15) | C1—Fe1—C7—C8 | −169.44 (17) |
| C5—Fe1—C2—C1 | 37.16 (14) | C10—Fe1—C7—C8 | 80.91 (19) |
| C9—Fe1—C2—C1 | 166.6 (4) | C2—Fe1—C7—C8 | −128.99 (18) |
| C6—Fe1—C2—C1 | −84.32 (18) | C6—C7—C8—C9 | 0.4 (3) |
| C3—Fe1—C2—C1 | 119.7 (2) | Fe1—C7—C8—C9 | −60.04 (18) |
| C10—Fe1—C2—C1 | −51.8 (3) | C6—C7—C8—Fe1 | 60.48 (18) |
| C4—Fe1—C2—C3 | −37.91 (14) | C4—Fe1—C8—C9 | −85.15 (18) |
| C7—Fe1—C2—C3 | 113.89 (17) | C7—Fe1—C8—C9 | 119.1 (2) |
| C8—Fe1—C2—C3 | 74.12 (19) | C5—Fe1—C8—C9 | −47.3 (3) |
| C5—Fe1—C2—C3 | −82.52 (15) | C6—Fe1—C8—C9 | 81.44 (18) |
| C9—Fe1—C2—C3 | 46.9 (5) | C3—Fe1—C8—C9 | −128.94 (16) |
| C6—Fe1—C2—C3 | 156.00 (16) | C1—Fe1—C8—C9 | 153.4 (4) |
| C1—Fe1—C2—C3 | −119.7 (2) | C10—Fe1—C8—C9 | 37.73 (16) |
| C10—Fe1—C2—C3 | −171.4 (2) | C2—Fe1—C8—C9 | −170.38 (15) |
| C1—C2—C3—C4 | −0.2 (3) | C4—Fe1—C8—C7 | 155.77 (17) |
| Fe1—C2—C3—C4 | 58.74 (15) | C5—Fe1—C8—C7 | −166.4 (2) |
| C1—C2—C3—Fe1 | −58.98 (16) | C9—Fe1—C8—C7 | −119.1 (2) |
| C4—Fe1—C3—C2 | 119.3 (2) | C6—Fe1—C8—C7 | −37.64 (17) |
| C7—Fe1—C3—C2 | −83.65 (19) | C3—Fe1—C8—C7 | 111.99 (18) |
| C8—Fe1—C3—C2 | −126.80 (18) | C1—Fe1—C8—C7 | 34.3 (5) |
| C5—Fe1—C3—C2 | 81.00 (16) | C10—Fe1—C8—C7 | −81.35 (18) |
| C9—Fe1—C3—C2 | −167.16 (17) | C2—Fe1—C8—C7 | 70.5 (2) |
| C6—Fe1—C3—C2 | −49.0 (3) | C7—C8—C9—C10 | −0.5 (3) |
| C1—Fe1—C3—C2 | 37.26 (14) | Fe1—C8—C9—C10 | −60.22 (18) |
| C10—Fe1—C3—C2 | 161.5 (4) | C7—C8—C9—Fe1 | 59.72 (18) |
| C7—Fe1—C3—C4 | 157.10 (15) | C4—Fe1—C9—C8 | 113.68 (17) |
| C8—Fe1—C3—C4 | 113.95 (16) | C7—Fe1—C9—C8 | −37.70 (17) |
| C5—Fe1—C3—C4 | −38.26 (13) | C5—Fe1—C9—C8 | 158.13 (16) |
| C9—Fe1—C3—C4 | 73.59 (19) | C6—Fe1—C9—C8 | −81.09 (19) |
| C6—Fe1—C3—C4 | −168.2 (2) | C3—Fe1—C9—C8 | 71.81 (19) |
| C1—Fe1—C3—C4 | −81.99 (15) | C1—Fe1—C9—C8 | −165.2 (2) |
| C10—Fe1—C3—C4 | 42.2 (5) | C10—Fe1—C9—C8 | −118.6 (2) |
| C2—Fe1—C3—C4 | −119.3 (2) | C2—Fe1—C9—C8 | 33.9 (5) |
| C2—C3—C4—C5 | 0.1 (3) | C4—Fe1—C9—C10 | −127.68 (17) |
| Fe1—C3—C4—C5 | 59.59 (15) | C7—Fe1—C9—C10 | 80.94 (18) |
| C2—C3—C4—C11 | −175.0 (2) | C8—Fe1—C9—C10 | 118.6 (2) |
| Fe1—C3—C4—C11 | −115.4 (2) | C5—Fe1—C9—C10 | −83.24 (18) |
| C2—C3—C4—Fe1 | −59.54 (15) | C6—Fe1—C9—C10 | 37.54 (17) |
| C7—Fe1—C4—C3 | −49.2 (3) | C3—Fe1—C9—C10 | −169.56 (16) |
