In the crystal structure of the title compound, [Fe(dmbpy)(H2O)4][SO4], the charged components form an extensive hydrogen-bonding network. Eight O—H⋯O hydrogen bonds [d(O⋯H) < 2.00 Å], form a two-dimensional network parallel to the ab plane.
Keywords: crystal structure; 5,5′-dimethyl-2,2′-dipyridyl; tetraaquairon(II) complex; sulfate; bipyridine ligand; hydrogen bonding; π–π interactions
Abstract
In the title compound, [Fe(C12H12N2)(H2O)4]SO4, the central FeII ion is coordinated by two N atoms from the 5,5′-dimethyl-2,2′-bipyridine ligand and four water O atoms in a distorted octahedral geometry. The Fe—O coordination bond lengths vary from 2.080 (3) to 2.110 (3) Å, while the two Fe—N coordination bonds have practically identical lengths [2.175 (3) and 2.177 (3) Å]. The chelating N—Fe—N angle of 75.6 (1)° shows the largest deviation from an ideal octahedral geometry; the other coordination angles deviate from ideal values by 0.1 (1) to 9.1 (1)°. O—H⋯O hydrogen bonding between the four aqua ligands of the cationic complex and four O-atom acceptors of the anion leads to the formation of layers parallel to the ab plane. Neighbouring layers further interact by means of C—H⋯O and π–π interactions involving the laterally positioned bipyridine rings. The perpendicular distance between π–π interacting rings is 3.365 (2) Å, with a centroid–centroid distance of 3.702 (3) Å.
Chemical context
Coordination compounds containing polynitrile anions as ligands are of current interest for their magnetic properties and their rich architectures and topologies (Setifi et al., 2003 ▶; Gaamoune et al., 2010 ▶; Váhovská & Potočňák, 2012 ▶; Setifi, Setifi et al., 2013 ▶; Setifi, Domasevitsch et al., 2013 ▶; Potočňák et al., 2014 ▶). Given the crucial role of these anionic ligands, we are interested in using them in combination with other chelating or bridging neutral co-ligands to explore their structural and electronic characteristics in the large field of molecular materials exhibiting the spin crossover (SCO) phenomenon. In an attempt to prepare such a complex, we obtained the title compound, [Fe(dmbpy)(H2O)4]SO4, (I), where dmbpy is 5,5′-dimethyl-2,2′-bipyridyl. 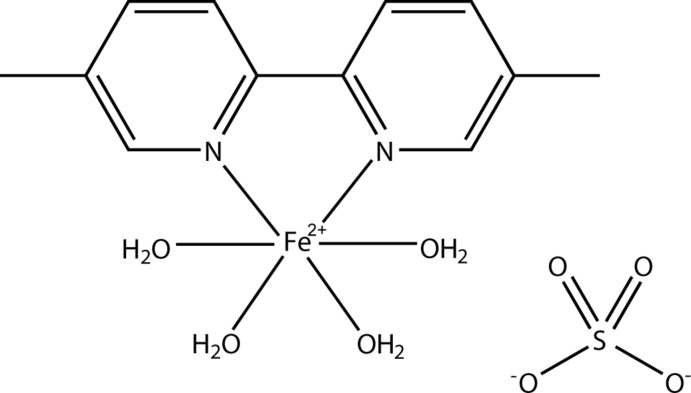
The crystal structures of several complexes with general formula [M(bpy)(H2O)4]2+ comprising bipyridine derivatives as ligands have been reported previously (Boonlue et al., 2012 ▶; Harvey et al., 1999 ▶; Kwak et al., 2007 ▶; Suarez et al., 2013 ▶; Xiao et al., 2003 ▶; Yang, 2009 ▶; Yu et al., 2007 ▶; Zhang et al., 2008 ▶; Zhao & Bai, 2009 ▶). This is the first complex of this type with FeII as the central ion.
