Abstract
In the title compound, C14H14Cl2N2O3, the chlorophenyl ring makes a dihedral angle of 87.08 (9)° with the tetrahydropyrimidine ring. There is a short intramolecular C—H⋯O contact present. In the crystal, molecules are linked via pairs of N—H⋯O hydrogen bonds, forming inversion dimers with an R 2 2(8) ring motif. The dimers are linked via a second pair of N—H⋯O hydrogen bonds, this time enclosing an R 4 4(20) ring motif, forming ribbons along [100]. The ribbons are linked via C—H⋯O hydrogen bonds, forming sheets lying parallel to (001). The terminal ethyl group is disordered over two positions with an occupancy ratio of 0.654 (17):0.346 (17).
Keywords: crystal structure, tetrahydropyrimidine, inversion dimers, anticarcinogenic, antihypertensive, calcium channel modulators.
Related literature
For the many biological activities of dihydropyrimidinone derivatives, see: Atwal et al. (1991 ▶); Jauk et al. (2000 ▶); Kato (1984 ▶); Wipf & Cunningham (1995 ▶); Bedia et al. (2006 ▶); For related structures, see: Nayak et al. (2009 ▶); Yuvaraj et al. (2010 ▶);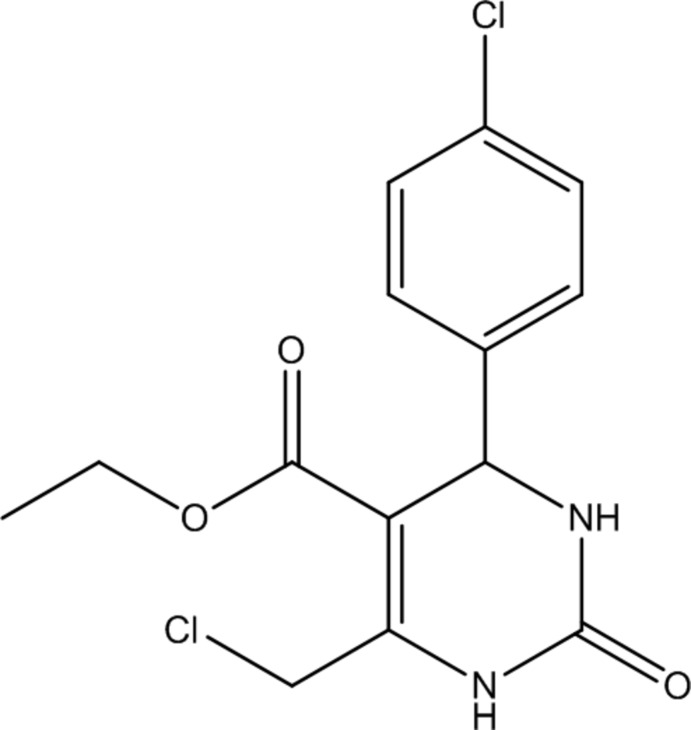
Experimental
Crystal data
C14H14Cl2N2O3
M r = 329.17
Triclinic,

a = 7.4698 (3) Å
b = 9.1436 (3) Å
c = 12.6085 (4) Å
α = 107.147 (2)°
β = 99.941 (2)°
γ = 105.331 (2)°
V = 763.71 (5) Å3
Z = 2
Mo Kα radiation
μ = 0.44 mm−1
T = 295 K
0.30 × 0.25 × 0.20 mm
Data collection
Bruker APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.897, T max = 0.917
20157 measured reflections
3945 independent reflections
2911 reflections with I > 2σ(I)
R int = 0.022
Refinement
R[F 2 > 2σ(F 2)] = 0.054
wR(F 2) = 0.153
S = 1.03
3945 reflections
211 parameters
26 restraints
H-atom parameters constrained
Δρmax = 0.64 e Å−3
Δρmin = −0.53 e Å−3
Data collection: APEX2 (Bruker, 2008 ▶); cell refinement: SAINT (Bruker, 2008 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: PLATON (Spek, 2009 ▶); software used to prepare material for publication: SHELXL97 and PLATON.
