In three potentially anticonvulsant compounds, of which two are isoindoline derivatives and one an isoquinoline derivative, the central moiety is planar. In the crystals of all three compounds, there are C—H⋯O hydrogen bonds present linking the molecules into two-dimensional slabs for the isoindoline derivatives, and into a three-dimensional framework for the isoquinoline derivative.
Keywords: crystal structure, anticonvulsant, isoindoline, isoquinoline, indoline
Abstract
The title compounds, C9H7NO3, (1), C10H7NO5, (2), and C14H9NO5, (3), are three potentially anticonvulsant compounds. Compounds (1) and (2) are isoindoline derivatives and (3) is an isoquinoline derivative. Compounds (2) and (3) crystallize with two independent molecules (A and B) in their asymmetric units. In all three cases, the isoindoline and benzoisoquinoline moieties are planar [r.m.s. deviations are 0.021 Å for (1), 0.04 and 0.018 Å for (2), and 0.033 and 0.041 Å for (3)]. The substituents attached to the N atom are almost perpendicular to the mean planes of the heterocycles, with dihedral angles of 89.7 (3)° for the N—O—Cmethyl group in (1), 71.01 (4) and 80.00 (4)° for the N—O—C(=O)O—Cmethyl groups in (2), and 75.62 (14) and 74.13 (4)° for the same groups in (3). In the crystal of (1), there are unusual intermolecular C=O⋯C contacts of 2.794 (1) and 2.873 (1) Å present in molecules A and B, respectively. There are also C—H⋯O hydrogen bonds and π–π interactions [inter-centroid distance = 3.407 (3) Å] present, forming slabs lying parallel to (001). In the crystal of (2), the A and B molecules are linked by C—H⋯O hydrogen bonds, forming slabs parallel to (10-1), which are in turn linked via a number of π–π interactions [the most significant centroid–centroid distances are 3.4202 (7) and 3.5445 (7) Å], forming a three-dimensional structure. In the crystal of (3), the A and B molecules are linked via C—H⋯O hydrogen bonds, forming a three-dimensional structure, which is consolidated by π–π interactions [the most significant inter-centroid distances are 3.575 (3) and 3.578 (3) Å].
Chemical context
Traumatic brain injury (TBI) is a neurological disorder that is defined as damage to the brain resulting from external mechanical force, including accelerating, decelerating and rotating forces (Langlois et al., 2003 ▶, 2005 ▶; Ashman et al., 2006 ▶; Coronado et al., 2011 ▶). TBI also exacerbates seizure severity in individuals with pre-existing epilepsy (Ferraro et al., 1999 ▶), being one example of the process of epileptogenesis (Christensen et al., 2009 ▶). In this context, it has been demonstrated that early lesions in the central nervous system (CNS) alter the transport dynamic of the blood–brain barrier (BBB) and deteriorate the balance of the inhibitory and excitatory neurotransmitter system (Scantlebury et al., 2005 ▶]. This neuronal dysfunction predisposes to subsequent development of spontaneous recurrent seizures in the presence of prior subtle brain malformation (Love, 2005 ▶].
TBI is the major cause of death in young individuals (14–24 years) from industrialized countries, with head injuries accounting for 25–33% of all trauma-related deaths (Abdul-Muneer et al., 2014 ▶). Disorders like memory loss, depression and seizures are some of the side effects to TBI. TBI affects people over 75 years of age because of falls and of 17–25 years of age because of accidents (Langlois et al., 2003 ▶, 2005 ▶; Ashman et al., 2006 ▶; Coronado et al., 2011 ▶). At present, there are no effective treatments available for TBI and there is thus a critical need to develop novel and effective strategies to alter the disease course. As indicated above, this health condition is quite similar to epilepsy in some instances and thus our earlier work (Alexander et al., 2013 ▶; Jackson et al., 2012 ▶; Edafiogho et al., 2007 ▶) on developing anticonvulsant compounds for the treatment of epilepsy is relevant.
Our research on pharmacologically active compounds is a multi-pronged approach, which involves synthesis, chemical characterization, computer modeling, pharmacological evaluation, and structure determination (North et al., 2012 ▶; Gibson et al., 2009 ▶). From this comprehensive approach, structure–activity correlations can be made to improve the existing pharmacologically active compounds. From our studies, we identified three imidooxy derivatives as potential drug candidates for TBI that underwent anticonvulsant evaluation to test their ability to inhibit the onset of seizures in the in vivo MES, scPTZ test models. The MES (maximal electroshock seizure evaluation) test presented activity in animals in phase 1 testing.
2-Methoxyisoindoline-1,3-dione, (1), studied by X-ray techniques, was inactive in MES and scPTZ in mice, but showed MES protection in rat studies at 50 mg kg−1 at 4 h and also protected 1/4 mice at three different time intervals (0.50, 1 and 2 h) in the 6 Hz test (Jackson, 2009 ▶). For scPTZ studies, the compound was Class III (no activity at 300 mg kg−1). The compound is a dual MES/6Hz active compound. Compounds (2) and (3) showed similar activity.
The title compounds, containing either an isoindoline-1,3-dione moiety, (1) (Fig. 1 ▶) and (2) (Fig. 2 ▶), or an isoquinoline-1,3-dione moiety, (3) (Fig. 3 ▶), have been studied extensively for their anticonvulsant effects with promising results. Herein, we report on the crystal structures of these new structurally related compounds.
Figure 1.
The molecular structure of compound (1), with atom labelling. Displacement ellipsoids are drawn at the 30% probability level.
Figure 2.
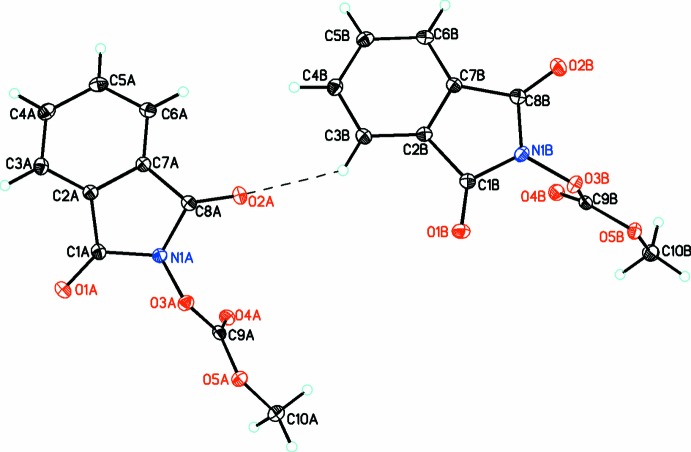
The molecular structures of the two independent molecules (A and B) of compound (2), with atom labelling. Displacement ellipsoids are drawn at the 30% probability level. The C—H⋯O hydrogen bond is shown as a dashed line (see Table 2 ▶ for details).
Figure 3.