Structural commentary
A molecular view of complex (I), together with the atom-numbering scheme is given in Fig. 1 ▶. The crystal structure of (I) consists of the cationic complex [Fe(dmbpy)(H2O)4]2+ and a free [SO4]2− counter-ion. The FeII atom is in a distorted octahedral coordination environment and the equatorial plane of the octahedron is formed by a pair of nitrogen donors from the 5,5′-dimethyl-2,2′-bipyridyl ligand and two molecules of water, while the axial sites are occupied by two other water molecules. The equatorial donor atoms are nearly coplanar (r.m.s. deviation = 0.0062 Å), while the deviation of the Fe atom from the least-squares plane is somewhat larger [0.021 (2) Å]. The bipyridine chelating angle N1—Fe—N2 of 75.6 (1)° shows the most significant deviation from an ideal octahedral geometry. The other angular distortions from an ideal octahedral geometry are in the range 0.1 (1) to 9.1 (1)°. The S—O bond lengths [1.466 (3)–1.480 (3) Å] and O—S—O angles [108.8 (2)–109.9 (2)°] indicate a nearly ideal tetrahedral geometry for the anion.
Figure 1.
The molecular structure of (I), with atom labels and 50% probability displacement ellipsoids for non-H atoms. Hydrogen bonds are indicated by dashed lines.
Supramolecular features
Within the crystal packing, the charged components are connected by an extensive hydrogen-bonding network (Table 1 ▶). Each of the [Fe(dmbpy)(H2O)4]2+ cations engages all four coordinating water molecules in hydrogen bonding to four [SO4]2− anions (Fig. 2 ▶
a). The anions surrounding the cationic unit are positioned at similar Fe⋯S distance of  4.9 Å. On the other hand, each of the [SO4]2− anions appears surrounded with four cationic units, where its four O atoms engage as acceptors in bifurcated O—H⋯O hydrogen bonds towards neighbouring cations (Fig. 2 ▶
a). Such a mutual arrangement leads to the formation of a two-dimensional hydrogen-bonded network parallel to the ab plane (Fig. 2 ▶
b). Laterally arranged aromatic rings of the 5,5′-dimethyl-2,2′-bipyridine ligand in neighbouring layers interact by means of weak C—H⋯O and π–π interactions, forming the three-dimensional crystal packing (Table 1 ▶ and Fig. 3 ▶). The centroid–centroid distance for the latter interaction is 3.702 (3) Å.
4.9 Å. On the other hand, each of the [SO4]2− anions appears surrounded with four cationic units, where its four O atoms engage as acceptors in bifurcated O—H⋯O hydrogen bonds towards neighbouring cations (Fig. 2 ▶
a). Such a mutual arrangement leads to the formation of a two-dimensional hydrogen-bonded network parallel to the ab plane (Fig. 2 ▶
b). Laterally arranged aromatic rings of the 5,5′-dimethyl-2,2′-bipyridine ligand in neighbouring layers interact by means of weak C—H⋯O and π–π interactions, forming the three-dimensional crystal packing (Table 1 ▶ and Fig. 3 ▶). The centroid–centroid distance for the latter interaction is 3.702 (3) Å.
Table 1. Hydrogen-bond geometry (, ).
| DHA | DH | HA | D A | DHA |
|---|---|---|---|---|
| O5H1O5O4i | 0.83 | 1.92 | 2.734(4) | 165 |
| O5H2O5O2ii | 0.96 | 1.94 | 2.794(4) | 147 |
| O6H1O6O1 | 0.94 | 1.90 | 2.820(4) | 167 |
| O6H2O6O3iii | 0.83 | 1.95 | 2.765(4) | 165 |
| O7H1O7O4ii | 0.83 | 1.89 | 2.722(4) | 175 |
| O7H2O7O2 | 0.82 | 1.89 | 2.697(4) | 167 |
| O8H1O8O1iii | 0.77 | 1.95 | 2.719(4) | 175 |
| O8H2O8O3i | 0.89 | 1.91 | 2.792(5) | 174 |
| C4H4O4iv | 0.93 | 2.54 | 3.232(5) | 132 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  .