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S1600536814023046/su5005sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536814023046/su5005Isup2.hkl
Supporting information file. DOI: 10.1107/S1600536814023046/su5005Isup3.cml
. DOI: 10.1107/S1600536814023046/su5005fig1.tif
The molecular structure of the title molecule, with atom labelling. Displacement ellipsoids are drawn at the 30% probability level.
a . DOI: 10.1107/S1600536814023046/su5005fig2.tif
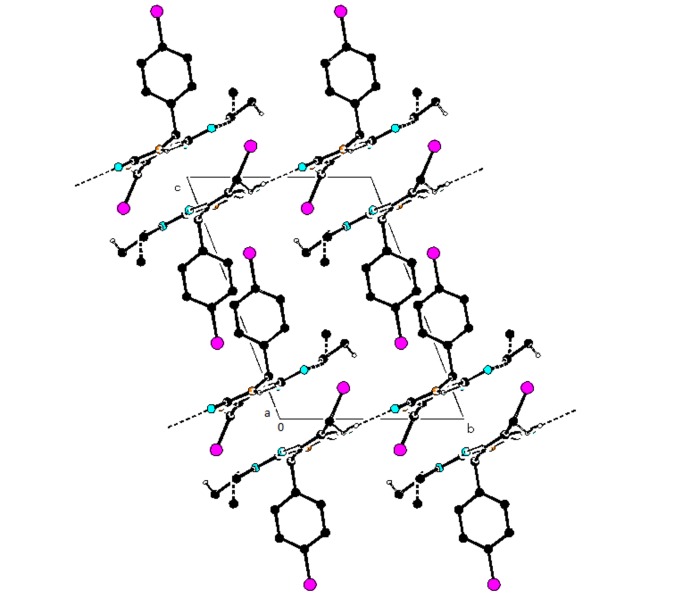
The crystal packing of the title compound, viewed along the a axis. Hydrogen bonds are shown as dashed lines (see Table 1 for details).
CCDC reference: 1030125
Additional supporting information: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (, ).
| DHA | DH | HA | D A | DHA |
|---|---|---|---|---|
| N2H2AO1i | 0.86 | 2.04 | 2.885(2) | 166 |
| N1H1O2ii | 0.86 | 2.23 | 3.070(2) | 166 |
| C11H11BO1iii | 0.97 | 2.50 | 3.069(3) | 117 |
| C11H11AO2 | 0.97 | 2.14 | 2.814(3) | 126 |
Symmetry codes: (i) 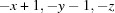 ; (ii)
; (ii) 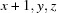 ; (iii)
; (iii)  .
.
Acknowledgments
SB thanks Professor K. Ramamurthi, Department of Physics and Nanotechnology, SRM University, Chennai, for his guidance and valuable suggestions.
supplementary crystallographic information
S1. Comment
Dihydropyrimidinones (DHPM) and their derivatives are important on account of their wide range of applications in therapeutic and pharmacology, namely because of their anticarcinogenic (Kato, 1984), antihypertensive (Atwal et al., 1991) and calcium channel modulators (Jauk et al., 2000) activities. These derivatives have also been screened for anti-bacterial (Wipf & Cunningham, 1995), and anti-tuberculosis activity (Bedia et al., 2006).
The geometric parameters of the title molecule (Fig. 1) agree well with those reported fro similar structures (Nayak et al., 2009; Yuvaraj et al., 2010). The chlorophenyl ring makes a dihedral angles of 87.08 (9) ° with the tetrahydropyrimidine ring. There is a short intramolecular C—H···O contact (Table 1).
In the crystal, molecules are linked via a pair of N—H···O hydrogen bonds forming inversion dimers with an R22(8) ring motif. The dimers are linked via a second pair of N—H···O hydrogen bonds, this time enclosing an R44(20) ring motif, forming ribbons along [100]. The ribbons are linked via C—H···O hydrogen bonds forming sheets lying parallel to (001); see Table 1 and Fig. 2 for details.
S2. Experimental
A mixture of ethyl-4-chloro acetoacetate (4.1 ml, 0.025 mol), 4-chlorobenzaldehyde (3.6 g, 0.025 mol), and urea (4.5 g, 0.075 mol) in ethanol (5 ml) was heated under reflux in the presence of concentrated HCl (1 mL) for 5 h (monitored by TLC). The reaction mixture, after being cooled to room temperature, was poured onto crushed ice and stirred for 5–10 min. The solid was separated and filtered under suction, washed with ice-cold water (50 ml), and then recrystallized from hot ethanol to afford pure product [m.p. 437 K; yield 76%].