The molecular structures of the two independent molecules (A and B) of compound (3), with atom labelling. Displacement ellipsoids are drawn at the 30% probability level. The C—H⋯O hydrogen bond is shown as a dashed line (see Table 3 ▶ for details).
Structural commentary
In compound (1), the isoindoline ring is planar [r.m.s. deviation = 0.017 (4) Å]. The methoxy O atom, O3, deviates from this plane by 0.176 (6) Å while the methyl C atom, C9, is out of the plane by 1.105 (9) Å. The methoxy substituent is oriented almost perpendicular to the indoline ring with the dihedral angle between the mean planes of the indoline ring and the methoxy substituent being 89.7 (3)°.
In compound (2), there are two molecules (A and B) in the asymmetric unit. The isoindoline ring is planar [r.m.s. deviation = 0.0327 (9) for A and 0.0147 (9) Å for B] with the dione O atoms significantly out of the plane for molecule A but not for molecule B [0.172 (1) and 0.123 (1) Å for atoms O1 and O2, respectively, in A but by only 0.013 (1) and 0.002 (1) Å, respectively, in B]. The carbonato moiety is planar in both molecules [r.m.s. deviations of 0.0066 (2) and 0.0027 (5) Å for A and B, respectively] and makes dihedral angles of 71.50 (3) and 80.03 (4)° with the benzoisoquinoline ring in A and B, respectively, indicating that these substituents are oriented almost perpendicular to the benzoisoquinoline ring system.
In compound (3), there are also two molecules (A and B) in the asymmetric unit. In both molecules, the benzoisoquinoline ring systems are planar (r.m.s. deviations for A and B = 0.033 and 0.015 Å, respectively). The methoxy O atom deviates from this plane by 0.126 (1) for atom O5A in A and 0.156 (1) Å for atom O5B in B. The methyl carbonate moieties are planar [r.m.s. deviations of 0.007 (1) and 0.003 (1) Å for A and B, respectively] and these substituents are oriented almost perpendicular to the isoquinoline rings, making dihedral angles of 71.50 (3) and 80.04 (4)° for A and B, respectively. As in (2), these dihedral angles are significantly smaller than that found for (1).
Supramolecular features
In the crystal of (1), there are C—H⋯O hydrogen bonds (Fig. 4 ▶ and Table 1 ▶) and π–π interactions present, forming slabs lying parallel to (001) [Cg1⋯Cg2i,ii = 3.407 (3) Å; Cg1 and Cg2 are the centroids of rings N1/C1/C2/C7/C8 and C2–C7, respectively; symmetry codes: (i) x − 1, y, z; (ii) x + 1, y, z].
Figure 4.
A view along the a axis of the crystal packing of compound (1), showing the formation of the three-dimensional array by an extensive network of C—H⋯O hydrogen bonds (shown as dashed lines; see Table 1 ▶ for details).
Table 1. Hydrogen-bond geometry (, ) for (1) .
| DHA | DH | HA | D A | DHA |
|---|---|---|---|---|
| C4H4AO2i | 0.95 | 2.38 | 3.190(4) | 143 |
| C9H9AO1ii | 0.98 | 2.54 | 3.428(7) | 151 |
| C9H9BO1iii | 0.98 | 2.53 | 3.260(8) | 131 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  .
.
In the crystal of (2), the A and B molecules are linked by C—H⋯O hydrogen bonds (Fig. 5 ▶ and Table 2 ▶), forming slabs parallel to (10 ). The slabs are in turn linked via π–π interactions, forming a three-dimensional structure with centroid–centroid distances of 3.4202 (7) for Cg1⋯Cg5ii and 3.5445 (7) Å for Cg2⋯Cg4ii [Cg1, Cg2, Cg4 and Cg5 are the centroids of rings N1A/C1A/C2A/C7A/C8A, C2A–C7A, N1B/C1B/C2B/C7B/C8B and C2B–C7B, respectively; symmetry code: (ii) x + 1, y, z − 1].
). The slabs are in turn linked via π–π interactions, forming a three-dimensional structure with centroid–centroid distances of 3.4202 (7) for Cg1⋯Cg5ii and 3.5445 (7) Å for Cg2⋯Cg4ii [Cg1, Cg2, Cg4 and Cg5 are the centroids of rings N1A/C1A/C2A/C7A/C8A, C2A–C7A, N1B/C1B/C2B/C7B/C8B and C2B–C7B, respectively; symmetry code: (ii) x + 1, y, z − 1].
Figure 5.

A view along the a axis of the crystal packing of compound (2), showing the three-dimensional array formed by an extensive network of C—H⋯O hydrogen bonds (dashed lines; see Table 2 ▶ for details).
Table 2. Hydrogen-bond geometry (, ) for (2) .
| DHA | DH | HA | D A | DHA |
|---|---|---|---|---|
| C5AH5AAO3B i | 0.95 | 2.54 | 3.3341(15) | 141 |
| C6AH6AAO4A ii | 0.95 | 2.51 | 3.4091(15) | 158 |
| C3BH3BAO2A iii | 0.95 | 2.59 | 3.2281(14) | 125 |
| C6BH6BAO3A iv | 0.95 | 2.55 | 3.3086(14) | 137 |
| C10BH10FO2B v | 0.98 | 2.57 | 3.4956(16) | 157 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  ; (v)
; (v)  .
.
In the crystal of (3), the A and B molecules are linked by C—H⋯O hydrogen bonds (Fig. 6 ▶ and Table 3 ▶), forming a three-dimensional structure, which is consolidated by π–π interactions [Cg1⋯Cg3iii = 3.578 (3), Cg2⋯Cg3iii = 3.575 (3) Å and Cg9⋯Cg10iv; Cg1, Cg2, Cg3, Cg9 and Cg10 are the centroids of rings N1A/C1A–C5A, C2A/C3A/C6A–C9A, C3A/C4A/C9A–C12A, C2B/C3B/C6B–C9B and C3B/C4B/C9B–C12B, respectively; symmetry codes: (iii) x, −y +  , z −
, z −  ; (iv) x, −y +
; (iv) x, −y +  , z +
, z +  ].
].
Figure 6.
For molecule A in compound (2), perpendicular interactions between atoms O1A and C9A (shown as dashed lines) link the molecules into inversion dimers [symmetry code: (A) − x + 1, − y + 2, −z].
Table 3. Hydrogen-bond geometry (, ) for (3) .
| DHA | DH | HA | D A | DHA |
|---|---|---|---|---|
| C6AH6AAO4A i | 0.95 | 2.51 | 3.159(5) | 125 |
| C7BH7BAO2B ii | 0.95 | 2.51 | 3.229(5) | 133 |
| C10BH10BO5B ii | 0.95 | 2.60 | 3.428(5) | 146 |
| C11BH11BO1A iii | 0.95 | 2.48 | 3.270(6) | 141 |
| C14AH14AO1B iv | 0.98 | 2.51 | 3.481(5) | 169 |
| C14BH14EO4A iv | 0.98 | 2.51 | 3.306(6) | 138 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  .