| C8—Fe1—C4—C3 | −85.19 (17) | C1—Fe1—C9—C10 | −46.6 (3) |
| C5—Fe1—C4—C3 | 118.92 (19) | C2—Fe1—C9—C10 | 152.6 (4) |
| C9—Fe1—C4—C3 | −128.09 (16) | C8—C9—C10—C6 | 0.4 (3) |
| C6—Fe1—C4—C3 | 156.4 (4) | Fe1—C9—C10—C6 | −59.46 (18) |
| C1—Fe1—C4—C3 | 80.97 (15) | C8—C9—C10—Fe1 | 59.82 (17) |
| C10—Fe1—C4—C3 | −168.86 (15) | C7—C6—C10—C9 | −0.1 (3) |
| C2—Fe1—C4—C3 | 37.51 (14) | Fe1—C6—C10—C9 | 59.44 (17) |
| C7—Fe1—C4—C5 | −168.1 (2) | C7—C6—C10—Fe1 | −59.53 (19) |
| C8—Fe1—C4—C5 | 155.88 (16) | C4—Fe1—C10—C9 | 73.2 (2) |
| C9—Fe1—C4—C5 | 112.99 (16) | C7—Fe1—C10—C9 | −81.66 (19) |
| C6—Fe1—C4—C5 | 37.5 (4) | C8—Fe1—C10—C9 | −37.79 (17) |
| C3—Fe1—C4—C5 | −118.92 (19) | C5—Fe1—C10—C9 | 114.70 (18) |
| C1—Fe1—C4—C5 | −37.95 (14) | C6—Fe1—C10—C9 | −119.2 (2) |
| C10—Fe1—C4—C5 | 72.22 (18) | C3—Fe1—C10—C9 | 38.7 (5) |
| C2—Fe1—C4—C5 | −81.41 (15) | C1—Fe1—C10—C9 | 157.75 (17) |
| C7—Fe1—C4—C11 | 69.1 (3) | C2—Fe1—C10—C9 | −166.9 (2) |
| C8—Fe1—C4—C11 | 33.1 (3) | C4—Fe1—C10—C6 | −167.59 (16) |
| C5—Fe1—C4—C11 | −122.8 (3) | C7—Fe1—C10—C6 | 37.55 (17) |
| C9—Fe1—C4—C11 | −9.8 (2) | C8—Fe1—C10—C6 | 81.42 (19) |
| C6—Fe1—C4—C11 | −85.3 (4) | C5—Fe1—C10—C6 | −126.09 (18) |
| C3—Fe1—C4—C11 | 118.3 (3) | C9—Fe1—C10—C6 | 119.2 (2) |
| C1—Fe1—C4—C11 | −160.7 (2) | C3—Fe1—C10—C6 | 158.0 (4) |
| C10—Fe1—C4—C11 | −50.6 (3) | C1—Fe1—C10—C6 | −83.04 (19) |
| C2—Fe1—C4—C11 | 155.8 (2) | C2—Fe1—C10—C6 | −47.7 (3) |
| C2—C1—C5—C4 | −0.3 (3) | C12—O2—C11—O1 | 0.4 (3) |
| Fe1—C1—C5—C4 | −59.37 (15) | C12—O2—C11—C4 | −179.32 (19) |
| C2—C1—C5—Fe1 | 59.06 (16) | C3—C4—C11—O1 | −3.3 (4) |
| C3—C4—C5—C1 | 0.2 (3) | C5—C4—C11—O1 | −177.3 (2) |
| C11—C4—C5—C1 | 174.9 (2) | Fe1—C4—C11—O1 | −89.5 (3) |
| Fe1—C4—C5—C1 | 60.13 (16) | C3—C4—C11—O2 | 176.36 (19) |
| C3—C4—C5—Fe1 | −59.97 (15) | C5—C4—C11—O2 | 2.4 (3) |
| C11—C4—C5—Fe1 | 114.8 (2) | Fe1—C4—C11—O2 | 90.1 (2) |
| C4—Fe1—C5—C1 | −118.4 (2) | C11—O2—C12—C13 | 72.1 (3) |
| C7—Fe1—C5—C1 | 41.8 (4) | C11—O2—C12—C17 | −111.6 (3) |
| C8—Fe1—C5—C1 | −171.8 (2) | C17—C12—C13—C14 | 0.5 (4) |
| C9—Fe1—C5—C1 | 155.16 (16) | O2—C12—C13—C14 | 176.7 (2) |
| C6—Fe1—C5—C1 | 72.29 (19) | C20—O3—C14—C13 | −178.0 (2) |
| C3—Fe1—C5—C1 | −80.49 (15) | C20—O3—C14—C15 | 1.4 (3) |
| C10—Fe1—C5—C1 | 112.95 (17) | C12—C13—C14—O3 | 178.7 (2) |
