Abstract
In the title molecular salt, C22H22O2P+·PF6 −, the side chain of the cation adopts an anti–gauche conformation [P—C—C—C and C—C—C—C torsion angles = −179.11 (10) and −77.18 (16)°, respectively]. In the crystal, the cations are linked into carboxylic acid inversion dimers by pairs of O—H⋯O hydrogen bonds. Weak C—H⋯F and C—H⋯(F,F) hydrogen bonds connect the components into a three-dimensional network, but there are no aromatic π–π stacking interactions.
Keywords: crystal structure, phosphonium salt, hydrogen bonding
Related literature
For structures of related compounds, see: Li & Mak (1996 ▶); Wu et al. (2007 ▶). For compounds containing related metallated structures, see: Li & Mak (1997 ▶); Sabounchei et al. (2011 ▶). For the use of phosphonium compounds as Wittig reagents, see: Hoffman (2001 ▶), as biocodal agents, see: Kanazawa et al. (1993 ▶) and as phase transfer agents, see: Starks (1971 ▶).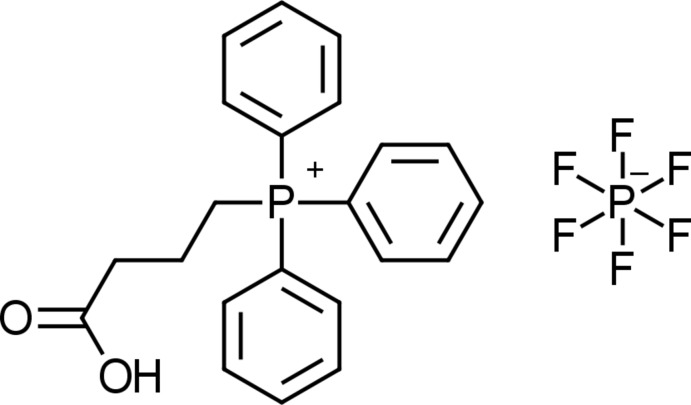
Experimental
Crystal data
C22H22O2P+·PF6 −
M r = 494.33
Triclinic,

a = 9.3307 (1) Å
b = 10.6773 (2) Å
c = 12.8129 (2) Å
α = 72.460 (1)°
β = 82.307 (1)°
γ = 65.495 (1)°
V = 1107.46 (3) Å3
Z = 2
Mo Kα radiation
μ = 0.26 mm−1
T = 100 K
0.29 × 0.16 × 0.07 mm
Data collection
Bruker APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2014 ▶) T min = 0.865, T max = 0.947
37843 measured reflections
5269 independent reflections
4426 reflections with I > 2σ(I)
R int = 0.035
Refinement
R[F 2 > 2σ(F 2)] = 0.034
wR(F 2) = 0.087
S = 1.06
5269 reflections
290 parameters
H-atom parameters constrained
Δρmax = 0.42 e Å−3
Δρmin = −0.36 e Å−3
Data collection: APEX2 (Bruker, 2014 ▶); cell refinement: SAINT (Bruker, 2013 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXT (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL2014 (Sheldrick, 2008 ▶); molecular graphics: OLEX2 (Dolomanov et al., 2009 ▶); software used to prepare material for publication: OLEX2.
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S160053681402323X/hb7304sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S160053681402323X/hb7304Isup2.hkl
Supporting information file. DOI: 10.1107/S160053681402323X/hb7304Isup3.mol
. DOI: 10.1107/S160053681402323X/hb7304fig1.tif
Crystal structure and labeling scheme of compound (1). 50% probablility ellipsoids. Phosphorous is in green, oxygen in red, fluorine in purple, and carbon in grey.
CCDC reference: 1030392
Additional supporting information: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (, ).
| DHA | DH | HA | D A | DHA |
|---|---|---|---|---|
| O1H1O2i | 0.84 | 1.80 | 2.6285(15) | 171 |
| C1H1AF2 | 0.99 | 2.48 | 3.455(2) | 168 |
| C1H1AF3 | 0.99 | 2.50 | 3.1656(19) | 124 |
| C22H22F4ii | 0.95 | 2.51 | 3.3924(18) | 155 |
Symmetry codes: (i) 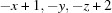 ; (ii)
; (ii)  .