.
Interestingly, in the crystal of (2) one of the two dione moieties for each molecule (O1A and O1B) has a short intermolecular interactions with the central C atom of the carbonato group [O1A⋯C9A = 2.794 (1), O1B⋯C9B = 2.873 (1) Å], which is perpendicular to the carbonato plane indicating that both atoms, C9A and C9B, must have significant positive character. These interactions link the molecules into dimers as shown in Figs. 6 ▶ and 7 ▶, respectively. This is also noticed to a lesser extent in (3) (Fig. 8 ▶) for molecule A (but not for molecule B), where a longer intermolecular interaction of 3.060 (3) Å is observed between atoms O2A and C13A, resulting in weakly associated dimers similar to that seen in the case of (2).
Figure 7.
For molecule B in compound (2), perpendicular interactions between atoms O1B and C9B (shown as dashed lines) link the molecules into inversion dimers [symmetry code: (A) −x, −y + 1, −z − 1].
Figure 8.
A view along the a axis of the crystal packing of compound (3), showing the formation of the three-dimensional array by an extensive network of C—H⋯O hydrogen bonds (dashed lines; see Table 3 ▶ for details).
Database survey
A search of the Cambridge Structural Database (Version 5.35; Groom & Allen, 2014 ▶) for the indoline skeleton gave 26 hits. In all cases, the geometrical parameters of the indoline skeleton are similar to those observed in compounds (1) and (2). In the case of the isoquinoline structure, there are only two structures containing the planar isoquinoline moiety with similar geometrical parameters to the present structure, (3).
Synthesis and crystallization
Compound (1):
To a freshly prepared solution of sodium (2.3 g, 0.10 mol) in absolute ethanol (60 ml) was added a solution of N-hydroxyphthalimide (16.3 g, 0.10 mol) in absolute ethanol (350 ml), and the red reaction mixture was stirred at room temperature for 30 min. The brick-red precipitate was collected, washed with water, and dried in the oven at 373 K for 30 min to give 17.45 g (95%) of sodium phthalimide oxide as brick-red crystals; m.p. > 573 K. To the solution of sodium phthalimide oxide (0.92 g, 5 mmol) in water (15 ml) was added acetone (10 ml), followed by a solution of bromomethane (0.66 g, 7 mmol). The reaction mixture was stirred at room temperature for 16 h, during which the red color disappeared. On standing at room temperature for 48 h, the product solidified in the aqueous mixture and was collected. Recrystallization from 2-propanol gave 0.72 g (78%) of compound (1) as plate-like colorless crystals: m.p. 395–397 K; 1H NMR (CDC13) δ 3.36 (s, 3H, J = 6 Hz, OCH3), 5.52, s, 1 H,CH, 7.87 (m, 4 H, phthalimido ring).
Compound (2):
To a solution of sodium phthalimide oxide (0.92 g, 5 mmol) in water (15 ml) was added acetone (10 ml), followed by a solution of bromo(methoxy)methanone (0.97 g, 7 mmol). The reaction mixture was stirred at room temperature for 16 h, during which the red color disappeared. On standing at room temperature for 48 h, the product solidified in the aqueous mixture and was collected. Recrystallization from ethanol gave 0.82 g (74%) of compound (2) as colorless crystals: m.p. 410–411 K; 1H NMR (CDC13) δ 3.8 (s, 3H,OCH3), 7.86 (m, 4H, phthalimido ring).
Compound (3):
To a solution of sodium naphthalimide oxide, (1.18 g, 5 mmol), in water (50 ml), was added bromo(methoxy)methanone (1.25g, 7 mmol) in acetone (10 ml). The red reaction mixture was stirred at room temperature. The red color disappeared within 5 min and the reaction mixture was filled with a white precipitate. After standing for 4 h, the white precipitate was collected, washed with water, and recrystallized from ethanol to give 1.46 g (89%) of compound (3) as colorless crystals: m.p. 483–485 K; 1H NMR (CDCl3) δ 3.79 (s, 3H, OCH3), 5.66 (s, 1H, CH), 7.65–8.50 (m, 6 H, naphthalimido ring).
Refinement
Crystal data, data collection and structure refinement details for (1), (2) and (3) are summarized in Table 4 ▶. For all three compounds, the H atoms were positioned geometrically and refined as riding: C—H = 0.93–0.99 Å with Uiso(H) = 1.5U eq(C) for methyl H atoms and = 1.2Ueq(C) for other H atoms.
Table 4. Experimental details.
| (1) | (2) | (3) | |
|---|---|---|---|
| Crystal data | |||
| Chemical formula | C9H7NO3 | C10H7NO5 | C14H9NO5 |
| M r | 177.16 | 221.17 | 271.22 |
| Crystal system, space group | Orthorhombic, P212121 | Triclinic, P

|
Monoclinic, P21/c |
| Temperature (K) | 123 | 123 | 123 |
| a, b, c () | 4.2987(4), 7.0243(10), 27.587(4) | 7.0363(4), 11.0082(5), 12.4239(6) | 16.512(3), 18.579(3), 7.6156(13) |
| , , () | 90, 90, 90 | 98.884(4), 96.159(4), 93.009(4) | 90, 99.434(17), 90 |
| V (3) | 832.98(19) | 942.95(8) | 2304.6(7) |
| Z | 4 | 4 | 8 |
| Radiation type | Mo K | Cu K | Mo K |
| (mm1) | 0.11 | 1.10 | 0.12 |
| Crystal size (mm) | 0.66 0.23 0.04 | 0.35 0.25 0.08 | 0.44 0.12 0.07 |
| Data collection | |||
| Diffractometer | Agilent Xcalibur (Ruby, Gemini) | SuperNova (Dual, Cu at zero, Atlas) | Agilent Xcalibur (Ruby, Gemini) |
| Absorption correction | Analytical (CrysAlis PRO; Agilent, 2012 ▶) | Multi-scan (CrysAlis PRO; Agilent, 2012 ▶) | Analytical (CrysAlis PRO; Agilent, 2012 ▶) |
| T min, T max | 0.946, 0.996 | 0.807, 1.000 | 0.995, 0.999 |
| No. of measured, independent and observed [I > 2(I)] reflections | 5145, 2259, 1989 | 6437, 3803, 3516 | 9949, 4156, 1898 |
| R int | 0.087 | 0.018 | 0.091 |
| (sin /)max (1) | 0.727 | 0.631 | 0.600 |
| Refinement | |||
| R[F 2 > 2(F 2)], wR(F 2), S | 0.099, 0.229, 1.13 | 0.033, 0.089, 1.06 | 0.080, 0.224, 1.00 |
| No. of reflections | 2259 | 3803 | 4156 |
| No. of parameters | 119 | 291 | 363 |
| H-atom treatment | H-atom parameters constrained | H-atom parameters constrained | H-atom parameters constrained |
| max, min (e 3) | 0.50, 0.34 | 0.29, 0.21 | 0.33, 0.39 |
Supplementary Material
Crystal structure: contains datablock(s) 1, 2, 3. DOI: 10.1107/S1600536814023769/su2795sup1.cif
Structure factors: contains datablock(s) 1. DOI: 10.1107/S1600536814023769/su27951sup2.hkl
Structure factors: contains datablock(s) 2. DOI: 10.1107/S1600536814023769/su27952sup3.hkl
Structure factors: contains datablock(s) 3. DOI: 10.1107/S1600536814023769/su27953sup4.hkl
Supporting information file. DOI: 10.1107/S1600536814023769/su27951sup5.cml
Supporting information file. DOI: 10.1107/S1600536814023769/su27952sup6.cml
Supporting information file. DOI: 10.1107/S1600536814023769/su27953sup7.cml
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
FE wishes to acknowledge Dr Ivan Edafiogho, University of Saint Joseph SOP and Professor Emeritus Kenneth R. Scott, for their generous donation of the compounds studied. The authors are indebted to Mr James P. Stables (retired), Epilepsy Branch, Division of Convulsive, Developmental and Neuromuscular Disorders, National Institute of Neurological Disorders and Stroke, for helpful discussions and initial data. The authors wish to acknowledge Drs Ivan Edafiogho and Mariano S. Alexander, for their generous assistance and support in completing this project. RJB is grateful to the NSF–MRI program (grant CHE-0619278) for funds to purchase the diffractometer and the Howard University Nanoscience Facility for access to liquid nitrogen.
