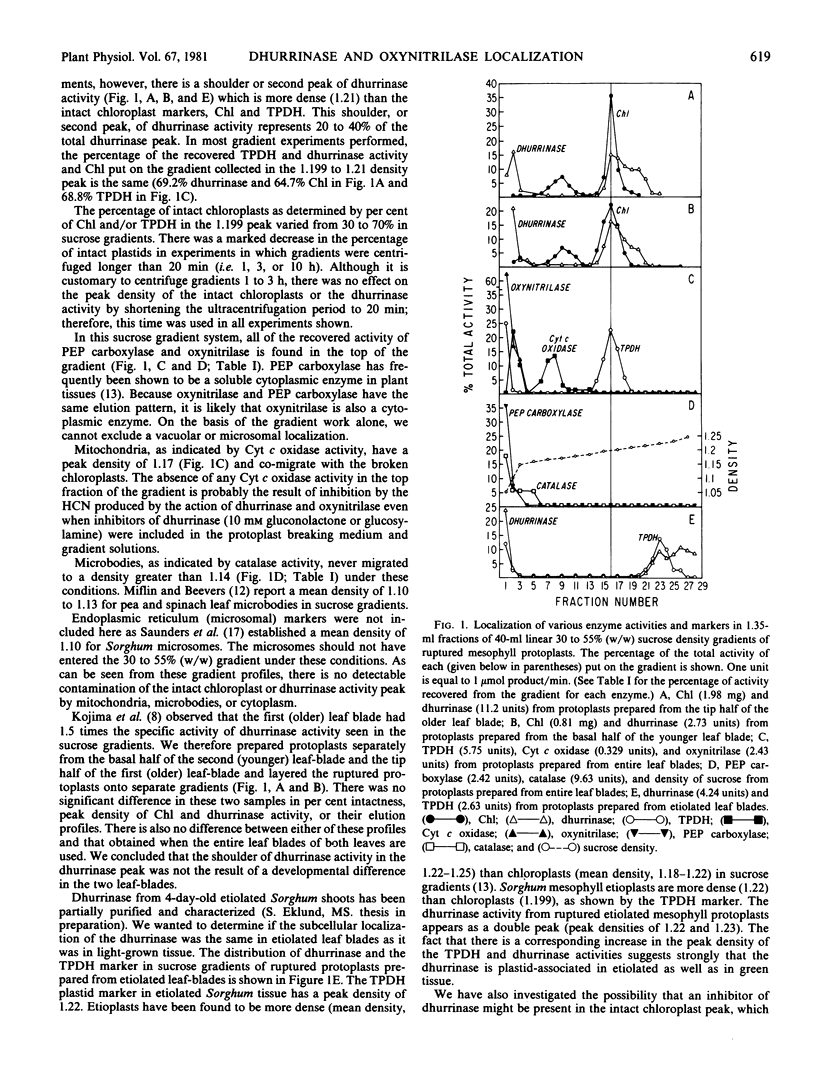
Abstract
Studies with purified mesophyll and epidermal protoplasts and bundle sheath strands have shown that the cyanogenic glucoside dhurrin (p-hydroxy-(S)-mandelonitrile-β-d-glucoside) is localized in the epidermis of sorghum leaves whereas the enzymes involved in its degradation (dhurrin β-glucosidase and hydroxynitrile lyase) are localized in the mesophyll tissue (Kojima M, JE Poulton, SS Thayer, EE Conn 1979 Plant Physiol 63: 1022-1028). The subcellular localization of these enzymes has now been examined using linear 30 to 55% (w/w) sucrose gradients by fractionation of mesophyll protoplast components. The hydroxynitrile lyase is found in the supernatant fractions suggesting a cytoplasmic (soluble cytoplasm, microsomal or vacuolar location). The dhurrin β-glucosidase (dhurrinase) is particulate and mostly chloroplast-associated. The dhurrinase activity peak has a shoulder of activity more dense than that of the intact chloroplasts. This shoulder does not coincide with markers of any other cell fraction.