.
Figure 2.
(a) O—H⋯O interactions (dashed lines) connect the cations and anions into layers parallel to the ab plane. (b) View of a single layer down the a axis.
Figure 3.

(a) The bipyridine rings from neighbouring layers interact via C—H⋯O and π–π interactions. (b) Orthogonal projection of the central fragment.
Synthesis and crystallization
The title compound, (I), was synthesized hydrothermally from a mixture of iron(II) sulfate heptahydrate (28 mg, 0.1 mmol), 5,5′-dimethyl-2,2′-bipyridyl (18 mg, 0.1 mmol) and potassium tricyanomethanide KC(CN)3 (26 mg, 0.2 mmol) in water–ethanol (4:1 v/v, 20 ml). The mixture was transferred to a Teflon-lined autoclave and heated at 410 K for 3 d. The autoclave was then allowed to cool to ambient temperature. Red crystals of (I) were collected by filtration, washed with water and dried in air (yield 35%).
Refinement details
Crystal data, data collection and structure refinement details are summarized in Table 2 ▶. H atoms bonded to C atoms were placed at geometrically calculated positions and refined using a riding model. C—H distances were fixed at 0.93 and 0.96 Å from aromatic and methyl C atoms, respectively. The U iso(H) values were equal to 1.2 and 1.5 times U eq of the corresponding C(sp 2) and C(sp 3) atoms. The H atoms of the four water molecules were initially located in a difference Fourier map. During the refinement, these H atoms were allowed to ride on their parent O atoms and also to rotate about the corresponding Fe—O bonds. The U iso(H) values were set equal to 1.2 times U eq of the parent O atom. The reflections (100) and (002) were excluded from the refinement because they were nearly completely obscured by the beamstop.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | [Fe(C12H12N2)(H2O)4]SO4 |
| M r | 408.21 |
| Crystal system, space group | Monoclinic, P21/c |
| Temperature (K) | 293 |
| a, b, c () | 9.5790(7), 9.6190(9), 18.5500(12) |
| () | 101.527(5) |
| V (3) | 1674.7(2) |
| Z | 4 |
| Radiation type | Mo K |
| (mm1) | 1.07 |
| Crystal size (mm) | 0.28 0.14 0.09 |
| Data collection | |
| Diffractometer | Bruker APEXII CCD |
| Absorption correction | Multi-scan (SADABS; Bruker, 2009 ▶) |
| T min, T max | 0.792, 0.881 |
| No. of measured, independent and observed [I > 2(I)] reflections | 14477, 4868, 3305 |
| R int | 0.117 |
| (sin /)max (1) | 0.706 |
| Refinement | |
| R[F 2 > 2(F 2)], wR(F 2), S | 0.065, 0.196, 1.08 |
| No. of reflections | 4867 |
| No. of parameters | 223 |
| H-atom treatment | H-atom parameters constrained |
| max, min (e 3) | 0.84, 1.33 |
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536814024982/vn2087sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536814024982/vn2087Isup2.hkl
CCDC reference: 1034106
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
The authors acknowledge the Algerian Ministry of Higher Education and Scientific Research, the Algerian Directorate General for Scientific Research and Technological Development and Ferhat Abbas Sétif 1 University for financial support. The Ministry of Education and Science of the Republic of Serbia is also thanked for support of the work of BMF and SBN (project Nos. 172014 and 172035).