supplementary crystallographic information
Crystal data
| C14H9NO5 | F(000) = 1120 |
| Mr = 271.22 | Dx = 1.563 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 16.512 (3) Å | Cell parameters from 1261 reflections |
| b = 18.579 (3) Å | θ = 3.4–26.9° |
| c = 7.6156 (13) Å | µ = 0.12 mm−1 |
| β = 99.434 (17)° | T = 123 K |
| V = 2304.6 (7) Å3 | Needle, colorless |
| Z = 8 | 0.44 × 0.12 × 0.07 mm |
Data collection
| Agilent Xcalibur (Ruby, Gemini) diffractometer | 4156 independent reflections |
| Radiation source: Enhance (Mo) X-ray Source | 1898 reflections with I > 2σ(I) |
| Detector resolution: 10.5081 pixels mm-1 | Rint = 0.091 |
| ω scans | θmax = 25.3°, θmin = 3.3° |
| Absorption correction: analytical (CrysAlis PRO; Agilent, 2012) | h = −15→19 |
| Tmin = 0.995, Tmax = 0.999 | k = −22→21 |
| 9949 measured reflections | l = −9→9 |
Refinement
| Refinement on F2 | 0 restraints |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.080 | H-atom parameters constrained |
| wR(F2) = 0.224 | w = 1/[σ2(Fo2) + (0.0796P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 1.00 | (Δ/σ)max < 0.001 |
| 4156 reflections | Δρmax = 0.33 e Å−3 |
| 363 parameters | Δρmin = −0.39 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1A | 0.3491 (2) | 0.31532 (15) | 0.1257 (5) | 0.0428 (9) | |
| O2A | 0.5630 (2) | 0.44236 (15) | 0.4206 (4) | 0.0441 (9) | |
| O3A | 0.4152 (2) | 0.43894 (14) | 0.2299 (4) | 0.0384 (9) | |
| O4A | 0.3270 (2) | 0.41710 (15) | 0.4258 (4) | 0.0413 (9) | |
| O5A | 0.31114 (19) | 0.50794 (14) | 0.2257 (4) | 0.0367 (8) | |
| N1A | 0.4539 (3) | 0.37502 (17) | 0.2925 (5) | 0.0375 (10) | |
| C1A | 0.4144 (3) | 0.3116 (2) | 0.2245 (7) | 0.0370 (12) | |
| C2A | 0.4578 (3) | 0.2448 (2) | 0.2868 (6) | 0.0334 (11) | |
| C3A | 0.5339 (3) | 0.2484 (2) | 0.4016 (6) | 0.0335 (11) | |
| C4A | 0.5716 (3) | 0.3145 (2) | 0.4574 (6) | 0.0359 (12) | |
| C5A | 0.5329 (3) | 0.3829 (2) | 0.3934 (6) | 0.0344 (12) | |
| C6A | 0.4237 (3) | 0.1799 (2) | 0.2309 (6) | 0.0361 (12) | |
| H6AA | 0.3722 | 0.1785 | 0.1543 | 0.043* | |
| C7A | 0.4646 (3) | 0.1149 (2) | 0.2864 (7) | 0.0400 (13) | |
| H7AA | 0.4415 | 0.0700 | 0.2451 | 0.048* | |
| C8A | 0.5375 (3) | 0.1174 (2) | 0.3996 (7) | 0.0369 (12) | |
| H8AA | 0.5642 | 0.0735 | 0.4376 | 0.044* | |
| C9A | 0.5748 (3) | 0.1828 (2) | 0.4622 (7) | 0.0365 (12) | |
| C10A | 0.6507 (3) | 0.1873 (2) | 0.5762 (7) | 0.0411 (13) | |
| H10A | 0.6786 | 0.1443 | 0.6171 | 0.049* | |
| C11A | 0.6852 (3) | 0.2519 (2) | 0.6293 (6) | 0.0385 (12) | |
| H11A | 0.7365 | 0.2534 | 0.7069 | 0.046* | |
| C12A | 0.6457 (3) | 0.3158 (2) | 0.5705 (6) | 0.0378 (12) | |
| H12A | 0.6701 | 0.3606 | 0.6087 | 0.045* | |
| C13A | 0.3473 (3) | 0.4514 (2) | 0.3079 (7) | 0.0354 (12) | |
| C14A | 0.2353 (3) | 0.5276 (2) | 0.2859 (6) | 0.0427 (13) | |
| H14A | 0.2134 | 0.5717 | 0.2253 | 0.064* | |
| H14B | 0.2459 | 0.5358 | 0.4147 | 0.064* | |
| H14C | 0.1953 | 0.4886 | 0.2583 | 0.064* | |
| O1B | 0.1669 (2) | 0.82517 (15) | 0.5198 (4) | 0.0392 (8) | |
| O2B | −0.0410 (2) | 0.95254 (15) | 0.2005 (4) | 0.0468 (10) | |
| O3B | 0.1021 (2) | 0.94818 (14) | 0.4052 (4) | 0.0384 (9) | |
| O4B | 0.1866 (2) | 0.92546 (16) | 0.2003 (4) | 0.0434 (9) | |
| O5B | 0.1998 (2) | 1.02184 (14) | 0.3847 (4) | 0.0392 (9) | |
| N1B | 0.0622 (3) | 0.88420 (17) | 0.3479 (5) | 0.0363 (10) | |
| C1B | 0.1017 (3) | 0.8213 (2) | 0.4202 (7) | 0.0359 (12) | |
| C2B | 0.0594 (3) | 0.7545 (2) | 0.3564 (6) | 0.0325 (11) | |
| C3B | −0.0186 (3) | 0.7576 (2) | 0.2461 (6) | 0.0358 (12) | |
| C4B | −0.0567 (3) | 0.8244 (2) | 0.1926 (6) | 0.0349 (12) | |
| C5B | −0.0156 (3) | 0.8933 (2) | 0.2436 (7) | 0.0376 (12) | |
| C6B | 0.0945 (3) | 0.6896 (2) | 0.4060 (6) | 0.0363 (12) | |