| C2—Fe1—C5—C1 | −36.90 (14) | C12—C13—C14—C15 | −0.8 (4) |
| C7—Fe1—C5—C4 | 160.2 (4) | O3—C14—C15—C16 | −179.2 (2) |
| C8—Fe1—C5—C4 | −53.5 (3) | C13—C14—C15—C16 | 0.2 (4) |
| C9—Fe1—C5—C4 | −86.45 (17) | O3—C14—C15—C18 | 0.6 (3) |
| C6—Fe1—C5—C4 | −169.33 (14) | C13—C14—C15—C18 | 180.0 (2) |
| C3—Fe1—C5—C4 | 37.89 (13) | C14—C15—C16—C17 | 0.8 (4) |
| C1—Fe1—C5—C4 | 118.4 (2) | C18—C15—C16—C17 | −179.1 (2) |
| C10—Fe1—C5—C4 | −128.67 (15) | C15—C16—C17—C12 | −1.1 (4) |
| C2—Fe1—C5—C4 | 81.48 (15) | C13—C12—C17—C16 | 0.4 (4) |
| C4—Fe1—C6—C7 | 162.3 (3) | O2—C12—C17—C16 | −175.9 (2) |
| C8—Fe1—C6—C7 | 37.96 (18) | C14—C15—C18—C19 | −1.5 (3) |
| C5—Fe1—C6—C7 | −167.46 (16) | C16—C15—C18—C19 | 178.3 (2) |
| C9—Fe1—C6—C7 | 81.50 (19) | C14—C15—C18—C21 | 178.6 (2) |
| C3—Fe1—C6—C7 | −51.0 (3) | C16—C15—C18—C21 | −1.6 (4) |
| C1—Fe1—C6—C7 | −126.98 (17) | C15—C18—C19—C20 | 0.5 (4) |
| C10—Fe1—C6—C7 | 118.9 (2) | C21—C18—C19—C20 | −179.6 (2) |
| C2—Fe1—C6—C7 | −84.58 (19) | C14—O3—C20—O4 | 178.6 (2) |
| C4—Fe1—C6—C10 | 43.5 (5) | C14—O3—C20—C19 | −2.4 (3) |
| C7—Fe1—C6—C10 | −118.9 (2) | C18—C19—C20—O4 | −179.7 (3) |
| C8—Fe1—C6—C10 | −80.92 (19) | C18—C19—C20—O3 | 1.4 (4) |
Footnotes
Supporting information for this paper is available from the IUCr electronic archives (Reference: WM5068).
References
- Bruker (2007). APEX2, SADABS and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Chen, W. Y. & Lu, J. (2004). Chin. Chem. Lett. 15, 1146–1148.
- Imrie, C., Elago, E. R. T., McCleland, C. W. & Williams, N. (2002). Green Chem. 4, 159–160.
- Imrie, C., Elago, E. R. T., Williams, N., McCleland, C. W. & Engelbrecht, P. (2005). J. Organomet. Chem. 690, 4959–4966.
- Peng, X. M. L. V., Damu, G. & Zhou, H. (2013). Curr. Pharm. Des. 19, 3884–3930. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Štěpnička, P. (2002). New J. Chem. 26, 567–575.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, Global. DOI: 10.1107/S1600536814022120/wm5068sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536814022120/wm5068Isup2.hkl
. DOI: 10.1107/S1600536814022120/wm5068fig1.tif
The molecular structure of the title compound showing atoms as ellipsoids at the 30% probability level.
CCDC reference: 1027955
Additional supporting information: crystallographic information; 3D view; checkCIF report


