Abstract
The rodent medial prefrontal cortex (mPFC) has been implicated in working memory function; lesions and inactivation of this region have been shown to result in impairments in spatial working memory tasks. Our laboratory has developed a tactile-visual conditional discrimination task, which uses floor insert cues to signal the correct goal-arm choice in a T-maze. This task can be manipulated by altering the floor insert cues to be present throughout the trial (CDSTANDARD) or present only at the beginning of the trial (CDWM), thus making the task either working-memory independent or working-memory dependent, respectively. This ability to manipulate the working memory demand of the task while holding all other task features constant allows us to rule out the possibility that confounding performance variables contributed to the observed impairment. A previous study from our lab showed that mPFC inactivation did not impair performance on CDSTANDARD, confirming that mPFC inactivation does not induce sensori-motor or motivational deficits which could impact task performance. In order to examine whether mPFC inactivation impairs CDWM, the current study transiently inactivated the mPFC with bilateral microinfusions of muscimol immediately prior to testing on the CDWM task. As predicted, CDWM task performance was significantly impaired during the muscimol-infusion session compared to the control saline-infusion sessions. Together with our previous demonstration that the mPFC in not required for CDSTANDARD, these results not only confirm that the mPFC is crucial for working memory, but also set the stage for using the task-comparison approach to investigate cortico-limbic interactions during working memory.
Keywords: prefrontal cortex, muscimol, working memory, conditional discrimination, inactivation
The rodent medial prefrontal cortex (mPFC) has been implicated in a variety of functions, including working memory, decision-making, and integration of various sensory cues into long-term memory (Euston, Gruber, & McNaughton, 2012; Funahashi & Kubota, 1994; Horst & Laubach, 2009; Jo et al., 2007). Lesions or inactivation of the mPFC produce deficits in spatial working memory tasks (Dunnett, Nathwani, & Brasted, 1999; Rogers et al., 1992; Shaw, Watson, Hallock, Cline, & Griffin, 2013; Sloan, Good, & Dunnett, 2006). These results suggest that the mPFC is important for the retention of situation-specific information that can be used to guide goal-directed behavior. However, previous studies have not used control tasks to rule out the possibility that task impairments were due in part to confounding performance variables such as sensorimotor deficits or motivational factors.
Our laboratory recently developed a working-memory-dependent conditional discrimination task (CDWM). This task differs from the standard version of conditional discrimination (CDSTANDARD) only in its working memory demand. Thus, the novelty of our approach is that we are able to compare performance across the CDWM and CDSTANDARD tasks, allowing us to conclude that differences observed between the tasks are due to a working-memory deficit and not other potential confounding factors such as motivational issues or sensori-motor deficits. It has been shown that mPFC inactivation does not impair performance on the CDSTANDARD (Shaw et al., 2013), and that the CDSTANDARD task is striatal-dependent (Hallock et al., 2013a). However, the role of the mPFC in performance of the CDWM task has not been established. Therefore, we trained rats to perform the CDWM task, implanted cannulae bilaterally into the mPFC and transiently inactivated the mPFC with muscimol. We predicted that mPFC inactivation would impair CDWM task performance.
Methods
Subjects
15 male Long-Evans hooded rats (Harlan, Indianapolis) were ordered to arrive at PD32. Rats were allowed 7 days of acclimation to the colony room. They were housed individually in standard laboratory caging on a 12:12 hour light/dark cycle. During acclimation, rats were given ad libitum access to food and water; thereafter they were food restricted to maintain at 80% of their free-feeding body weight but given ad libitum access to water. All procedures were carried out in accordance with the University of Delaware Institutional Animal Care and Use Committee.