| H6BA | 0.1462 | 0.6879 | 0.4820 | 0.044* | |
| C7B | 0.0547 (3) | 0.6251 (2) | 0.3450 (7) | 0.0392 (13) | |
| H7BA | 0.0803 | 0.5802 | 0.3780 | 0.047* | |
| C8B | −0.0194 (3) | 0.6266 (2) | 0.2403 (7) | 0.0400 (13) | |
| H8BA | −0.0454 | 0.5825 | 0.2016 | 0.048* | |
| C9B | −0.0595 (3) | 0.6923 (2) | 0.1864 (6) | 0.0356 (12) | |
| C10B | −0.1376 (3) | 0.6966 (2) | 0.0811 (7) | 0.0432 (13) | |
| H10B | −0.1652 | 0.6535 | 0.0395 | 0.052* | |
| C11B | −0.1745 (3) | 0.7611 (2) | 0.0371 (7) | 0.0459 (13) | |
| H11B | −0.2282 | 0.7626 | −0.0307 | 0.055* | |
| C12B | −0.1335 (3) | 0.8257 (2) | 0.0917 (7) | 0.0415 (13) | |
| H12B | −0.1592 | 0.8705 | 0.0585 | 0.050* | |
| C13B | 0.1661 (3) | 0.9614 (2) | 0.3143 (7) | 0.0365 (12) | |
| C14B | 0.2722 (3) | 1.0450 (2) | 0.3140 (7) | 0.0454 (14) | |
| H14D | 0.2953 | 1.0880 | 0.3779 | 0.068* | |
| H14E | 0.2569 | 1.0562 | 0.1871 | 0.068* | |
| H14F | 0.3132 | 1.0063 | 0.3292 | 0.068* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1A | 0.037 (2) | 0.0392 (18) | 0.047 (2) | 0.0021 (16) | −0.0087 (18) | −0.0011 (15) |
| O2A | 0.044 (2) | 0.0267 (16) | 0.058 (2) | −0.0019 (16) | −0.0013 (18) | −0.0040 (15) |
| O3A | 0.037 (2) | 0.0299 (16) | 0.046 (2) | 0.0043 (15) | −0.0004 (17) | 0.0045 (14) |
| O4A | 0.048 (2) | 0.0313 (16) | 0.044 (2) | 0.0042 (16) | 0.0033 (18) | 0.0056 (16) |
| O5A | 0.039 (2) | 0.0245 (15) | 0.045 (2) | 0.0049 (15) | 0.0022 (17) | 0.0062 (14) |
| N1A | 0.038 (3) | 0.0219 (19) | 0.049 (3) | 0.0043 (18) | −0.003 (2) | −0.0012 (17) |
| C1A | 0.041 (4) | 0.029 (2) | 0.040 (3) | 0.001 (2) | 0.003 (3) | 0.000 (2) |
| C2A | 0.034 (3) | 0.026 (2) | 0.040 (3) | −0.003 (2) | 0.005 (2) | 0.001 (2) |
| C3A | 0.036 (3) | 0.027 (2) | 0.037 (3) | 0.002 (2) | 0.005 (2) | 0.003 (2) |
| C4A | 0.036 (3) | 0.032 (2) | 0.038 (3) | −0.002 (2) | 0.000 (2) | −0.002 (2) |
| C5A | 0.038 (3) | 0.031 (3) | 0.033 (3) | −0.003 (2) | 0.004 (2) | 0.000 (2) |
| C6A | 0.036 (3) | 0.035 (3) | 0.037 (3) | −0.006 (2) | 0.005 (2) | −0.001 (2) |
| C7A | 0.043 (4) | 0.029 (2) | 0.048 (3) | −0.002 (2) | 0.010 (3) | −0.006 (2) |
| C8A | 0.043 (4) | 0.029 (2) | 0.038 (3) | 0.004 (2) | 0.006 (3) | 0.002 (2) |
| C9A | 0.037 (3) | 0.029 (2) | 0.044 (3) | 0.002 (2) | 0.007 (3) | −0.005 (2) |
| C10A | 0.039 (3) | 0.035 (3) | 0.048 (4) | 0.007 (2) | 0.006 (3) | 0.002 (2) |
| C11A | 0.029 (3) | 0.045 (3) | 0.039 (3) | 0.002 (2) | −0.005 (2) | −0.001 (2) |
| C12A | 0.042 (3) | 0.034 (2) | 0.037 (3) | 0.000 (2) | 0.004 (3) | −0.009 (2) |
| C13A | 0.036 (3) | 0.031 (2) | 0.038 (3) | −0.004 (2) | 0.003 (3) | −0.003 (2) |
| C14A | 0.041 (3) | 0.037 (3) | 0.046 (3) | 0.002 (2) | −0.002 (3) | 0.002 (2) |
| O1B | 0.035 (2) | 0.0361 (17) | 0.044 (2) | 0.0022 (16) | −0.0005 (17) | −0.0033 (15) |
| O2B | 0.050 (3) | 0.0270 (17) | 0.059 (3) | 0.0022 (16) | −0.0037 (19) | 0.0046 (15) |
| O3B | 0.040 (2) | 0.0257 (16) | 0.048 (2) | −0.0046 (15) | 0.0019 (18) | −0.0046 (14) |
| O4B | 0.050 (2) | 0.0384 (18) | 0.041 (2) | −0.0032 (16) | 0.0047 (18) | −0.0054 (16) |
| O5B | 0.046 (2) | 0.0265 (16) | 0.043 (2) | −0.0059 (15) | 0.0031 (17) | −0.0044 (14) |
| N1B | 0.037 (3) | 0.0213 (19) | 0.046 (3) | −0.0027 (18) | −0.006 (2) | −0.0020 (17) |
| C1B | 0.033 (3) | 0.036 (3) | 0.037 (3) | 0.005 (2) | 0.002 (3) | 0.001 (2) |
| C2B | 0.035 (3) | 0.027 (2) | 0.035 (3) | 0.001 (2) | 0.002 (2) | −0.007 (2) |
| C3B | 0.040 (3) | 0.030 (2) | 0.038 (3) | 0.001 (2) | 0.005 (2) | 0.003 (2) |
| C4B | 0.033 (3) | 0.032 (2) | 0.038 (3) | −0.004 (2) | 0.001 (2) | 0.000 (2) |
| C5B | 0.040 (4) | 0.032 (3) | 0.041 (3) | −0.001 (2) | 0.006 (3) | −0.002 (2) |
| C6B | 0.039 (3) | 0.031 (2) | 0.039 (3) | 0.001 (2) | 0.008 (2) | 0.003 (2) |
| C7B | 0.047 (4) | 0.025 (2) | 0.046 (3) | 0.002 (2) | 0.010 (3) | 0.003 (2) |