S3. Refinement
The terminal ethyl group is disordered over two position. The refined site occupancies of the disordered C atoms are C13/C14 = 0.654 (17) and C13A/C14A = 0.346 (17). The O3—C13A and C13A—C14A bond distances was restrained to be 1.400 (1) Å. In the refinement, ISOR was used for atoms C13, C14, C13A and C14A. The H atoms were positioned geometrically and refined using a riding model: N—H = 0.86 Å, C—H = 0.93 - 0.98 Å with Uiso(H) = 1.5Ueq(C) for methyl H atoms and = 1.2Ueq(N,C) for other H atoms.
Figures
Fig. 1.
The molecular structure of the title molecule, with atom labelling. Displacement ellipsoids are drawn at the 30% probability level.
Fig. 2.
The crystal packing of the title compound, viewed along the a axis. Hydrogen bonds are shown as dashed lines (see Table 1 for details).
Crystal data
| C14H14Cl2N2O3 | Z = 2 |
| Mr = 329.17 | F(000) = 340 |
| Triclinic, P1 | Dx = 1.431 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 7.4698 (3) Å | Cell parameters from 4865 reflections |
| b = 9.1436 (3) Å | θ = 2.5–30.1° |
| c = 12.6085 (4) Å | µ = 0.44 mm−1 |
| α = 107.147 (2)° | T = 295 K |
| β = 99.941 (2)° | Block, colourless |
| γ = 105.331 (2)° | 0.30 × 0.25 × 0.20 mm |
| V = 763.71 (5) Å3 |
Data collection
| Bruker APEXII CCD diffractometer | 3945 independent reflections |
| Radiation source: fine-focus sealed tube | 2911 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.022 |
| Detector resolution: 0 pixels mm-1 | θmax = 31.0°, θmin = 2.5° |
| ω and φ scans | h = −10→10 |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | k = −12→12 |
| Tmin = 0.897, Tmax = 0.917 | l = −18→17 |
| 20157 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.054 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.153 | H-atom parameters constrained |
| S = 1.03 | w = 1/[σ2(Fo2) + (0.0628P)2 + 0.6453P] where P = (Fo2 + 2Fc2)/3 |
| 3945 reflections | (Δ/σ)max < 0.001 |
| 211 parameters | Δρmax = 0.64 e Å−3 |
| 26 restraints | Δρmin = −0.53 e Å−3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| C1 | 0.8422 (5) | 0.1384 (4) | 0.5387 (2) | 0.0651 (8) | |
| C2 | 0.7145 (5) | −0.0099 (4) | 0.4712 (3) | 0.0725 (9) | |
| H2 | 0.6712 | −0.0878 | 0.5031 | 0.087* | |
| C3 | 0.6488 (4) | −0.0442 (3) | 0.3538 (2) | 0.0598 (7) | |
| H3 | 0.5588 | −0.1453 | 0.3073 | 0.072* | |
| C4 | 0.7137 (3) | 0.0674 (3) | 0.30506 (18) | 0.0361 (4) | |
| C5 | 0.8424 (5) | 0.2165 (4) | 0.3761 (3) | 0.0699 (9) | |
| H5 | 0.8876 | 0.2950 | 0.3452 | 0.084* | |