supplementary crystallographic information
Crystal data
| [Fe(C12H12N2)(H2O)4]SO4 | F(000) = 848 |
| Mr = 408.21 | Dx = 1.619 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 4857 reflections |
| a = 9.5790 (7) Å | θ = 2.2–29.9° |
| b = 9.6190 (9) Å | µ = 1.07 mm−1 |
| c = 18.5500 (12) Å | T = 293 K |
| β = 101.527 (5)° | Block, red |
| V = 1674.7 (2) Å3 | 0.28 × 0.14 × 0.09 mm |
| Z = 4 |
Data collection
| Bruker APEXII CCD diffractometer | 4868 independent reflections |
| Radiation source: fine-focus sealed tube | 3305 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.117 |
| ω–2θ scans | θmax = 30.1°, θmin = 2.2° |
| Absorption correction: multi-scan (SADABS; Bruker, 2009) | h = −13→13 |
| Tmin = 0.792, Tmax = 0.881 | k = −13→13 |
| 14477 measured reflections | l = −26→26 |
Refinement
| Refinement on F2 | 223 parameters |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.065 | w = 1/[σ2(Fo2) + (0.0821P)2 + 2.0315P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.196 | (Δ/σ)max = 0.002 |
| S = 1.08 | Δρmax = 0.84 e Å−3 |
| 4867 reflections | Δρmin = −1.33 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Fe1 | 0.77171 (5) | 0.13308 (6) | 0.80535 (3) | 0.02534 (17) | |
| S1 | 0.75114 (9) | −0.36313 (10) | 0.75262 (5) | 0.0251 (2) | |
| O3 | 0.6693 (3) | −0.4269 (3) | 0.80282 (17) | 0.0376 (7) | |
| O1 | 0.6620 (3) | −0.2600 (3) | 0.70522 (17) | 0.0364 (7) | |
| O2 | 0.8783 (3) | −0.2931 (3) | 0.79546 (16) | 0.0338 (6) | |
| O4 | 0.7980 (3) | −0.4722 (3) | 0.70641 (16) | 0.0347 (6) | |
| O5 | 0.8418 (3) | 0.2477 (3) | 0.72229 (16) | 0.0361 (7) | |
| H1O5 | 0.8129 | 0.3296 | 0.7171 | 0.043* | |
| H2O5 | 0.9194 | 0.2065 | 0.7043 | 0.043* | |
| O6 | 0.6213 (3) | 0.0261 (3) | 0.72705 (17) | 0.0399 (7) | |
| H1O6 | 0.6389 | −0.0652 | 0.7130 | 0.048* | |
| H2O6 | 0.5378 | 0.0556 | 0.7195 | 0.048* | |
| O7 | 0.9191 (3) | −0.0166 (3) | 0.7871 (2) | 0.0489 (9) | |
| H1O7 | 1.0046 | −0.0016 | 0.7863 | 0.059* | |
| H2O7 | 0.8929 | −0.0981 | 0.7879 | 0.059* | |
| O8 | 0.6224 (3) | 0.2876 (4) | 0.8109 (2) | 0.0619 (11) | |
| H1O8 | 0.5422 | 0.2701 | 0.8053 | 0.074* | |
| H2O8 | 0.6333 | 0.3793 | 0.8107 | 0.074* | |
| N1 | 0.7224 (4) | 0.0244 (4) | 0.90015 (19) | 0.0340 (8) | |
| N2 | 0.9173 (4) | 0.2218 (4) | 0.89908 (19) | 0.0337 (8) | |