| C8B | 0.040 (4) | 0.030 (2) | 0.050 (4) | −0.004 (2) | 0.006 (3) | −0.002 (2) |
| C9B | 0.037 (3) | 0.036 (3) | 0.032 (3) | −0.005 (2) | 0.003 (2) | −0.002 (2) |
| C10B | 0.041 (4) | 0.037 (3) | 0.049 (3) | −0.009 (2) | 0.000 (3) | 0.005 (2) |
| C11B | 0.042 (3) | 0.048 (3) | 0.045 (3) | −0.006 (3) | −0.001 (3) | 0.001 (2) |
| C12B | 0.040 (4) | 0.037 (3) | 0.046 (3) | 0.001 (2) | 0.002 (3) | 0.006 (2) |
| C13B | 0.036 (3) | 0.030 (3) | 0.042 (3) | 0.002 (2) | 0.000 (3) | 0.007 (2) |
| C14B | 0.044 (4) | 0.037 (3) | 0.055 (4) | −0.010 (2) | 0.007 (3) | 0.001 (2) |
Geometric parameters (Å, º)
| O1A—C1A | 1.211 (6) | O1B—C1B | 1.212 (5) |
| O2A—C5A | 1.215 (5) | O2B—C5B | 1.204 (5) |
| O3A—C13A | 1.371 (5) | O3B—C13B | 1.377 (5) |
| O3A—N1A | 1.395 (4) | O3B—N1B | 1.394 (4) |
| O4A—C13A | 1.193 (5) | O4B—C13B | 1.188 (5) |
| O5A—C13A | 1.316 (5) | O5B—C13B | 1.326 (5) |
| O5A—C14A | 1.449 (5) | O5B—C14B | 1.455 (5) |
| N1A—C1A | 1.403 (6) | N1B—C5B | 1.405 (6) |
| N1A—C5A | 1.408 (6) | N1B—C1B | 1.406 (6) |
| C1A—C2A | 1.473 (6) | C1B—C2B | 1.468 (6) |
| C2A—C6A | 1.370 (6) | C2B—C6B | 1.365 (6) |
| C2A—C3A | 1.409 (6) | C2B—C3B | 1.419 (6) |
| C3A—C4A | 1.410 (6) | C3B—C4B | 1.419 (6) |
| C3A—C9A | 1.432 (6) | C3B—C9B | 1.427 (6) |
| C4A—C12A | 1.377 (7) | C4B—C12B | 1.371 (7) |
| C4A—C5A | 1.471 (6) | C4B—C5B | 1.472 (6) |
| C6A—C7A | 1.413 (6) | C6B—C7B | 1.408 (6) |
| C6A—H6AA | 0.9500 | C6B—H6BA | 0.9500 |
| C7A—C8A | 1.362 (7) | C7B—C8B | 1.347 (7) |
| C7A—H7AA | 0.9500 | C7B—H7BA | 0.9500 |
| C8A—C9A | 1.410 (6) | C8B—C9B | 1.417 (6) |
| C8A—H8AA | 0.9500 | C8B—H8BA | 0.9500 |
| C9A—C10A | 1.405 (7) | C9B—C10B | 1.404 (7) |
| C10A—C11A | 1.361 (6) | C10B—C11B | 1.362 (6) |
| C10A—H10A | 0.9500 | C10B—H10B | 0.9500 |
| C11A—C12A | 1.392 (6) | C11B—C12B | 1.406 (6) |
| C11A—H11A | 0.9500 | C11B—H11B | 0.9500 |
| C12A—H12A | 0.9500 | C12B—H12B | 0.9500 |
| C14A—H14A | 0.9800 | C14B—H14D | 0.9800 |
| C14A—H14B | 0.9800 | C14B—H14E | 0.9800 |
| C14A—H14C | 0.9800 | C14B—H14F | 0.9800 |
| C13A—O3A—N1A | 110.9 (3) | C13B—O3B—N1B | 111.0 (3) |
| C13A—O5A—C14A | 113.6 (3) | C13B—O5B—C14B | 114.6 (4) |
| O3A—N1A—C1A | 115.4 (4) | O3B—N1B—C5B | 114.5 (3) |
| O3A—N1A—C5A | 115.4 (3) | O3B—N1B—C1B | 114.9 (4) |
| C1A—N1A—C5A | 128.4 (4) | C5B—N1B—C1B | 130.2 (4) |
| O1A—C1A—N1A | 119.6 (4) | O1B—C1B—N1B | 120.2 (4) |
| O1A—C1A—C2A | 125.8 (4) | O1B—C1B—C2B | 125.7 (4) |
| N1A—C1A—C2A | 114.6 (5) | N1B—C1B—C2B | 114.0 (5) |
| C6A—C2A—C3A | 120.9 (4) | C6B—C2B—C3B | 120.2 (4) |
| C6A—C2A—C1A | 119.3 (5) | C6B—C2B—C1B | 119.8 (5) |
| C3A—C2A—C1A | 119.8 (4) | C3B—C2B—C1B | 119.9 (4) |
| C2A—C3A—C4A | 122.2 (4) | C2B—C3B—C4B | 121.4 (4) |
| C2A—C3A—C9A | 119.0 (4) | C2B—C3B—C9B | 119.3 (4) |
| C4A—C3A—C9A | 118.8 (5) | C4B—C3B—C9B | 119.2 (5) |
| C12A—C4A—C3A | 120.5 (4) | C12B—C4B—C3B | 120.1 (4) |
| C12A—C4A—C5A | 119.1 (4) | C12B—C4B—C5B | 118.5 (4) |
| C3A—C4A—C5A | 120.4 (5) | C3B—C4B—C5B | 121.4 (5) |
| O2A—C5A—N1A | 120.3 (4) | O2B—C5B—N1B | 120.7 (4) |
| O2A—C5A—C4A | 125.9 (5) | O2B—C5B—C4B | 126.7 (5) |
| N1A—C5A—C4A | 113.8 (4) | N1B—C5B—C4B | 112.5 (4) |
| C2A—C6A—C7A | 120.5 (5) | C2B—C6B—C7B | 120.4 (5) |
| C2A—C6A—H6AA | 119.7 | C2B—C6B—H6BA | 119.8 |
| C7A—C6A—H6AA | 119.7 | C7B—C6B—H6BA | 119.8 |
| C8A—C7A—C6A | 119.3 (4) | C8B—C7B—C6B | 120.5 (4) |
| C8A—C7A—H7AA | 120.3 | C8B—C7B—H7BA | 119.8 |
| C6A—C7A—H7AA | 120.3 | C6B—C7B—H7BA | 119.8 |
| C7A—C8A—C9A | 122.3 (4) | C7B—C8B—C9B | 121.7 (4) |
| C7A—C8A—H8AA | 118.8 | C7B—C8B—H8BA | 119.1 |
| C9A—C8A—H8AA | 118.8 | C9B—C8B—H8BA | 119.1 |
| C10A—C9A—C8A | 123.8 (4) | C10B—C9B—C8B | 123.8 (4) |
| C10A—C9A—C3A | 118.3 (4) | C10B—C9B—C3B | 118.4 (4) |