Apparatus
The task was conducted on a wooden T-maze that consisted of a central stem (117 × 6 cm), two goal arms (79 × 6 cm) and two return arms (120 × 6 cm) (Figure 1A; Hallock, Wang, Shaw, & Griffin, 2013b). A start box consisted of a circular platform which abutted the central arm of the maze that was separated from the maze by a removable barrier. Plastic cups located at the end of each goal arm were baited with a chocolate sprinkle reward (Fig 1A). The room was illuminated by a single 60 W bulb and surrounded by a black curtain. The floor of the maze was covered with black vinyl. Three removable wooden floor inserts covered with black plastic mesh on one side and smooth wood on the other served as conditional cues. One insert (76 × 8 cm) extended halfway from the start box to the goal arm juncture. The other two inserts (31 × 8 cm) were placed at the ends of the goal arms leading up to the reward cups.
Figure 1.
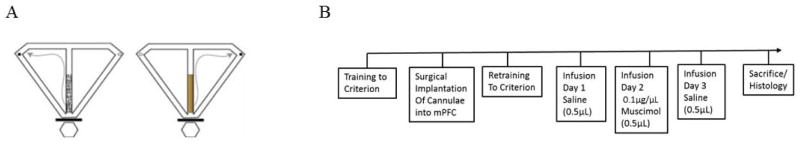
A, Schematic of the CDWM task. Rats are required to run up the central stem, choose a goal arm, consume the chocolate sprinkle reward (black dots on figures), and return to the start box via the return arms where they were confined until the start of the next trial. Rats are required to choose the goal arm that contains reward based on the texture and appearance of a floor insert (e.g., left on mesh, right on wood). B, Experimental timeline. All rats received a bilateral infusion of physiological saline on Day 1 (0.5μL), followed by muscimol on Day 2 (0.1μg/μL; total volume 0.5μL), and a final saline infusion on Day 3 (0.5μL).
Handling and pretraining
After a 1-week acclimation period to the colony room, rats were brought to the laboratory daily for 5–7 days and handled for 10–15 minutes by the experimenter. For the next 2–6 days, rats underwent goal box training; they were confined to the goal arms on the maze and allowed to eat sprinkles from the reward cups for a total of 6 daily trials, 3 per goal arm. Once rats consumed the reward in less than 90 seconds on every trial for two consecutive sessions, they progressed to the forced-run stage of pretraining. For forced run sessions (12 trials per day), one of the goal arms was blocked and the rat was encouraged to run up the start arm, select the available goal arm, consume the reward, and return to the start box via the return arm. Once rats were consuming rewards on 80% of forced run trials for 2 consecutive days, they progressed to the CDWM task.
Working memory conditional discrimination task
Prior to each trial, the experimenter placed the floor inserts into the central stem and both goal arms of the T-maze with either the wood or mesh side facing up. Rats learned to select either the left or right goal arm to obtain a food reward contingent upon the texture/color of the floor insert. Half of the rats were trained on the rule ‘left on wood/right on mesh’ and the other half was trained on the ‘right on wood/left on mesh’ rule. Because the floor insert extended approximately halfway up the stem, rats were required to retain the cue information until reaching at the goal arm juncture (Hallock et al., 2013b). During the inter-trial interval (ITI), a black wooden barricade was placed between the start box and the maze to obstruct the rats’ view while the experimenter prepared for the next trial. In order to prevent the rat from using auditory cues to anticipate the next trial, the experimenter flipped the insert during every ITI. The unrewarded goal zone was sham baited on each trial. Although trial duration, and thus delay length, was not measured in the current study, pilot data from our laboratory show that rats take ~1 s to traverse the insert-free portion of the maze stem, with minimal variance between subjects and trials (M = 1.0 sec, SD = .6 sec). Each ITI lasted for 8–10 s. Rats were given 24 trials (12 mesh, 12 wood) in a pseudorandom sequence (Fellows, 1967). Criterion was set at 80% correct for 2 consecutive days.