| C1 | 0.6251 (5) | −0.0763 (5) | 0.8969 (3) | 0.0390 (10) | |
| H1 | 0.5747 | −0.1028 | 0.8508 | 0.047* | |
| C2 | 0.5945 (5) | −0.1437 (5) | 0.9581 (3) | 0.0424 (10) | |
| C3 | 0.6691 (6) | −0.0975 (5) | 1.0262 (3) | 0.0452 (11) | |
| H3 | 0.6497 | −0.1362 | 1.0691 | 0.054* | |
| C4 | 0.7709 (5) | 0.0043 (5) | 1.0307 (2) | 0.0412 (10) | |
| H4 | 0.8219 | 0.0327 | 1.0763 | 0.049* | |
| C5 | 0.7973 (4) | 0.0650 (5) | 0.9666 (2) | 0.0341 (9) | |
| C6 | 0.9054 (4) | 0.1741 (4) | 0.9658 (2) | 0.0316 (8) | |
| C7 | 0.9931 (5) | 0.2257 (5) | 1.0288 (2) | 0.0440 (11) | |
| H7 | 0.9845 | 0.1926 | 1.0748 | 0.053* | |
| C8 | 1.0931 (5) | 0.3265 (5) | 1.0230 (3) | 0.0436 (11) | |
| H8 | 1.1512 | 0.3617 | 1.0653 | 0.052* | |
| C9 | 1.1071 (5) | 0.3750 (5) | 0.9549 (3) | 0.0411 (10) | |
| C10 | 1.0156 (5) | 0.3184 (5) | 0.8947 (2) | 0.0381 (9) | |
| H10 | 1.0231 | 0.3496 | 0.8482 | 0.046* | |
| C11 | 0.4852 (6) | −0.2566 (6) | 0.9492 (3) | 0.0594 (14) | |
| H11A | 0.3987 | −0.2243 | 0.9184 | 0.089* | |
| H11B | 0.4670 | −0.2817 | 0.9966 | 0.089* | |
| H11C | 0.5198 | −0.3363 | 0.9270 | 0.089* | |
| C12 | 1.2165 (5) | 0.4814 (6) | 0.9444 (3) | 0.0553 (13) | |
| H12A | 1.2919 | 0.4366 | 0.9261 | 0.083* | |
| H12B | 1.2548 | 0.5249 | 0.9907 | 0.083* | |
| H12C | 1.1726 | 0.5504 | 0.9099 | 0.083* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Fe1 | 0.0228 (3) | 0.0200 (3) | 0.0332 (3) | 0.0001 (2) | 0.00542 (19) | 0.0011 (2) |
| S1 | 0.0219 (4) | 0.0171 (4) | 0.0374 (5) | 0.0005 (3) | 0.0084 (3) | 0.0000 (4) |
| O3 | 0.0387 (16) | 0.0309 (16) | 0.0461 (17) | −0.0077 (13) | 0.0152 (13) | 0.0018 (13) |
| O1 | 0.0323 (15) | 0.0262 (15) | 0.0500 (17) | 0.0075 (12) | 0.0067 (12) | 0.0064 (13) |
| O2 | 0.0223 (12) | 0.0289 (16) | 0.0499 (17) | −0.0067 (11) | 0.0065 (11) | −0.0046 (13) |
| O4 | 0.0371 (15) | 0.0236 (14) | 0.0455 (16) | 0.0052 (12) | 0.0135 (12) | −0.0051 (12) |
| O5 | 0.0361 (15) | 0.0212 (14) | 0.0536 (18) | −0.0039 (12) | 0.0151 (13) | 0.0065 (13) |
| O6 | 0.0266 (14) | 0.0299 (16) | 0.0601 (19) | −0.0038 (12) | 0.0013 (13) | −0.0091 (15) |
| O7 | 0.0286 (15) | 0.0234 (15) | 0.098 (3) | 0.0025 (12) | 0.0207 (17) | 0.0004 (17) |
| O8 | 0.0270 (16) | 0.0275 (17) | 0.134 (4) | 0.0013 (13) | 0.022 (2) | 0.000 (2) |
| N1 | 0.0345 (17) | 0.0336 (19) | 0.0351 (18) | 0.0003 (15) | 0.0102 (14) | 0.0042 (15) |