.
Acknowledgments
The authors wish to acknowledge the Mississippi State University Department of Chemistry for funding.
supplementary crystallographic information
S1. Synthesis and crystallization
A 1.0g (2.3mmol) sample of 3-carboxypropyltriphenylphosphonium chloride and 0.4g (2.4mmol) of sodium hexafluorophosphate were dissolved in 40mL of water. A white precipitate immediately formed and the slurry was stirred for 1 hour. The mixture was filtered, the solid was washed with water (3 x 25mL), and dried under high vacuum to yield a white solid. Yield: 0.65g (80.8%). Single crystals suitable for X-ray diffraction were grown from slow evaporation of dichloromethane. 1H NMR (CHLOROFORM-d ,300MHz): δ = 7.90–8.02 (m, 9H), 7.77–7.89 (m, 6H), 3.54–3.72 (m, 2H), 2.66 (t, J = 6.6 Hz, 2H), 2.04–2.07 p.p.m. (m, 2H). 13C NMR (CHLOROFORM-d ,75MHz): δ = 174.0, 136.4, 134.9, 131.6, 120.3, 119.1, 34.2, 34.0, 22.0, 19.2 p.p.m. HRMS (ESI–TOF) m/z: [M+] Calcd for C22H22O2P+ 349.381; found 349.1355. [M-] Calcd for PF6 144.965; found 144.9632.
S2. Refinement
Carbon-bound H-atoms were placed in calculated positions (C—H = 0.95 to 0.99 Å, O—H = 0.84 Å) and were included in the refinement in the riding model approximation with Uiso(H) = 1.2Ueq(C) or 1.5Ueq(O).
S3. Comment
Organic phosphonium cations have been used as phase transfer catalysts (Starks, 1971), biocidal agents (Kanazawa et al. 1993), and as reagents for Wittig reactions (Hoffman, 2001). There are few examples in the crystallographic literature, however, of triphenylphosphonium cations bearing a carboxylic acid functional group.
In the title compound, 3-carboxypropyltriphenylphosphonium hexafluorophosphate (Fig. 1), crystallizes in a triclinic unit cell with a single cation-anion pair in the asymmetric unit. The dominant intermolecular interactions is hydrogen bonding from the carboxylic acid moiety on the cation (Table 1). The alkyl chain attached to the phosphorous deviates from the expected staggered conformation, showing a rotation at the C1—C2 carbons. This twist in the carbons is likely the cause of the unusual torsion angles observed in the three phenyl rings (Table 2). The phenyl ring that is located under the C2 hydrogens is nearly perpendicular when compared to the other two rings. It is suspected that this perpendicular arrangement of the phenyl ring is assumed to minimize potential steric interactions with the bent portion of the alkyl chain. Interestingly, there are no observed π–π interactions from any of the phenyl rings and there are no weak C—H···F interactions.
Figures
Fig. 1.
Crystal structure and labeling scheme of compound (1). 50% probablility ellipsoids. Phosphorous is in green, oxygen in red, fluorine in purple, and carbon in grey.