Cannula implantation
Bilateral cannulae were implanted into the mPFC accordingly to published procedures (Shaw et al., 2013). Briefly, rats were anesthetized with continuous-flow isoflurane (1.5–3% in oxygen). The skull was exposed and four small holes were drilled near the skull ridge using a stereotaxic-mounted drill (Fine Science Tools) and anchor screws were inserted. Circular holes were drilled using a 1.8-mm-diameter trephine (Fine Science Tools) at the following coordinates: 3.0 mm anterior to bregma, ±1.8 mm lateral to bregma (Paxinos, 2005). A 26-gauge stainless steel guide cannula (PlasticsOne, Roanoke, Virginia), held in a stereotaxic arm at a 14° angle, was lowered into each hemisphere 2.0 mm ventral to the brain surface. The guide cannulae and anchor screws were affixed to the skull with dental acrylic (Lang Dental, Wheeling, Illinois). A subcutaneous injection of Banamine (2.5 mg/kg) was given approximately 30 min prior to the end of surgery and children’s ibuprofen (20 mg/mL) was given in the drinking water two days postoperatively for analgesia. Rats were allowed to recover for 5 days.
Microinfusions
Muscimol, a GABAA agonist, was dissolved in phosphate-buffered saline (PBS) and the solution was infused bilaterally via a 31-gauge injector cannula connected to a 10-μL Hamilton syringe by a polyethylene tube. The injector cannula extended 1.5 mm beyond the tip of the guide cannula. The infusion volume and rate was controlled by an infusion pump (World Precision Instruments) programmed to deliver the infusate, either muscimol (0.1 μg/μL) or PBS, at a rate of 0.25 μL/min for 2 min, for a total volume of 0.50 μL for each hemisphere. Injector cannulae were left in place for 2 min after the infusion to allow for diffusion and were withdrawn slowly to prevent capillary diffusion of the solution back up into the cannula. Rats were placed back into their home cages for 40 minutes post-infusion to allow for drug effects to take hold. Each rat was given a saline infusion on Day 1, muscimol on Day 2, and saline again on Day 3 (Fig 1B). The second saline day was given to confirm that muscimol-induced deficits were not a result of mechanical tissue damage resulting from multiple infusions.
Histology
After the final infusion day, rats were anesthetized with isoflurane and given an overdose of sodium pentobarbital (200 mg/kg, ip) and perfused using 0.9% saline followed by 10% buffered formalin. The brains were then removed and placed in 10% buffered formalin. After at least 24 h in formalin, the brains were placed in 30% buffered sucrose solution. After sinking, the brains were frozen and sectioned (40 μm) using a cryostat. The sections were mounted on slides, stained with cresyl violet (0.5%) and photographed using a camera mounted on a microscope. Cannulae placements were verified by overlaying digital photographs of each section with digital atlas plates from the Paxinos and Watson rat brain atlas (Paxinos, 2005) in Adobe Illustrator.
Data Analysis
The number of correct trials per 6-trial block across the saline, muscimol, and second saline-infusion sessions was analyzed using a two-way repeated-measures ANOVA. Posthoc pairwise comparisons were done using Bonferroni-corrected t-tests. An alpha level of 0.05 was used for all statistical tests.
RESULTS
Cannula Placements
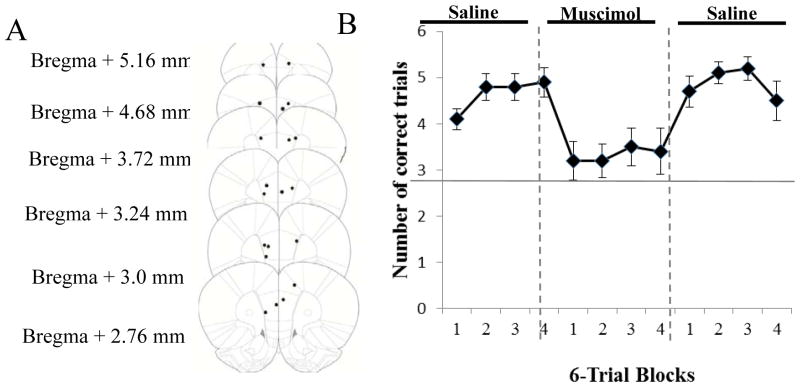
15 rats were implanted with guide cannulae, given muscimol infusions, and tested on the CDWM task. Of these, 10 rats had cannula tracks that terminated bilaterally in the mPFC. 9 rats had bilateral cannulae placements in the prelimbic region of the mPFC, and 1 rat had a unilateral placement in the prelimbic region of the mPFC and a unilateral placement in the anterior cingulate (AC) region of the mPFC (Figure 2A). Only rats with cannulae placements in either mPFC or AC were included in the statistical analysis.