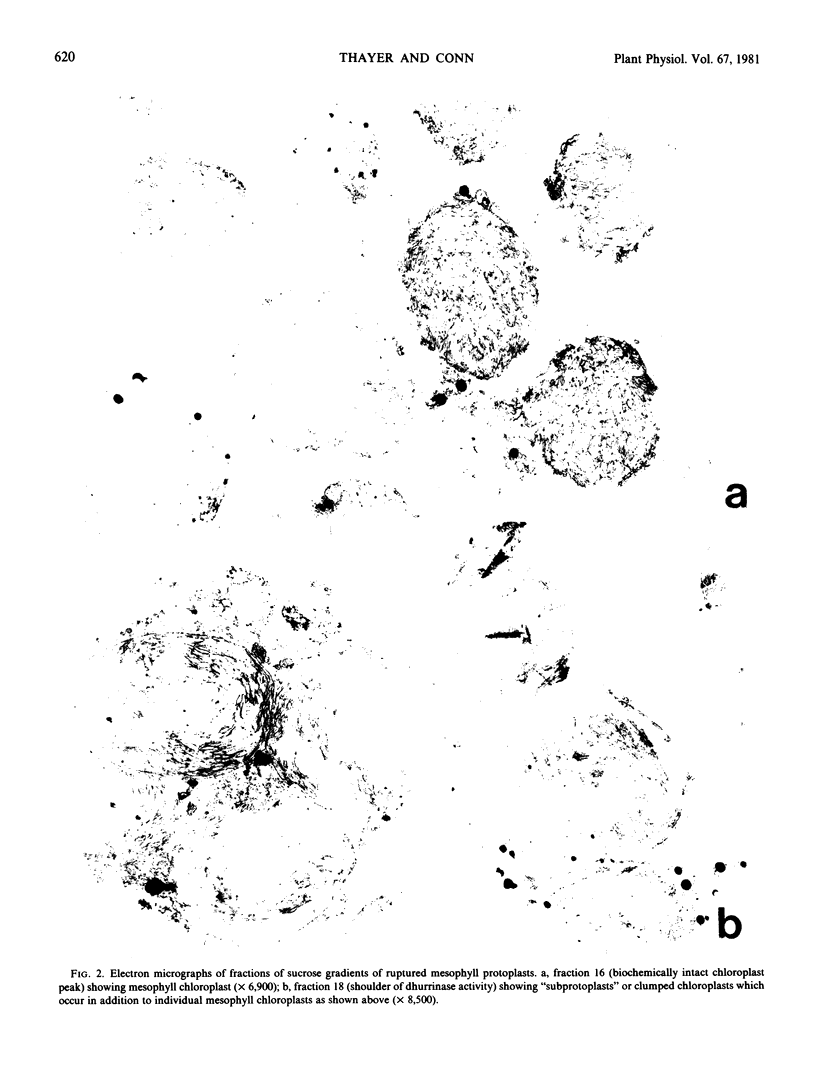
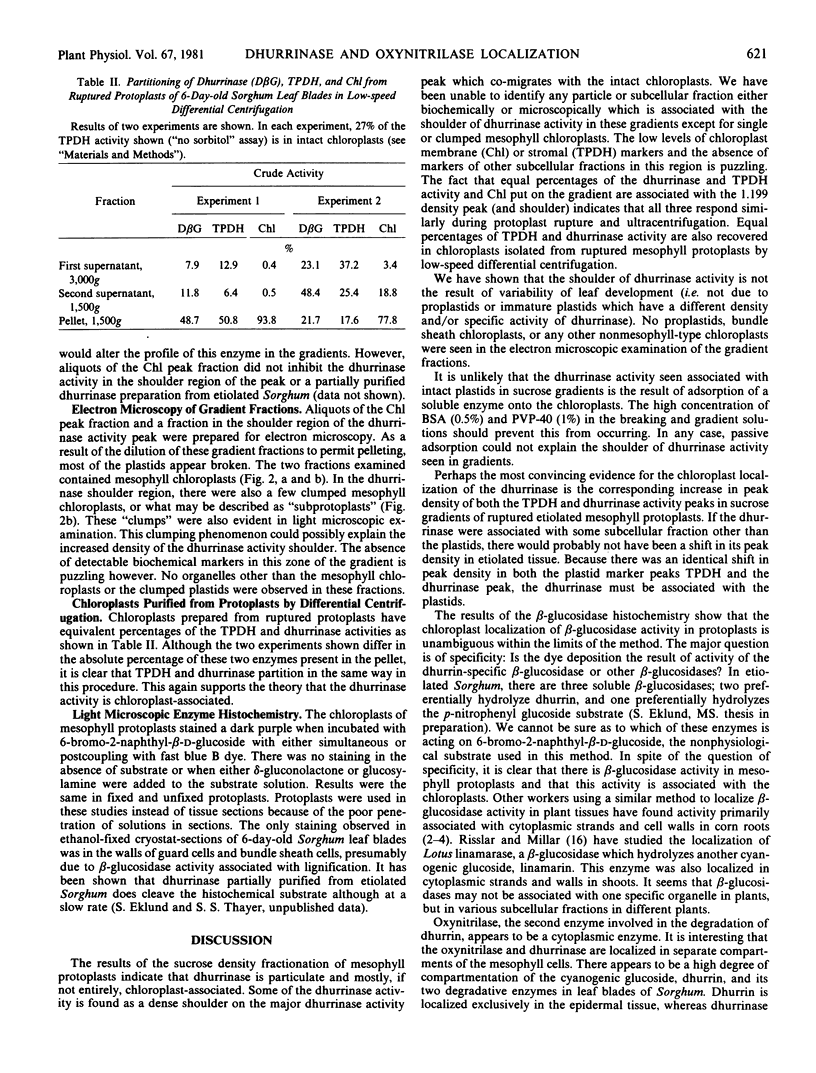
In studies of chloroplasts isolated from ruptured mesophyll protoplasts by differential, low-speed centrifugation, the dhurrinase partitions in the same manner as the chloroplast marker triose phosphate dehydrogenase. Chloroplast localization of the β-glucosidase has also been shown in histochemical studies using 6-bromo-2-naphthyl-β-d-glucoside substrate coupled with fast Blue B.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford A. E. Histochemical localization of beta-glycosidases in roots of Zea mays. I. A simultaneous coupling azo-dye technique for the localization of beta-glucosidase and beta-galactosidase. Protoplasma. 1970;71(3):281–293. doi: 10.1007/BF01279637. [DOI] [PubMed] [Google Scholar]
- Heber U., Pon N. G., Heber M. Localization of Carboxydismutase & Triosephosphate Dehydrogenases in Chloroplasts. Plant Physiol. 1963 May;38(3):355–360. doi: 10.1104/pp.38.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M., Poulton J. E., Thayer S. S., Conn E. E. Tissue Distributions of Dhurrin and of Enzymes Involved in Its Metabolism in Leaves of Sorghum bicolor. Plant Physiol. 1979 Jun;63(6):1022–1028. doi: 10.1104/pp.63.6.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miflin B. J., Beevers H. Isolation of intact plastids from a range of plant tissues. Plant Physiol. 1974 Jun;53(6):870–874. doi: 10.1104/pp.53.6.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. A., Conn E. E., Lin C. H., Shimada M. Localization of Cinnamic Acid 4-Monooxygenase and the Membrane-bound Enzyme System for Dhurrin Biosynthesis in Sorghum Seedlings. Plant Physiol. 1977 Oct;60(4):629–634. doi: 10.1104/pp.60.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Ting I. P. CO(2) Metabolism in Corn Roots. III. Inhibition of P-enolpyruvate Carboxylase by l-malate. Plant Physiol. 1968 Dec;43(12):1919–1924. doi: 10.1104/pp.43.12.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]