Crystal data
| C22H22O2P+·PF6− | Z = 2 |
| Mr = 494.33 | F(000) = 508 |
| Triclinic, P1 | Dx = 1.482 Mg m−3 |
| a = 9.3307 (1) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 10.6773 (2) Å | Cell parameters from 9960 reflections |
| c = 12.8129 (2) Å | θ = 2.4–27.8° |
| α = 72.460 (1)° | µ = 0.26 mm−1 |
| β = 82.307 (1)° | T = 100 K |
| γ = 65.495 (1)° | Block, colourless |
| V = 1107.46 (3) Å3 | 0.29 × 0.16 × 0.07 mm |
Data collection
| Bruker APEXII CCD diffractometer | 5269 independent reflections |
| Radiation source: fine-focus sealed tube | 4426 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.035 |
| Detector resolution: 7.9 pixels mm-1 | θmax = 27.9°, θmin = 1.7° |
| ω and φ scans | h = −12→11 |
| Absorption correction: multi-scan (SADABS; Bruker, 2014) | k = −14→14 |
| Tmin = 0.865, Tmax = 0.947 | l = −16→16 |
| 37843 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.034 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.087 | H-atom parameters constrained |
| S = 1.06 | w = 1/[σ2(Fo2) + (0.0364P)2 + 0.4848P] where P = (Fo2 + 2Fc2)/3 |
| 5269 reflections | (Δ/σ)max = 0.001 |
| 290 parameters | Δρmax = 0.42 e Å−3 |
| 0 restraints | Δρmin = −0.36 e Å−3 |
Special details
| Experimental. Absorption correction: SADABS-2014/2 (Bruker, 2014) was used for absorption correction. wR2(int) was 0.0583 before and 0.0488 after correction. The Ratio of minimum to maximum transmission is 0.9133. The λ/2 correction factor is 0.00150. |
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| P1 | 0.42475 (4) | 0.61911 (4) | 0.72374 (3) | 0.01582 (9) | |
| P2 | 0.21110 (5) | 0.22893 (4) | 0.72342 (3) | 0.02490 (10) | |
| F2 | 0.18704 (11) | 0.33829 (11) | 0.79338 (8) | 0.0344 (2) | |
| F6 | 0.13566 (12) | 0.36150 (10) | 0.61884 (8) | 0.0352 (2) | |
| F4 | 0.23378 (12) | 0.12048 (10) | 0.65303 (8) | 0.0342 (2) | |
| F5 | 0.03862 (12) | 0.23173 (12) | 0.76017 (8) | 0.0387 (3) | |
| F3 | 0.38172 (12) | 0.22834 (11) | 0.68553 (9) | 0.0406 (3) | |
| O2 | 0.48983 (12) | 0.17127 (10) | 0.96794 (8) | 0.0235 (2) | |
| F1 | 0.28391 (14) | 0.09741 (11) | 0.82789 (9) | 0.0453 (3) | |
| O1 | 0.66794 (13) | −0.01961 (11) | 0.91753 (10) | 0.0295 (3) | |
| H1 | 0.6104 | −0.0598 | 0.9557 | 0.044* | |
| C5 | 0.58121 (16) | 0.67621 (14) | 0.71818 (11) | 0.0172 (3) | |
| C18 | 0.23325 (17) | 0.57402 (16) | 0.90634 (11) | 0.0208 (3) | |
| H18 | 0.2785 | 0.4755 | 0.9084 | 0.025* | |