| N2 | 0.0352 (18) | 0.0307 (18) | 0.0336 (17) | −0.0043 (14) | 0.0033 (14) | −0.0016 (14) |
| C1 | 0.039 (2) | 0.036 (2) | 0.042 (2) | −0.0060 (19) | 0.0111 (18) | 0.0020 (19) |
| C2 | 0.045 (2) | 0.035 (2) | 0.051 (3) | 0.001 (2) | 0.017 (2) | 0.009 (2) |
| C3 | 0.059 (3) | 0.042 (3) | 0.040 (2) | 0.002 (2) | 0.024 (2) | 0.012 (2) |
| C4 | 0.050 (3) | 0.043 (3) | 0.033 (2) | 0.003 (2) | 0.0133 (19) | 0.0032 (19) |
| C5 | 0.037 (2) | 0.032 (2) | 0.034 (2) | 0.0064 (17) | 0.0093 (16) | 0.0015 (17) |
| C6 | 0.0318 (19) | 0.030 (2) | 0.034 (2) | 0.0048 (16) | 0.0078 (16) | 0.0014 (16) |
| C7 | 0.049 (3) | 0.047 (3) | 0.034 (2) | 0.002 (2) | 0.0034 (19) | −0.003 (2) |
| C8 | 0.044 (2) | 0.040 (3) | 0.044 (3) | −0.005 (2) | 0.0012 (19) | −0.012 (2) |
| C9 | 0.040 (2) | 0.035 (2) | 0.047 (3) | −0.0016 (19) | 0.0050 (18) | −0.005 (2) |
| C10 | 0.040 (2) | 0.036 (2) | 0.038 (2) | −0.0048 (19) | 0.0059 (17) | 0.0020 (19) |
| C11 | 0.061 (3) | 0.050 (3) | 0.073 (4) | −0.006 (3) | 0.028 (3) | 0.014 (3) |
| C12 | 0.042 (3) | 0.051 (3) | 0.069 (3) | −0.017 (2) | 0.003 (2) | −0.007 (3) |
Geometric parameters (Å, º)
| Fe1—O8 | 2.080 (3) | C1—H1 | 0.9300 |
| Fe1—O7 | 2.091 (3) | C2—C3 | 1.394 (7) |
| Fe1—O6 | 2.099 (3) | C2—C11 | 1.494 (7) |
| Fe1—O5 | 2.110 (3) | C3—C4 | 1.373 (7) |
| Fe1—N2 | 2.175 (3) | C3—H3 | 0.9300 |
| Fe1—N1 | 2.177 (3) | C4—C5 | 1.392 (6) |
| S1—O3 | 1.466 (3) | C4—H4 | 0.9300 |
| S1—O2 | 1.477 (3) | C5—C6 | 1.477 (6) |
| S1—O1 | 1.479 (3) | C6—C7 | 1.388 (6) |
| S1—O4 | 1.480 (3) | C7—C8 | 1.382 (7) |
| O5—H1O5 | 0.8346 | C7—H7 | 0.9300 |
| O5—H2O5 | 0.9588 | C8—C9 | 1.379 (7) |
| O6—H1O6 | 0.9409 | C8—H8 | 0.9300 |
| O6—H2O6 | 0.8339 | C9—C10 | 1.385 (6) |
| O7—H1O7 | 0.8346 | C9—C12 | 1.504 (7) |
| O7—H2O7 | 0.8248 | C10—H10 | 0.9300 |
| O8—H1O8 | 0.7727 | C11—H11A | 0.9600 |
| O8—H2O8 | 0.8889 | C11—H11B | 0.9600 |
| N1—C1 | 1.337 (6) | C11—H11C | 0.9600 |
| N1—C5 | 1.354 (5) | C12—H12A | 0.9600 |
| N2—C10 | 1.336 (5) | C12—H12B | 0.9600 |
| N2—C6 | 1.345 (5) | C12—H12C | 0.9600 |
| C1—C2 | 1.389 (6) | ||
| O8—Fe1—O7 | 173.50 (16) | C2—C1—H1 | 117.9 |
| O8—Fe1—O6 | 90.06 (14) | C1—C2—C3 | 116.0 (4) |
| O7—Fe1—O6 | 86.71 (13) | C1—C2—C11 | 120.5 (5) |
| O8—Fe1—O5 | 89.18 (14) | C3—C2—C11 | 123.6 (4) |
| O7—Fe1—O5 | 85.26 (13) | C4—C3—C2 | 120.7 (4) |