| C6 | 0.9060 (6) | 0.2518 (4) | 0.4938 (3) | 0.0895 (12) | |
| H6 | 0.9925 | 0.3537 | 0.5414 | 0.107* | |
| C7 | 0.6508 (3) | 0.0279 (2) | 0.17587 (17) | 0.0306 (4) | |
| H7 | 0.7032 | 0.1280 | 0.1613 | 0.037* | |
| C8 | 0.6382 (3) | −0.2509 (2) | 0.06968 (18) | 0.0342 (4) | |
| C9 | 0.3408 (3) | −0.1913 (2) | 0.06544 (17) | 0.0315 (4) | |
| C10 | 0.4341 (3) | −0.0329 (2) | 0.12644 (16) | 0.0300 (4) | |
| C11 | 0.1275 (3) | −0.2650 (3) | 0.0111 (2) | 0.0454 (5) | |
| H11A | 0.0752 | −0.1799 | 0.0068 | 0.055* | |
| H11B | 0.0673 | −0.3162 | 0.0593 | 0.055* | |
| C12 | 0.3328 (3) | 0.0848 (3) | 0.15276 (18) | 0.0360 (4) | |
| N1 | 0.7306 (2) | −0.0910 (2) | 0.11509 (15) | 0.0338 (4) | |
| H1 | 0.8450 | −0.0555 | 0.1081 | 0.041* | |
| N2 | 0.4415 (2) | −0.2989 (2) | 0.05067 (17) | 0.0375 (4) | |
| H2A | 0.3781 | −0.4009 | 0.0285 | 0.045* | |
| O1 | 0.7187 (2) | −0.35121 (19) | 0.04214 (16) | 0.0488 (4) | |
| O2 | 0.1610 (2) | 0.0561 (2) | 0.13615 (16) | 0.0508 (4) | |
| O3 | 0.4586 (2) | 0.23490 (19) | 0.20251 (17) | 0.0554 (5) | |
| C13 | 0.374 (3) | 0.3618 (16) | 0.2478 (12) | 0.086 (4) | 0.654 (17) |
| H13A | 0.2924 | 0.3745 | 0.1847 | 0.103* | 0.654 (17) |
| H13B | 0.2959 | 0.3318 | 0.2971 | 0.103* | 0.654 (17) |
| C14 | 0.5303 (12) | 0.5098 (7) | 0.3121 (11) | 0.116 (4) | 0.654 (17) |
| H14A | 0.6081 | 0.4964 | 0.3752 | 0.174* | 0.654 (17) |
| H14B | 0.4803 | 0.5955 | 0.3415 | 0.174* | 0.654 (17) |
| H14C | 0.6076 | 0.5371 | 0.2627 | 0.174* | 0.654 (17) |
| C13A | 0.394 (4) | 0.368 (3) | 0.2319 (11) | 0.070 (7) | 0.346 (17) |
| H13C | 0.2598 | 0.3357 | 0.1900 | 0.084* | 0.346 (17) |
| H13D | 0.4684 | 0.4531 | 0.2098 | 0.084* | 0.346 (17) |
| C14A | 0.414 (4) | 0.428 (3) | 0.3506 (12) | 0.127 (10) | 0.346 (17) |
| H14D | 0.3199 | 0.3533 | 0.3697 | 0.190* | 0.346 (17) |
| H14E | 0.3937 | 0.5314 | 0.3713 | 0.190* | 0.346 (17) |
| H14F | 0.5409 | 0.4415 | 0.3920 | 0.190* | 0.346 (17) |
| Cl1 | 0.92366 (19) | 0.18204 (17) | 0.68608 (7) | 0.1114 (4) | |
| Cl2 | 0.07043 (8) | −0.41074 (8) | −0.12863 (5) | 0.0522 (2) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0719 (18) | 0.085 (2) | 0.0334 (12) | 0.0407 (17) | 0.0004 (12) | 0.0089 (13) |
| C2 | 0.095 (2) | 0.079 (2) | 0.0475 (16) | 0.0299 (19) | 0.0186 (15) | 0.0296 (15) |
| C3 | 0.0751 (18) | 0.0529 (15) | 0.0402 (13) | 0.0083 (13) | 0.0088 (12) | 0.0163 (11) |
| C4 | 0.0315 (10) | 0.0396 (11) | 0.0348 (10) | 0.0151 (8) | 0.0040 (8) | 0.0098 (8) |
| C5 | 0.076 (2) | 0.0498 (15) | 0.0523 (16) | −0.0029 (14) | −0.0110 (14) | 0.0120 (12) |