Figure 2.
A, Cannula placements in mPFC. Cannula placements ranged from Bregma +5.16 mm to Bregma +2.76 mm, in the prelimbic and anterior cingulate regions of the mPFC. From The Rat Brain in Stereotaxic Coordinates (5th ed.), pp. 45–52, by G. Paxinos & C. Watson, 2005, New York, NY: Academic Press. Copyright 2005 by Elsevier Academic Press. Adapted with permission. B, Muscimol significantly impaired performance on the CDWM task. The number of correct trials per 6-trial block is shown for the saline, muscimol and second saline-infusion sessions. Muscimol infusion resulted in a significant drop in performance when compared to the other two sessions. The first saline-infusion day did not differ from the final saline-infusion day. The solid horizontal line indicates chance-level performance (3 out of 6 trials). Error bars represent SEM.
Inactivation of the mPFC Significantly Impaired CDWM task performance
Rats required an average of 21.8 + 2.69 days to reach criterion on the CDWM task. Most of the rats (7/10 rats) required only 2–3 days of post-surgery training to reach criterion once again before infusions began. The remaining 3 rats required more days of post-surgery training (6, 12, and 24 days). A two-way repeated measures ANOVA on the number of correct trials per 6-trial block revealed a significant main effect of session, F(2,18) = 41.34, p < .01. There was no significant main effect of block, F(3,27) = 1.01, p = .41, and no significant block-by-session interaction, F(6,54) = .50, p = .81. Bonferroni-corrected pairwise comparisons showed that the number of correct trials on the muscimol-infusion session (M = 13.3, SD = .52) was significantly lower than the first saline-infusion session (M = 18.6, SD = .52, p < .01) and the second saline-infusion session (M =19.5, SD = .65, p < .001). The first and second saline-infusion sessions were not significantly different from one another (Fig. 2B).
Discussion
Transient bilateral inactivation of the mPFC with muscimol impaired performance on a working-memory-dependent conditional discrimination task (CDWM). This version of the conditional discrimination task utilizes floor inserts that extend only halfway up the central stem of the T-maze, requiring the rat to hold the information of the cue in memory for a short time before making a goal-arm choice. These results are consistent with previous studies demonstrating that inactivation or lesions of the mPFC results in impairments on spatial working memory tasks (Clark et al., 2008; D’Esposito, Cooney, Gazzaley, Gibbs, & Postle, 2006; Eichenbaum et al., 1983; Kolb et al., 1974; Kyd & Bilkey, 2003). However, our findings add two additional insights. First, our laboratory uses a task-comparison approach in which performance is compared across two tasks that differ only in their reliance on working memory. Our lab has previously demonstrated that performance on the CDSTANDARD task, which differs from the CDWM task only in its reliance on working memory, does not require the mPFC (Shaw et al., 2013). Together, these findings support the notion that failure to perform the CDWM task resulted from the inability to use working memory, excluding the possibility that the impaired performance was due to other potential confounding performance variables such as motivational or sensory deficits. A second novel insight is that the delay length in the current study was appreciably shorter in duration than delays used in most of the previous studies that have found prefrontal-dependent working-memory deficits. However, a recent study found working memory deficits after mPFC inactivation for delays as short as 3 seconds using a delayed spatial alternation task (Yoon et al., 2008). The fact that we find near-chance performance following muscimol infusion with only a 1–2 second delay between cue presentation and response suggests that the ability to use a conditional cue to guide spatial behavior may tax working memory to a greater extent than traditional delayed-response tasks. Moreover, our results suggest that the ability to hold the conditional cue “online” is compromised after mPFC inactivation regardless of the duration of time that the cue needs to be retained.