| O6—Fe1—O5 | 91.46 (12) | C4—C3—H3 | 119.6 |
| O8—Fe1—N2 | 90.98 (15) | C2—C3—H3 | 119.6 |
| O7—Fe1—N2 | 93.10 (14) | C3—C4—C5 | 119.7 (4) |
| O6—Fe1—N2 | 170.92 (13) | C3—C4—H4 | 120.1 |
| O5—Fe1—N2 | 97.58 (13) | C5—C4—H4 | 120.1 |
| O8—Fe1—N1 | 92.32 (15) | N1—C5—C4 | 120.2 (4) |
| O7—Fe1—N1 | 93.60 (14) | N1—C5—C6 | 116.1 (4) |
| O6—Fe1—N1 | 95.39 (13) | C4—C5—C6 | 123.7 (4) |
| O5—Fe1—N1 | 172.99 (13) | N2—C6—C7 | 120.3 (4) |
| N2—Fe1—N1 | 75.55 (14) | N2—C6—C5 | 116.1 (4) |
| O3—S1—O2 | 109.72 (18) | C7—C6—C5 | 123.6 (4) |
| O3—S1—O1 | 109.88 (18) | C8—C7—C6 | 119.8 (4) |
| O2—S1—O1 | 109.25 (18) | C8—C7—H7 | 120.1 |
| O3—S1—O4 | 109.51 (18) | C6—C7—H7 | 120.1 |
| O2—S1—O4 | 108.76 (17) | C9—C8—C7 | 120.3 (4) |
| O1—S1—O4 | 109.70 (18) | C9—C8—H8 | 119.8 |
| Fe1—O5—H1O5 | 115.4 | C7—C8—H8 | 119.8 |
| Fe1—O5—H2O5 | 114.8 | C8—C9—C10 | 116.3 (4) |
| H1O5—O5—H2O5 | 127.9 | C8—C9—C12 | 123.1 (4) |
| Fe1—O6—H1O6 | 120.9 | C10—C9—C12 | 120.6 (4) |
| Fe1—O6—H2O6 | 116.9 | N2—C10—C9 | 124.4 (4) |
| H1O6—O6—H2O6 | 119.3 | N2—C10—H10 | 117.8 |
| Fe1—O7—H1O7 | 125.5 | C9—C10—H10 | 117.8 |
| Fe1—O7—H2O7 | 115.7 | C2—C11—H11A | 109.5 |
| H1O7—O7—H2O7 | 118.0 | C2—C11—H11B | 109.5 |
| Fe1—O8—H1O8 | 120.9 | H11A—C11—H11B | 109.5 |
| Fe1—O8—H2O8 | 128.8 | C2—C11—H11C | 109.5 |
| H1O8—O8—H2O8 | 109.3 | H11A—C11—H11C | 109.5 |
| C1—N1—C5 | 119.2 (4) | H11B—C11—H11C | 109.5 |
| C1—N1—Fe1 | 125.0 (3) | C9—C12—H12A | 109.5 |
| C5—N1—Fe1 | 115.8 (3) | C9—C12—H12B | 109.5 |
| C10—N2—C6 | 118.9 (4) | H12A—C12—H12B | 109.5 |
| C10—N2—Fe1 | 124.9 (3) | C9—C12—H12C | 109.5 |
| C6—N2—Fe1 | 116.3 (3) | H12A—C12—H12C | 109.5 |
| N1—C1—C2 | 124.1 (4) | H12B—C12—H12C | 109.5 |
| N1—C1—H1 | 117.9 |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O5—H1O5···O4i | 0.83 | 1.92 | 2.734 (4) | 165 |
| O5—H2O5···O2ii | 0.96 | 1.94 | 2.794 (4) | 147 |
| O6—H1O6···O1 | 0.94 | 1.90 | 2.820 (4) | 167 |
| O6—H2O6···O3iii | 0.83 | 1.95 | 2.765 (4) | 165 |
| O7—H1O7···O4ii | 0.83 | 1.89 | 2.722 (4) | 175 |
| O7—H2O7···O2 | 0.82 | 1.89 | 2.697 (4) | 167 |
| O8—H1O8···O1iii | 0.77 | 1.95 | 2.719 (4) | 175 |
| O8—H2O8···O3i | 0.89 | 1.91 | 2.792 (5) | 174 |
| C4—H4···O4iv | 0.93 | 2.54 | 3.232 (5) | 132 |
Symmetry codes: (i) x, y+1, z; (ii) −x+2, y+1/2, −z+3/2; (iii) −x+1, y+1/2, −z+3/2; (iv) x, −y−1/2, z+1/2.