| C6 | 0.099 (3) | 0.069 (2) | 0.0506 (18) | 0.0008 (19) | −0.0216 (17) | −0.0003 (16) |
| C7 | 0.0246 (9) | 0.0292 (9) | 0.0363 (10) | 0.0085 (7) | 0.0058 (7) | 0.0110 (8) |
| C8 | 0.0261 (9) | 0.0363 (10) | 0.0398 (10) | 0.0112 (8) | 0.0095 (8) | 0.0122 (8) |
| C9 | 0.0228 (8) | 0.0364 (10) | 0.0361 (10) | 0.0107 (7) | 0.0095 (7) | 0.0124 (8) |
| C10 | 0.0255 (9) | 0.0331 (9) | 0.0325 (9) | 0.0112 (7) | 0.0066 (7) | 0.0126 (8) |
| C11 | 0.0262 (10) | 0.0453 (12) | 0.0516 (13) | 0.0079 (9) | 0.0099 (9) | 0.0027 (10) |
| C12 | 0.0343 (10) | 0.0374 (10) | 0.0370 (10) | 0.0163 (8) | 0.0061 (8) | 0.0123 (8) |
| N1 | 0.0207 (7) | 0.0351 (9) | 0.0416 (9) | 0.0079 (6) | 0.0086 (6) | 0.0094 (7) |
| N2 | 0.0237 (8) | 0.0284 (8) | 0.0552 (11) | 0.0069 (6) | 0.0101 (7) | 0.0096 (7) |
| O1 | 0.0303 (8) | 0.0385 (8) | 0.0734 (12) | 0.0151 (6) | 0.0150 (7) | 0.0103 (8) |
| O2 | 0.0333 (8) | 0.0521 (10) | 0.0681 (11) | 0.0229 (7) | 0.0135 (7) | 0.0155 (8) |
| O3 | 0.0431 (9) | 0.0336 (8) | 0.0738 (12) | 0.0180 (7) | −0.0026 (8) | 0.0034 (8) |
| C13 | 0.071 (6) | 0.051 (6) | 0.116 (9) | 0.039 (4) | 0.008 (6) | −0.001 (5) |
| C14 | 0.100 (5) | 0.041 (3) | 0.171 (9) | 0.017 (3) | 0.030 (5) | −0.003 (4) |
| C13A | 0.077 (14) | 0.052 (10) | 0.063 (8) | 0.048 (10) | −0.016 (7) | −0.009 (7) |
| C14A | 0.18 (2) | 0.138 (18) | 0.075 (9) | 0.115 (18) | 0.028 (9) | 0.000 (9) |
| Cl1 | 0.1383 (10) | 0.1531 (11) | 0.0355 (4) | 0.0742 (8) | 0.0001 (5) | 0.0144 (5) |
| Cl2 | 0.0426 (3) | 0.0510 (4) | 0.0510 (3) | 0.0153 (3) | −0.0023 (2) | 0.0100 (3) |
Geometric parameters (Å, º)
| C1—C6 | 1.342 (5) | C11—Cl2 | 1.766 (2) |
| C1—C2 | 1.352 (5) | C11—H11A | 0.9700 |
| C1—Cl1 | 1.739 (3) | C11—H11B | 0.9700 |
| C2—C3 | 1.388 (4) | C12—O2 | 1.207 (3) |
| C2—H2 | 0.9300 | C12—O3 | 1.330 (3) |
| C3—C4 | 1.366 (3) | N1—H1 | 0.8600 |
| C3—H3 | 0.9300 | N2—H2A | 0.8600 |
| C4—C5 | 1.369 (3) | O3—C13A | 1.4000 (10) |
| C4—C7 | 1.518 (3) | O3—C13 | 1.482 (8) |
| C5—C6 | 1.387 (4) | C13—C14 | 1.430 (19) |
| C5—H5 | 0.9300 | C13—H13A | 0.9700 |
| C6—H6 | 0.9300 | C13—H13B | 0.9700 |
| C7—N1 | 1.461 (2) | C14—H14A | 0.9600 |
| C7—C10 | 1.513 (2) | C14—H14B | 0.9600 |
| C7—H7 | 0.9800 | C14—H14C | 0.9600 |
| C8—O1 | 1.225 (2) | C13A—C14A | 1.4000 (10) |
| C8—N1 | 1.333 (3) | C13A—H13C | 0.9700 |
| C8—N2 | 1.373 (2) | C13A—H13D | 0.9700 |
| C9—C10 | 1.341 (3) | C14A—H14D | 0.9600 |
| C9—N2 | 1.379 (2) | C14A—H14E | 0.9600 |
| C9—C11 | 1.498 (3) | C14A—H14F | 0.9600 |
| C10—C12 | 1.466 (3) | ||