| C11 | 0.32167 (16) | 0.70037 (15) | 0.59658 (11) | 0.0184 (3) | |
| C17 | 0.28593 (16) | 0.67064 (15) | 0.82977 (11) | 0.0182 (3) | |
| C4 | 0.60945 (17) | 0.11522 (15) | 0.91839 (11) | 0.0200 (3) | |
| C1 | 0.51033 (16) | 0.42771 (14) | 0.74813 (12) | 0.0188 (3) | |
| H1A | 0.4262 | 0.3910 | 0.7713 | 0.023* | |
| H1B | 0.5569 | 0.4039 | 0.6789 | 0.023* | |
| C12 | 0.25839 (16) | 0.62504 (16) | 0.55808 (12) | 0.0217 (3) | |
| H12 | 0.2804 | 0.5273 | 0.5944 | 0.026* | |
| C2 | 0.63811 (16) | 0.35274 (15) | 0.83609 (12) | 0.0207 (3) | |
| H2A | 0.7222 | 0.3896 | 0.8137 | 0.025* | |
| H2B | 0.5918 | 0.3743 | 0.9060 | 0.025* | |
| C6 | 0.62009 (17) | 0.69945 (15) | 0.80985 (12) | 0.0213 (3) | |
| H6 | 0.5526 | 0.7019 | 0.8723 | 0.026* | |
| C19 | 0.11368 (17) | 0.62379 (17) | 0.97956 (12) | 0.0243 (3) | |
| H19 | 0.0781 | 0.5585 | 1.0325 | 0.029* | |
| C22 | 0.22021 (17) | 0.81526 (15) | 0.82747 (12) | 0.0239 (3) | |
| H22 | 0.2574 | 0.8806 | 0.7762 | 0.029* | |
| C13 | 0.16332 (17) | 0.69379 (18) | 0.46650 (12) | 0.0255 (3) | |
| H13 | 0.1208 | 0.6429 | 0.4394 | 0.031* | |
| C3 | 0.70880 (17) | 0.19153 (15) | 0.85265 (13) | 0.0240 (3) | |
| H3A | 0.7292 | 0.1726 | 0.7799 | 0.029* | |
| H3B | 0.8116 | 0.1506 | 0.8893 | 0.029* | |
| C14 | 0.13030 (18) | 0.83680 (18) | 0.41448 (12) | 0.0276 (3) | |
| H14 | 0.0626 | 0.8843 | 0.3530 | 0.033* | |
| C16 | 0.29145 (19) | 0.84353 (16) | 0.54243 (13) | 0.0262 (3) | |
| H16 | 0.3363 | 0.8942 | 0.5677 | 0.031* | |
| C7 | 0.75827 (17) | 0.71902 (16) | 0.80929 (13) | 0.0253 (3) | |
| H7 | 0.7852 | 0.7355 | 0.8714 | 0.030* | |
| C8 | 0.85698 (18) | 0.71457 (17) | 0.71847 (14) | 0.0275 (3) | |
| H8 | 0.9528 | 0.7254 | 0.7193 | 0.033* | |
| C10 | 0.67893 (18) | 0.67535 (17) | 0.62542 (12) | 0.0251 (3) | |
| H10 | 0.6511 | 0.6618 | 0.5622 | 0.030* | |
| C21 | 0.10050 (19) | 0.86258 (17) | 0.90047 (13) | 0.0296 (3) | |
| H21 | 0.0552 | 0.9609 | 0.8990 | 0.035* | |
| C20 | 0.04614 (17) | 0.76728 (18) | 0.97598 (12) | 0.0271 (3) | |
| H20 | −0.0373 | 0.8007 | 1.0251 | 0.033* | |
| C9 | 0.81677 (19) | 0.69444 (19) | 0.62641 (14) | 0.0316 (4) | |
| H9 | 0.8839 | 0.6937 | 0.5637 | 0.038* | |
| C15 | 0.1951 (2) | 0.91087 (18) | 0.45130 (13) | 0.0315 (4) | |
| H15 | 0.1735 | 1.0083 | 0.4141 | 0.038* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| P1 | 0.01569 (17) | 0.01745 (17) | 0.01619 (17) | −0.00773 (13) | 0.00018 (13) | −0.00564 (13) |