Because the CDWM task has a major spatial component, successful task performance may require both the integrity of the mPFC itself and interactions between the mPFC and the hippocampus, a notion that is supported by the known anatomical connectivity between these two structures (Jay, Glowinski, & Thierry, 1989; Jay & Witter, 1991; Thierry, Gioanni, Degenetais, & Glowinski, 2000). Our lab previously reported that inactivation of the nucleus reuniens/rhomboid nucleus (Re/Rh) impaired performance on the CDWM task, without impacting performance on the working-memory-independent CDSTANDARD task (Hallock et al., 2013b). These midline thalamic nuclei are bidirectionally connected to both the hippocampus and mPFC (Cavdar et al., 2008; Vertes, 2002; Vertes, Hoover, Szigeti-Buck, & Leranth, 2007). This anatomical connectivity likely enables the RE/Rh to coordinate and modulate hippocampal-prefrontal synchrony, which has been shown to be correlated with working memory performance (Hyman, Zilli, Paley, & Hasselmo, 2010; Jones & Wilson, 2005; Parnaudeau et al., 2013; Sigurdsson, Stark, Karayiorgou, Gogos, & Gordon, 2010). These findings set the stage for future investigations using comparisons across the CDWM and CDSTANDARD task to examine circuit-level interactions between the hippocampus, higher-order thalamic nuclei, and the mPFC during working memory.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant 1P20GM103653 – 01A1 (A.L.G.).
References
- Cavdar S, Onat FY, Cakmak YO, Yananli HR, Gulcebi M, Aker R. The pathways connecting the hippocampal formation, the thalamic reuniens nucleus and the thalamic reticular nucleus in the rat. J Anat. 2008;212(3):249–256. doi: 10.1111/j.1469-7580.2008.00858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Bechara A, Damasio H, Aitken MR, Sahakian BJ, Robbins TW. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain. 2008;131(Pt 5):1311–1322. doi: 10.1093/brain/awn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Cooney JW, Gazzaley A, Gibbs SE, Postle BR. Is the prefrontal cortex necessary for delay task performance? Evidence from lesion and FMRI data. J Int Neuropsychol Soc. 2006;12(2):248–260. doi: 10.1017/S1355617706060322. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Nathwani F, Brasted PJ. Medial prefrontal and neostriatal lesions disrupt performance in an operant delayed alternation task in rats. Behav Brain Res. 1999;106(1–2):13–28. doi: 10.1016/s0166-4328(99)00076-5. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Clegg RA, Feeley A. Reexamination of functional subdivisions of the rodent prefrontal cortex. Exp Neurol. 1983;79(2):434–451. doi: 10.1016/0014-4886(83)90224-8. [DOI] [PubMed] [Google Scholar]
- Euston DR, Gruber AJ, McNaughton BL. The role of medial prefrontal cortex in memory and decision making. Neuron. 2012;76(6):1057–1070. doi: 10.1016/j.neuron.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows BJ. Chance stimulus sequence for discrimination tasks. Psychol Bull. 1967;67 doi: 10.1037/h0024098. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Kubota K. Working memory and prefrontal cortex. Neurosci Res. 1994;21(1):1–11. doi: 10.1016/0168-0102(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Hallock HL, Arreola AR, Shaw CL, Watson GDR, Griffin AL. Dissociable Roles of the Dorsal Striatum and Dorsal Hippocampus in Conditional Discrimination and Spatial Alternation T-Maze Tasks. Neurobiol Learn Mem. 2013a;100:108–116. doi: 10.1016/j.nlm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Hallock HL, Wang A, Shaw CL, Griffin AL. Transient inactivation of the thalamic nucleus reuniens and rhomboid nucleus produces deficits of a working-memory dependent tactile-visual conditional discrimination task. Behav Neurosci. 2013b;127(6):860–866. doi: 10.1037/a0034653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornak J, O’Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, Polkey CE. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. J Cogn Neurosci. 2004;16(3):463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- Horst NK, Laubach M. The role of rat dorsomedial prefrontal cortex in spatial working memory. Neuroscience. 