| C6—C1—C2 | 121.0 (3) | Cl2—C11—H11A | 109.2 |
| C6—C1—Cl1 | 119.8 (3) | C9—C11—H11B | 109.2 |
| C2—C1—Cl1 | 119.2 (3) | Cl2—C11—H11B | 109.2 |
| C1—C2—C3 | 119.1 (3) | H11A—C11—H11B | 107.9 |
| C1—C2—H2 | 120.5 | O2—C12—O3 | 122.43 (19) |
| C3—C2—H2 | 120.5 | O2—C12—C10 | 127.3 (2) |
| C4—C3—C2 | 121.3 (3) | O3—C12—C10 | 110.28 (17) |
| C4—C3—H3 | 119.4 | C8—N1—C7 | 124.46 (16) |
| C2—C3—H3 | 119.4 | C8—N1—H1 | 117.8 |
| C3—C4—C5 | 118.0 (2) | C7—N1—H1 | 117.8 |
| C3—C4—C7 | 121.6 (2) | C8—N2—C9 | 123.10 (17) |
| C5—C4—C7 | 120.4 (2) | C8—N2—H2A | 118.5 |
| C4—C5—C6 | 120.8 (3) | C9—N2—H2A | 118.4 |
| C4—C5—H5 | 119.6 | C12—O3—C13A | 120.4 (14) |
| C6—C5—H5 | 119.6 | C12—O3—C13 | 114.8 (8) |
| C1—C6—C5 | 119.8 (3) | C13A—O3—C13 | 10.8 (18) |
| C1—C6—H6 | 120.1 | C14—C13—O3 | 107.3 (12) |
| C5—C6—H6 | 120.1 | C14—C13—H13A | 110.3 |
| N1—C7—C10 | 109.45 (15) | O3—C13—H13A | 110.3 |
| N1—C7—C4 | 110.73 (15) | C14—C13—H13B | 110.3 |
| C10—C7—C4 | 113.47 (16) | O3—C13—H13B | 110.3 |
| N1—C7—H7 | 107.7 | H13A—C13—H13B | 108.5 |
| C10—C7—H7 | 107.7 | O3—C13A—C14A | 110.7 (9) |
| C4—C7—H7 | 107.7 | O3—C13A—H13C | 109.5 |
| O1—C8—N1 | 123.56 (18) | C14A—C13A—H13C | 109.5 |
| O1—C8—N2 | 120.66 (19) | O3—C13A—H13D | 109.5 |
| N1—C8—N2 | 115.73 (17) | C14A—C13A—H13D | 109.5 |
| C10—C9—N2 | 120.01 (17) | H13C—C13A—H13D | 108.1 |
| C10—C9—C11 | 124.57 (18) | C13A—C14A—H14D | 109.5 |
| N2—C9—C11 | 115.41 (18) | C13A—C14A—H14E | 109.5 |
| C9—C10—C12 | 122.35 (17) | H14D—C14A—H14E | 109.5 |
| C9—C10—C7 | 119.80 (17) | C13A—C14A—H14F | 109.5 |
| C12—C10—C7 | 117.78 (17) | H14D—C14A—H14F | 109.5 |
| C9—C11—Cl2 | 112.02 (15) | H14E—C14A—H14F | 109.5 |
| C9—C11—H11A | 109.2 | ||
| C6—C1—C2—C3 | −0.1 (5) | C10—C9—C11—Cl2 | 138.10 (19) |
| Cl1—C1—C2—C3 | 179.6 (3) | N2—C9—C11—Cl2 | −42.8 (3) |
| C1—C2—C3—C4 | 1.4 (5) | C9—C10—C12—O2 | 8.7 (4) |
| C2—C3—C4—C5 | −1.7 (5) | C7—C10—C12—O2 | −168.2 (2) |
| C2—C3—C4—C7 | 176.7 (3) | C9—C10—C12—O3 | −173.0 (2) |
| C3—C4—C5—C6 | 0.7 (5) | C7—C10—C12—O3 | 10.1 (3) |
| C7—C4—C5—C6 | −177.7 (3) | O1—C8—N1—C7 | −163.2 (2) |
| C2—C1—C6—C5 | −0.8 (6) | N2—C8—N1—C7 | 19.2 (3) |
| Cl1—C1—C6—C5 | 179.4 (3) | C10—C7—N1—C8 | −31.1 (3) |
| C4—C5—C6—C1 | 0.5 (6) | C4—C7—N1—C8 | 94.8 (2) |
| C3—C4—C7—N1 | −68.6 (3) | O1—C8—N2—C9 | −171.1 (2) |
| C5—C4—C7—N1 | 109.8 (3) | N1—C8—N2—C9 | 6.6 (3) |
| C3—C4—C7—C10 | 55.0 (3) | C10—C9—N2—C8 | −16.8 (3) |
| C5—C4—C7—C10 | −126.6 (3) | C11—C9—N2—C8 | 164.1 (2) |
| N2—C9—C10—C12 | −174.98 (18) | O2—C12—O3—C13A | −3.8 (9) |