| P2 | 0.0302 (2) | 0.0301 (2) | 0.0236 (2) | −0.01993 (18) | −0.00010 (16) | −0.00870 (16) |
| F2 | 0.0362 (5) | 0.0439 (6) | 0.0369 (5) | −0.0220 (5) | 0.0021 (4) | −0.0225 (5) |
| F6 | 0.0485 (6) | 0.0327 (5) | 0.0294 (5) | −0.0224 (5) | −0.0021 (4) | −0.0055 (4) |
| F4 | 0.0490 (6) | 0.0336 (5) | 0.0328 (5) | −0.0257 (5) | −0.0006 (4) | −0.0132 (4) |
| F5 | 0.0403 (6) | 0.0640 (7) | 0.0306 (5) | −0.0381 (5) | 0.0065 (4) | −0.0166 (5) |
| F3 | 0.0308 (5) | 0.0479 (6) | 0.0599 (7) | −0.0238 (5) | 0.0105 (5) | −0.0308 (5) |
| O2 | 0.0238 (5) | 0.0203 (5) | 0.0248 (5) | −0.0076 (4) | 0.0042 (4) | −0.0074 (4) |
| F1 | 0.0651 (7) | 0.0398 (6) | 0.0371 (6) | −0.0275 (5) | −0.0218 (5) | −0.0001 (5) |
| O1 | 0.0294 (6) | 0.0194 (5) | 0.0389 (7) | −0.0107 (5) | 0.0116 (5) | −0.0102 (5) |
| C5 | 0.0165 (6) | 0.0172 (6) | 0.0193 (7) | −0.0080 (5) | 0.0001 (5) | −0.0053 (5) |
| C18 | 0.0214 (7) | 0.0237 (7) | 0.0199 (7) | −0.0115 (6) | −0.0009 (5) | −0.0058 (6) |
| C11 | 0.0157 (6) | 0.0226 (7) | 0.0173 (6) | −0.0076 (5) | 0.0014 (5) | −0.0070 (5) |
| C17 | 0.0149 (6) | 0.0221 (7) | 0.0175 (6) | −0.0070 (5) | −0.0012 (5) | −0.0054 (5) |
| C4 | 0.0216 (7) | 0.0181 (6) | 0.0192 (7) | −0.0060 (5) | −0.0033 (5) | −0.0052 (5) |
| C1 | 0.0192 (7) | 0.0192 (6) | 0.0209 (7) | −0.0089 (5) | 0.0005 (5) | −0.0077 (5) |
| C12 | 0.0193 (7) | 0.0283 (7) | 0.0208 (7) | −0.0119 (6) | 0.0023 (5) | −0.0087 (6) |
| C2 | 0.0182 (7) | 0.0185 (7) | 0.0260 (7) | −0.0077 (5) | −0.0021 (5) | −0.0054 (6) |
| C6 | 0.0210 (7) | 0.0230 (7) | 0.0219 (7) | −0.0089 (6) | 0.0002 (6) | −0.0087 (6) |
| C19 | 0.0216 (7) | 0.0361 (8) | 0.0194 (7) | −0.0169 (6) | 0.0012 (6) | −0.0060 (6) |
| C22 | 0.0244 (7) | 0.0200 (7) | 0.0238 (7) | −0.0068 (6) | 0.0029 (6) | −0.0053 (6) |
| C13 | 0.0201 (7) | 0.0415 (9) | 0.0212 (7) | −0.0151 (7) | 0.0017 (6) | −0.0138 (6) |
| C3 | 0.0186 (7) | 0.0208 (7) | 0.0296 (8) | −0.0061 (6) | 0.0011 (6) | −0.0055 (6) |
| C14 | 0.0199 (7) | 0.0402 (9) | 0.0183 (7) | −0.0071 (6) | −0.0011 (6) | −0.0084 (6) |
| C16 | 0.0319 (8) | 0.0241 (7) | 0.0248 (8) | −0.0124 (6) | −0.0042 (6) | −0.0060 (6) |
| C7 | 0.0233 (7) | 0.0252 (7) | 0.0307 (8) | −0.0088 (6) | −0.0061 (6) | −0.0108 (6) |
| C8 | 0.0209 (7) | 0.0291 (8) | 0.0361 (9) | −0.0142 (6) | −0.0014 (6) | −0.0070 (7) |
| C10 | 0.0264 (8) | 0.0345 (8) | 0.0204 (7) | −0.0167 (7) | 0.0038 (6) | −0.0107 (6) |