References
- Boonlue, S., Theppitak, C. & Chainok, K. (2012). Acta Cryst. E68, m908. [DOI] [PMC free article] [PubMed]
- Bruker (2009). APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Gaamoune, B., Setifi, Z., Beghidja, A., El-Ghozzi, M., Setifi, F. & Avignant, D. (2010). Acta Cryst. E66, m1044–m1045. [DOI] [PMC free article] [PubMed]
- Harvey, M., Baggio, S., Baggio, R. & Mombrú, A. (1999). Acta Cryst. C55, 1457–1460.
- Kwak, O. K., Min, K. S. & Kim, B. G. (2007). Acta Cryst. E63, m17–m19.
- Macrae, C. F., Edgington, P. R., McCabe, P., Pidcock, E., Shields, G. P., Taylor, R., Towler, M. & van de Streek, J. (2006). J. Appl. Cryst. 39, 453–457.
- Potočňák, I., Váhovská, L. & Herich, P. (2014). Acta Cryst. C70, 432–436. [DOI] [PubMed]
- Setifi, Z., Domasevitch, K. V., Setifi, F., Mach, P., Ng, S. W., Petříček, V. & Dušek, M. (2013). Acta Cryst. C69, 1351–1356. [DOI] [PubMed]
- Setifi, F., Ouahab, L., Golhen, S., Miyazaki, A., Enoki, A. & Yamada, J. I. (2003). C. R. Chim. 6, 309–316.
- Setifi, Z., Setifi, F., Ng, S. W., Oudahmane, A., El-Ghozzi, M. & Avignant, D. (2013). Acta Cryst. E69, m12–m13. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Suarez, S., Doctorovich, F., Harvey, M. A. & Baggio, R. (2013). Acta Cryst. C69, 351–355. [DOI] [PubMed]
- Váhovská, L. & Potočňák, I. (2012). Acta Cryst. E68, m1524–m1525. [DOI] [PMC free article] [PubMed]
- Xiao, H.-P., Shi, Z., Zhu, L.-G., Xu, R.-R. & Pang, W.-Q. (2003). Acta Cryst. C59, m82–m83. [DOI] [PubMed]
- Yang, H. (2009). Acta Cryst. E65, m1207. [DOI] [PMC free article] [PubMed]
- Yu, M., Liu, S.-X., Xie, L.-H., Cao, R.-G. & Ren, Y.-H. (2007). Acta Cryst. E63, m2110.
- Zhang, B.-Y., Nie, J.-J. & Xu, D.-J. (2008). Acta Cryst. E64, m1003–m1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Q.-L. & Bai, H.-F. (2009). Acta Cryst. E65, m866. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536814024982/vn2087sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536814024982/vn2087Isup2.hkl
CCDC reference: 1034106
Additional supporting information: crystallographic information; 3D view; checkCIF report