| C11—C9—C10—C12 | 4.0 (3) | C10—C12—O3—C13A | 177.8 (9) |
| N2—C9—C10—C7 | 1.8 (3) | O2—C12—O3—C13 | 6.6 (7) |
| C11—C9—C10—C7 | −179.16 (19) | C10—C12—O3—C13 | −171.8 (7) |
| N1—C7—C10—C9 | 19.3 (3) | C12—O3—C13—C14 | 171.9 (10) |
| C4—C7—C10—C9 | −105.0 (2) | C13A—O3—C13—C14 | −65 (9) |
| N1—C7—C10—C12 | −163.80 (17) | C12—O3—C13A—C14A | 103 (2) |
| C4—C7—C10—C12 | 72.0 (2) | C13—O3—C13A—C14A | 42 (7) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H2A···O1i | 0.86 | 2.04 | 2.885 (2) | 166 |
| N1—H1···O2ii | 0.86 | 2.23 | 3.070 (2) | 166 |
| C11—H11B···O1iii | 0.97 | 2.50 | 3.069 (3) | 117 |
| C11—H11A···O2 | 0.97 | 2.14 | 2.814 (3) | 126 |
Symmetry codes: (i) −x+1, −y−1, −z; (ii) x+1, y, z; (iii) x−1, y, z.
Footnotes
Supporting information for this paper is available from the IUCr electronic archives (Reference: SU5005).
References
- Atwal, K. S., Swanson, B. N., Unger, S. E., Floyd, D. M., Moreland, S., Hedberg, A. & O’Reilly, B. C. (1991). J. Med. Chem. 34, 806–811. [DOI] [PubMed]
- Bedia, K. K., Elçin, O., Seda, U., Fatma, K., Nathaly, S., Sevim, R. & Dimoglo, A. (2006). Eur. J. Med. Chem. 41, 1253–1261. [DOI] [PubMed]
- Bruker (2008). APEX2 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Jauk, B., Pernat, T. & Kappe, C. O. (2000). Molecules, 5, 227–239.
- Kato, T. (1984). Chem. Abstr. 102, 132067.
- Nayak, S. K., Venugopala, K. N., Chopra, D., Govender, T., Kruger, H. G., Maguire, G. E. M. & Guru Row, T. N. (2009). Acta Cryst. E65, o2502. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (1996). SADABS, University of G\"ottingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Wipf, P. & Cunningham, A. (1995). Tetrahedron Lett. 36, 7819–7822.
- Yuvaraj, H., Sundaramoorthy, S., Velmurugan, D. & Kalkhambkar, R. G. (2010). Acta Cryst. E66, o3325. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S1600536814023046/su5005sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536814023046/su5005Isup2.hkl
Supporting information file. DOI: 10.1107/S1600536814023046/su5005Isup3.cml
. DOI: 10.1107/S1600536814023046/su5005fig1.tif
The molecular structure of the title molecule, with atom labelling. Displacement ellipsoids are drawn at the 30% probability level.
a . DOI: 10.1107/S1600536814023046/su5005fig2.tif
The crystal packing of the title compound, viewed along the a axis. Hydrogen bonds are shown as dashed lines (see Table 1 for details).
CCDC reference: 1030125
Additional supporting information: crystallographic information; 3D view; checkCIF report