| C21 | 0.0264 (8) | 0.0255 (8) | 0.0293 (8) | −0.0020 (6) | 0.0028 (6) | −0.0102 (6) |
| C20 | 0.0175 (7) | 0.0396 (9) | 0.0217 (7) | −0.0071 (6) | 0.0029 (6) | −0.0122 (7) |
| C9 | 0.0264 (8) | 0.0440 (10) | 0.0297 (8) | −0.0213 (7) | 0.0081 (7) | −0.0108 (7) |
| C15 | 0.0356 (9) | 0.0268 (8) | 0.0251 (8) | −0.0078 (7) | −0.0054 (7) | −0.0018 (6) |
Geometric parameters (Å, º)
| P1—C5 | 1.7867 (14) | C2—H2B | 0.9900 |
| P1—C11 | 1.7910 (14) | C2—C3 | 1.5227 (19) |
| P1—C17 | 1.7930 (14) | C6—H6 | 0.9500 |
| P1—C1 | 1.8000 (14) | C6—C7 | 1.388 (2) |
| P2—F2 | 1.6053 (10) | C19—H19 | 0.9500 |
| P2—F6 | 1.6054 (10) | C19—C20 | 1.382 (2) |
| P2—F4 | 1.6044 (10) | C22—H22 | 0.9500 |
| P2—F5 | 1.6058 (10) | C22—C21 | 1.384 (2) |
| P2—F3 | 1.5990 (10) | C13—H13 | 0.9500 |
| P2—F1 | 1.5951 (11) | C13—C14 | 1.386 (2) |
| O2—C4 | 1.2216 (17) | C3—H3A | 0.9900 |
| O1—H1 | 0.8400 | C3—H3B | 0.9900 |
| O1—C4 | 1.3140 (17) | C14—H14 | 0.9500 |
| C5—C6 | 1.3919 (19) | C14—C15 | 1.382 (2) |
| C5—C10 | 1.3977 (19) | C16—H16 | 0.9500 |
| C18—H18 | 0.9500 | C16—C15 | 1.388 (2) |
| C18—C17 | 1.3964 (19) | C7—H7 | 0.9500 |
| C18—C19 | 1.391 (2) | C7—C8 | 1.384 (2) |
| C11—C12 | 1.396 (2) | C8—H8 | 0.9500 |
| C11—C16 | 1.397 (2) | C8—C9 | 1.384 (2) |
| C17—C22 | 1.3975 (19) | C10—H10 | 0.9500 |
| C4—C3 | 1.496 (2) | C10—C9 | 1.385 (2) |
| C1—H1A | 0.9900 | C21—H21 | 0.9500 |
| C1—H1B | 0.9900 | C21—C20 | 1.390 (2) |
| C1—C2 | 1.5352 (19) | C20—H20 | 0.9500 |
| C12—H12 | 0.9500 | C9—H9 | 0.9500 |
| C12—C13 | 1.386 (2) | C15—H15 | 0.9500 |
| C2—H2A | 0.9900 | ||
| C5—P1—C11 | 110.19 (6) | C3—C2—C1 | 110.82 (12) |
| C5—P1—C17 | 110.68 (6) | C3—C2—H2A | 109.5 |
| C5—P1—C1 | 107.80 (6) | C3—C2—H2B | 109.5 |
| C11—P1—C17 | 107.83 (6) | C5—C6—H6 | 120.3 |
| C11—P1—C1 | 109.51 (6) | C7—C6—C5 | 119.50 (13) |
| C17—P1—C1 | 110.83 (7) | C7—C6—H6 | 120.3 |
| F2—P2—F6 | 89.92 (5) | C18—C19—H19 | 119.7 |
| F2—P2—F5 | 89.94 (5) | C20—C19—C18 | 120.63 (14) |
| F6—P2—F5 | 89.47 (6) | C20—C19—H19 | 119.7 |
| F4—P2—F2 | 179.53 (6) | C17—C22—H22 | 120.3 |
| F4—P2—F6 | 89.72 (5) | C21—C22—C17 | 119.49 (14) |
| F4—P2—F5 | 89.76 (5) | C21—C22—H22 | 120.3 |
| F3—P2—F2 | 89.89 (5) | C12—C13—H13 | 120.0 |
| F3—P2—F6 | 89.71 (6) | C12—C13—C14 | 119.99 (14) |
| F3—P2—F4 | 90.41 (5) | C14—C13—H13 | 120.0 |
| F3—P2—F5 | 179.17 (7) | C4—C3—C2 | 115.15 (12) |
| F1—P2—F2 | 89.99 (6) | C4—C3—H3A | 108.5 |