| C8A—C9A—C3A | 117.9 (5) | C8B—C9B—C3B | 117.8 (5) |
| C11A—C10A—C9A | 121.6 (4) | C11B—C10B—C9B | 121.5 (5) |
| C11A—C10A—H10A | 119.2 | C11B—C10B—H10B | 119.2 |
| C9A—C10A—H10A | 119.2 | C9B—C10B—H10B | 119.2 |
| C10A—C11A—C12A | 120.3 (5) | C10B—C11B—C12B | 120.2 (5) |
| C10A—C11A—H11A | 119.9 | C10B—C11B—H11B | 119.9 |
| C12A—C11A—H11A | 119.9 | C12B—C11B—H11B | 119.9 |
| C4A—C12A—C11A | 120.5 (4) | C4B—C12B—C11B | 120.5 (5) |
| C4A—C12A—H12A | 119.7 | C4B—C12B—H12B | 119.8 |
| C11A—C12A—H12A | 119.7 | C11B—C12B—H12B | 119.8 |
| O4A—C13A—O5A | 128.5 (4) | O4B—C13B—O5B | 128.3 (5) |
| O4A—C13A—O3A | 126.0 (4) | O4B—C13B—O3B | 126.9 (4) |
| O5A—C13A—O3A | 105.6 (4) | O5B—C13B—O3B | 104.7 (4) |
| O5A—C14A—H14A | 109.5 | O5B—C14B—H14D | 109.5 |
| O5A—C14A—H14B | 109.5 | O5B—C14B—H14E | 109.5 |
| H14A—C14A—H14B | 109.5 | H14D—C14B—H14E | 109.5 |
| O5A—C14A—H14C | 109.5 | O5B—C14B—H14F | 109.5 |
| H14A—C14A—H14C | 109.5 | H14D—C14B—H14F | 109.5 |
| H14B—C14A—H14C | 109.5 | H14E—C14B—H14F | 109.5 |
| C13A—O3A—N1A—C1A | 75.2 (5) | C13B—O3B—N1B—C5B | −107.2 (4) |
| C13A—O3A—N1A—C5A | −113.9 (4) | C13B—O3B—N1B—C1B | 79.7 (5) |
| O3A—N1A—C1A—O1A | −3.6 (6) | O3B—N1B—C1B—O1B | −2.2 (6) |
| C5A—N1A—C1A—O1A | −173.0 (5) | C5B—N1B—C1B—O1B | −173.9 (5) |
| O3A—N1A—C1A—C2A | 177.8 (4) | O3B—N1B—C1B—C2B | −179.2 (3) |
| C5A—N1A—C1A—C2A | 8.3 (7) | C5B—N1B—C1B—C2B | 9.1 (7) |
| O1A—C1A—C2A—C6A | 0.3 (8) | O1B—C1B—C2B—C6B | −1.7 (8) |
| N1A—C1A—C2A—C6A | 178.8 (4) | N1B—C1B—C2B—C6B | 175.1 (4) |
| O1A—C1A—C2A—C3A | 179.8 (5) | O1B—C1B—C2B—C3B | 177.2 (4) |
| N1A—C1A—C2A—C3A | −1.6 (6) | N1B—C1B—C2B—C3B | −6.0 (7) |
| C6A—C2A—C3A—C4A | 178.2 (4) | C6B—C2B—C3B—C4B | 180.0 (4) |
| C1A—C2A—C3A—C4A | −1.3 (7) | C1B—C2B—C3B—C4B | 1.1 (7) |
| C6A—C2A—C3A—C9A | −1.1 (7) | C6B—C2B—C3B—C9B | −0.5 (7) |
| C1A—C2A—C3A—C9A | 179.4 (4) | C1B—C2B—C3B—C9B | −179.4 (4) |
| C2A—C3A—C4A—C12A | 179.6 (4) | C2B—C3B—C4B—C12B | −177.4 (4) |
| C9A—C3A—C4A—C12A | −1.1 (7) | C9B—C3B—C4B—C12B | 3.1 (7) |
| C2A—C3A—C4A—C5A | −1.3 (7) | C2B—C3B—C4B—C5B | 2.3 (7) |
| C9A—C3A—C4A—C5A | 178.0 (4) | C9B—C3B—C4B—C5B | −177.2 (4) |
| O3A—N1A—C5A—O2A | −0.2 (7) | O3B—N1B—C5B—O2B | 4.6 (6) |
| C1A—N1A—C5A—O2A | 169.3 (4) | C1B—N1B—C5B—O2B | 176.3 (4) |
| O3A—N1A—C5A—C4A | 179.8 (3) | O3B—N1B—C5B—C4B | −177.6 (3) |
| C1A—N1A—C5A—C4A | −10.7 (7) | C1B—N1B—C5B—C4B | −5.9 (7) |
| C12A—C4A—C5A—O2A | 5.6 (8) | C12B—C4B—C5B—O2B | −3.0 (8) |
| C3A—C4A—C5A—O2A | −173.4 (5) | C3B—C4B—C5B—O2B | 177.3 (5) |
| C12A—C4A—C5A—N1A | −174.3 (4) | C12B—C4B—C5B—N1B | 179.4 (4) |
| C3A—C4A—C5A—N1A | 6.6 (7) | C3B—C4B—C5B—N1B | −0.3 (7) |
| C3A—C2A—C6A—C7A | −0.5 (7) | C3B—C2B—C6B—C7B | 1.4 (7) |
| C1A—C2A—C6A—C7A | 179.0 (4) | C1B—C2B—C6B—C7B | −179.7 (4) |
| C2A—C6A—C7A—C8A | 1.5 (7) | C2B—C6B—C7B—C8B | −1.5 (7) |
| C6A—C7A—C8A—C9A | −0.9 (7) | C6B—C7B—C8B—C9B | 0.6 (8) |
| C7A—C8A—C9A—C10A | −178.7 (5) | C7B—C8B—C9B—C10B | −178.4 (5) |
| C7A—C8A—C9A—C3A | −0.6 (7) | C7B—C8B—C9B—C3B | 0.3 (7) |
| C2A—C3A—C9A—C10A | 179.8 (4) | C2B—C3B—C9B—C10B | 178.4 (4) |
| C4A—C3A—C9A—C10A | 0.5 (7) | C4B—C3B—C9B—C10B | −2.1 (7) |
| C2A—C3A—C9A—C8A | 1.6 (7) | C2B—C3B—C9B—C8B | −0.4 (7) |
| C4A—C3A—C9A—C8A | −177.7 (4) | C4B—C3B—C9B—C8B | 179.2 (4) |
| C8A—C9A—C10A—C11A | 178.2 (4) | C8B—C9B—C10B—C11B | 178.1 (5) |
| C3A—C9A—C10A—C11A | 0.2 (8) | C3B—C9B—C10B—C11B | −0.6 (8) |
| C9A—C10A—C11A—C12A | −0.3 (7) | C9B—C10B—C11B—C12B | 2.3 (8) |
| C3A—C4A—C12A—C11A | 1.0 (8) | C3B—C4B—C12B—C11B | −1.4 (8) |
| C5A—C4A—C12A—C11A | −178.0 (4) | C5B—C4B—C12B—C11B | 178.9 (4) |
| C10A—C11A—C12A—C4A | −0.3 (7) | C10B—C11B—C12B—C4B | −1.3 (8) |
| C14A—O5A—C13A—O4A | −2.6 (7) | C14B—O5B—C13B—O4B | −1.5 (7) |
| C14A—O5A—C13A—O3A | 177.5 (3) | C14B—O5B—C13B—O3B | 177.3 (3) |