2009;164(2):444–456. doi: 10.1016/j.neuroscience.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Working Memory Performance Correlates with Prefrontal-Hippocampal Theta Interactions but not with Prefrontal Neuron Firing Rates. Front Integr Neurosci. 2010;4:2. doi: 10.3389/neuro.07.002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TM, Glowinski J, Thierry AM. Selectivity of the hippocampal projection to the prelimbic area of the prefrontal cortex in the rat. Brain Res. 1989;505(2):337–40. doi: 10.1016/0006-8993(89)91464-9. [DOI] [PubMed] [Google Scholar]
- Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1991;313(4):574–86. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- Jo YS, Park EH, Kim IH, Park SK, Kim H, Kim HT, Choi JS. The medial prefrontal cortex is involved in spatial memory retrieval under partial-cue conditions. J Neurosci. 2007;27(49):13567–13578. doi: 10.1523/JNEUROSCI.3589-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3(12):e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Nonneman AJ, Singh RK. Double dissociation of spatial impairments and perseveration following selective prefrontal lesions in rats. J Comp Physiol Psychol. 1974;87(4):772–780. doi: 10.1037/h0036970. [DOI] [PubMed] [Google Scholar]
- Kyd RJ, Bilkey DK. Prefrontal cortex lesions modify the spatial properties of hippocampal place cells. Cereb Cortex. 2003;13(5):444–451. doi: 10.1093/cercor/13.5.444. [DOI] [PubMed] [Google Scholar]
- Parnaudeau S, O’Neill PK, Bolkan SS, Ward RD, Abbas AI, Roth BL, Kellendonk C. Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron. 2013;77(6):1151–1162. doi: 10.1016/j.neuron.2013.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos JWC. The rat brain in stereotaxic coordinates. 5. New York, NY: Elsevier; 2005. [Google Scholar]
- Rogers DC, Wright PW, Roberts JC, Reavill C, Rothaul AL, Hunter AJ. Photothrombotic lesions of the frontal cortex impair the performance of the delayed non-matching to position task by rats. Behav Brain Res. 1992;49(2):231–235. doi: 10.1016/s0166-4328(05)80169-x. [DOI] [PubMed] [Google Scholar]
- Shaw CL, Watson GD, Hallock HL, Cline KM, Griffin AL. The role of the medial prefrontal cortex in the acquisition, retention, and reversal of a tactile visuospatial conditional discrimination task. Behav Brain Res. 2013;236(1):94–101. doi: 10.1016/j.bbr.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464(7289):763–767. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan HL, Good M, Dunnett SB. Double dissociation between hippocampal and prefrontal lesions on an operant delayed matching task and a water maze reference memory task. Behav Brain Res. 2006;171(1):116–126. doi: 10.1016/j.bbr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Gioanni Y, Dégénétais E, Glowinski J. Hippocampo-prefrontal cortex pathway: anatomical and electrophysiological characteristics. Hippocampus. 2000;10(4):411–9. doi: 10.1002/1098-1063(2000)10:4<411::AID-HIPO7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Verin M, Partiot A, Pillon B, Malapani C, Agid Y, Dubois B. Delayed response tasks and prefrontal lesions in man--evidence for self generated patterns of behaviour with poor environmental modulation. Neuropsychologia. 1993;31(12):1379–1396. doi: 10.1016/0028-3932(93)90105-9. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Analysis of projections from the medial prefrontal cortex to the thalamus in the rat, with emphasis on nucleus reuniens. J Comp Neurol. 2002;442(2):163–187. doi: 10.1002/cne.10083. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Szigeti-Buck K, Leranth C. Nucleus reuniens of the midline thalamus: link between the medial prefrontal cortex and the hippocampus. Brain Res Bull. 2007;71(6):601–609. doi: 10.1016/j.brainresbull.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon T, Okada J, Jung MW, Kim JJ. Prefrontal cortex and hippocampus subserve different components of working memory in rat. Learn Mem. 2014;15:97–105. doi: 10.1101/lm.850808. [DOI] [PMC free article] [PubMed] [Google Scholar]