| F1—P2—F6 | 179.28 (6) | C4—C3—H3B | 108.5 |
| F1—P2—F4 | 90.37 (6) | C2—C3—H3A | 108.5 |
| F1—P2—F5 | 89.82 (6) | C2—C3—H3B | 108.5 |
| F1—P2—F3 | 91.00 (6) | H3A—C3—H3B | 107.5 |
| C4—O1—H1 | 109.5 | C13—C14—H14 | 119.7 |
| C6—C5—P1 | 120.87 (11) | C15—C14—C13 | 120.53 (14) |
| C6—C5—C10 | 120.30 (13) | C15—C14—H14 | 119.7 |
| C10—C5—P1 | 118.10 (11) | C11—C16—H16 | 120.4 |
| C17—C18—H18 | 120.4 | C15—C16—C11 | 119.24 (15) |
| C19—C18—H18 | 120.4 | C15—C16—H16 | 120.4 |
| C19—C18—C17 | 119.17 (13) | C6—C7—H7 | 119.9 |
| C12—C11—P1 | 119.73 (11) | C8—C7—C6 | 120.18 (14) |
| C12—C11—C16 | 120.37 (13) | C8—C7—H7 | 119.9 |
| C16—C11—P1 | 119.58 (11) | C7—C8—H8 | 119.8 |
| C18—C17—P1 | 121.95 (11) | C7—C8—C9 | 120.35 (14) |
| C18—C17—C22 | 120.34 (13) | C9—C8—H8 | 119.8 |
| C22—C17—P1 | 117.45 (11) | C5—C10—H10 | 120.3 |
| O2—C4—O1 | 124.32 (13) | C9—C10—C5 | 119.44 (14) |
| O2—C4—C3 | 123.95 (13) | C9—C10—H10 | 120.3 |
| O1—C4—C3 | 111.72 (12) | C22—C21—H21 | 119.8 |
| P1—C1—H1A | 109.1 | C22—C21—C20 | 120.45 (14) |
| P1—C1—H1B | 109.1 | C20—C21—H21 | 119.8 |
| H1A—C1—H1B | 107.8 | C19—C20—C21 | 119.90 (14) |
| C2—C1—P1 | 112.67 (9) | C19—C20—H20 | 120.1 |
| C2—C1—H1A | 109.1 | C21—C20—H20 | 120.1 |
| C2—C1—H1B | 109.1 | C8—C9—C10 | 120.20 (14) |
| C11—C12—H12 | 120.2 | C8—C9—H9 | 119.9 |
| C13—C12—C11 | 119.56 (14) | C10—C9—H9 | 119.9 |
| C13—C12—H12 | 120.2 | C14—C15—C16 | 120.28 (15) |
| C1—C2—H2A | 109.5 | C14—C15—H15 | 119.9 |
| C1—C2—H2B | 109.5 | C16—C15—H15 | 119.9 |
| H2A—C2—H2B | 108.1 | ||
| P1—C5—C6—C7 | 168.72 (11) | C17—P1—C11—C12 | −92.86 (12) |
| P1—C5—C10—C9 | −168.71 (12) | C17—P1—C11—C16 | 80.66 (13) |
| P1—C11—C12—C13 | 172.55 (11) | C17—P1—C1—C2 | −77.68 (11) |
| P1—C11—C16—C15 | −172.12 (12) | C17—C18—C19—C20 | 0.8 (2) |
| P1—C17—C22—C21 | 173.20 (12) | C17—C22—C21—C20 | 0.3 (2) |
| P1—C1—C2—C3 | −179.11 (10) | C1—P1—C5—C6 | −96.79 (12) |
| O2—C4—C3—C2 | −9.1 (2) | C1—P1—C5—C6 | −96.79 (12) |
| O1—C4—C3—C2 | 171.88 (13) | C1—P1—C5—C10 | 73.39 (13) |
| C5—P1—C11—C12 | 146.24 (11) | C1—P1—C11—C12 | 27.83 (13) |
| C5—P1—C11—C16 | −40.23 (13) | C1—P1—C11—C12 | 27.83 (13) |
| C5—P1—C17—C18 | −131.04 (12) | C1—P1—C11—C16 | −158.65 (11) |
| C5—P1—C17—C22 | 54.76 (13) | C1—P1—C17—C18 | −11.48 (14) |
| C5—P1—C1—C2 | 43.58 (12) | C1—P1—C17—C18 | −11.48 (14) |
| C5—C6—C7—C8 | −0.4 (2) | C1—P1—C17—C22 | 174.32 (11) |
| C5—C10—C9—C8 | −0.2 (2) | C1—C2—C3—C4 | −77.18 (16) |