| N1A—O3A—C13A—O4A | 6.8 (6) | N1B—O3B—C13B—O4B | 0.7 (7) |
| N1A—O3A—C13A—O5A | −173.3 (3) | N1B—O3B—C13B—O5B | −178.1 (3) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C6A—H6AA···O4Ai | 0.95 | 2.51 | 3.159 (5) | 125 |
| C7B—H7BA···O2Bii | 0.95 | 2.51 | 3.229 (5) | 133 |
| C10B—H10B···O5Bii | 0.95 | 2.60 | 3.428 (5) | 146 |
| C11B—H11B···O1Aiii | 0.95 | 2.48 | 3.270 (6) | 141 |
| C14A—H14A···O1Biv | 0.98 | 2.51 | 3.481 (5) | 169 |
| C14B—H14E···O4Aiv | 0.98 | 2.51 | 3.306 (6) | 138 |
Symmetry codes: (i) x, −y+1/2, z−1/2; (ii) −x, y−1/2, −z+1/2; (iii) −x, −y+1, −z; (iv) x, −y+3/2, z−1/2.
References
- Abdul-Muneer, P. M., Namas, C. & James, H. (2014). Mol. Neurobiol. 10.1007/s12035-014-8752-3.
- Agilent (2012). CrysAlis PRO. Agilent Technologies, Yarnton, England.
- Alexander, M. S., Scott, K. R., Harkless, J., Butcher, R. J. & Jackson-Ayotunde, P. L. (2013). Bioorg. Med. Chem. 21, 3272–3279. [DOI] [PubMed]
- Ashman, T. A., Gordon, W. A., Cantor, J. B. & Hibbard, M. R. (2006). Mt Sinai J. Med. 73, 999–1005. [PubMed]
- Christensen, J., Pedersen, M. G., Pedersen, C. B., Sidenius, P., Olsen, J. & Vestergaard, M. (2009). Lancet, 373, 1105–1110. [DOI] [PubMed]
- Coronado, V. G., Xu, L., Basavaraju, S. V., McGuire, L. C., Wald, M. M., Faul, M. D., Guzman, B. R. & Hemphill, J. D. (2011). MMWR Surveill. Summ. 60, 1–32. [PubMed]
- Edafiogho, I. O., Kombian, S. B., Ananthalakshmi, K. V. V., Salama, N. N., Eddington, N. D., Wilson, T. L., Alexander, M. S., Jackson, P. L., Hanson, C. D. & Scott, K. R. (2007). J. Pharm. Sci. 96, 2509–2531. [DOI] [PubMed]
- Ferraro, T. N., Golden, G. T., Smith, G. G., St Jean, P., Schork, N. J., Mulholland, N., Ballas, C., Schill, J., Buono, R. J. & Berrettini, W. H. (1999). J. Neurosci. 19, 6733–6739. [DOI] [PMC free article] [PubMed]
- Gibson, A., Harkless, J., Alexander, M. & Scott, K. R. (2009). Bioorg. Med. Chem. 17, 5342–5346. [DOI] [PubMed]
- Groom, C. R. & Allen, F. H. (2014). Angew. Chem. Int. Ed. 53, 662–671. [DOI] [PubMed]
- Jackson, P. L. (2009). PhD thesis, Howard University, Washington, DC, USA.
- Jackson, P. L., Hanson, C. D., Farrell, A. K., Butcher, R. J., Stables, J. P., Eddington, N. D. & Scott, K. R. (2012). Eur. J. Med. Chem. 51, 42–51. [DOI] [PubMed]
- Langlois, J. A., Kegler, S. R., Butler, J. A., Gotsch, K. E., Johnson, R. L., Reichard, A. A., Webb, K. W., Coronado, V. G., Selassie, A. W. & Thurman, D. J. (2003). MMWR Surveill. Summ. 52, 1–20. [PubMed]
- Langlois, J. A., Rutland-Brown, W. & Thomas, K. E. (2005). J. Head Trauma. Rehabil. 20, 229–238. [DOI] [PubMed]
- Love, R. (2005). Lancet Neurol. 4, 458. [DOI] [PubMed]
- North, H., Scott, K. R., Stables, J. P. & Wang, X. S. (2012). Abstracts of Papers, 243rd ACS National Meeting & Exposition, San Diego, CA, United States, March 25–29, 2012, COMP-299.
- Palatinus, L. & Chapuis, G. (2007). J. Appl. Cryst. 40, 786–790.
- Scantlebury, M. H., Gibbs, S. A., Foadjo, B., Lema, P., Psarropoulou, C. & Carmant, L. (2005). Ann. Neurol. 58, 41–49. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) 1, 2, 3. DOI: 10.1107/S1600536814023769/su2795sup1.cif
Structure factors: contains datablock(s) 1. DOI: 10.1107/S1600536814023769/su27951sup2.hkl
Structure factors: contains datablock(s) 2. DOI: 10.1107/S1600536814023769/su27952sup3.hkl
Structure factors: contains datablock(s) 3. DOI: 10.1107/S1600536814023769/su27953sup4.hkl
Supporting information file. DOI: 10.1107/S1600536814023769/su27951sup5.cml
Supporting information file. DOI: 10.1107/S1600536814023769/su27952sup6.cml
Supporting information file. DOI: 10.1107/S1600536814023769/su27953sup7.cml
Additional supporting information: crystallographic information; 3D view; checkCIF report