| C18—C17—C22—C21 | −1.1 (2) | C12—C11—C16—C15 | 1.4 (2) |
| C18—C19—C20—C21 | −1.6 (2) | C12—C13—C14—C15 | 1.8 (2) |
| C11—P1—C5—C6 | 143.74 (11) | C6—C5—C10—C9 | 1.5 (2) |
| C11—P1—C5—C10 | −46.07 (13) | C6—C7—C8—C9 | 1.7 (2) |
| C11—P1—C17—C18 | 108.38 (12) | C19—C18—C17—P1 | −173.47 (11) |
| C11—P1—C17—C22 | −65.82 (13) | C19—C18—C17—C22 | 0.6 (2) |
| C11—P1—C1—C2 | 163.47 (10) | C22—C21—C20—C19 | 1.1 (2) |
| C11—C12—C13—C14 | −0.7 (2) | C13—C14—C15—C16 | −1.4 (2) |
| C11—C16—C15—C14 | −0.2 (2) | C16—C11—C12—C13 | −0.9 (2) |
| C17—P1—C5—C6 | 24.57 (14) | C7—C8—C9—C10 | −1.5 (3) |
| C17—P1—C5—C10 | −165.24 (11) | C10—C5—C6—C7 | −1.2 (2) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1···O2i | 0.84 | 1.80 | 2.6285 (15) | 171 |
| C1—H1A···F2 | 0.99 | 2.48 | 3.455 (2) | 168 |
| C1—H1A···F3 | 0.99 | 2.50 | 3.1656 (19) | 124 |
| C22—H22···F4ii | 0.95 | 2.51 | 3.3924 (18) | 155 |
Symmetry codes: (i) −x+1, −y, −z+2; (ii) x, y+1, z.
Footnotes
Supporting information for this paper is available from the IUCr electronic archives (Reference: HB7304).
References
- Bruker (2013). SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2014). APEX2 and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Hoffman, R. W. (2001). Angew. Chem. Int. Ed. 40, 1411–1416.
- Kanazawa, A., Ikeda, T. & Endo, T. (1993). J. Polym. Sci. A Polym. Chem. 31, 1467–1472.
- Li, S.-L. & Mak, T. C. W. (1996). J. Mol. Struct. 384, 135–148.
- Li, S.-L. & Mak, T. C. W. (1997). Polyhedron, 16, 199–205.
- Sabounchei, S. J., Salehzadeh, S., Hosseinzadeh, M., Bagherjeri, F. A. & Khavasi, H. R. (2011). Polyhedron, 30, 2486–2492.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Starks, C. M. (1971). J. Am. Chem. Soc. 93, 195–199.
- Wu, D.-Y., Li, F.-S., Xia, J.-Y., Mao, N.-W. & Yao, H.-L. (2007). Acta Cryst. E63, o4532.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S160053681402323X/hb7304sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S160053681402323X/hb7304Isup2.hkl
Supporting information file. DOI: 10.1107/S160053681402323X/hb7304Isup3.mol
. DOI: 10.1107/S160053681402323X/hb7304fig1.tif
Crystal structure and labeling scheme of compound (1). 50% probablility ellipsoids. Phosphorous is in green, oxygen in red, fluorine in purple, and carbon in grey.
CCDC reference: 1030392
Additional supporting information: crystallographic information; 3D view; checkCIF report



