Abstract
Background
The potential epidemiological impact of isoniazid preventive therapy (IPT), delivered at levels that could be feasibly scaled up among people living with HIV (PLHIV) in modern, moderate-burden settings, remains uncertain.
Methods
We used routine surveillance and implementation data from a cluster-randomized trial of IPT among HIV-infected clinic patients with good access to antiretroviral therapy in Rio de Janeiro, Brazil, to populate a parsimonious transmission model of TB/HIV. We modeled IPT delivery as a constant process capturing a proportion of the eligible population every year. We projected feasible reductions in tuberculosis (TB) incidence and mortality in the general population and among PLHIV specifically at the end of five years after implementing an IPT program.
Results
Data on time to IPT fit an exponential curve well, suggesting that IPT was delivered at a rate covering 20% (95% confidence interval: 16%, 24%) of the 2,500 eligible individuals each year. By the end of year 5 after modeled program roll-out, IPT had reduced TB incidence by 3.0% (95% uncertainty range, UR: 1.6%, 7.2%) in the general population and by 15.6% (95%UR: 15.5%, 36.5%) among PLHIV. Corresponding reductions in TB mortality were 4.0% (95%UR: 2.2%, 10.3%) and 14.3% (14.6%, 33.7%). Results were robust to wide variations in parameter values on sensitivity analysis.
Conclusions
TB screening and IPT delivery can substantially reduce TB incidence and mortality among PLHIV in urban, moderate-burden settings. In such settings, IPT can be an important component of a multi-faceted strategy to feasibly reduce the burden of TB in PLHIV.
Keywords: Tuberculosis, Infectious Disease Transmission, HIV, Theoretical Models, Brazil, Isoniazid
INTRODUCTION
Isoniazid preventive therapy (IPT) is recommended by the World Health Organization (WHO) as a cornerstone of the “Three I’s” approach to management of tuberculosis (TB) in people living with HIV (PLHIV).1 The individual-level effectiveness of IPT in preventing active TB is well-established,2,3 including among PLHIV.4–7 However, although IPT showed dramatic population-level effects in the populations of Alaska and Greenland in the 1960s,8,9 the population-level impact of IPT among PLHIV in the modern era is less clear. HIV co-infection decreases both the duration and sputum bacillary burden of TB,10,11 such that prevention of TB in PLHIV may have less impact on transmission. Individual-level effectiveness of IPT does not necessarily translate to population-level reductions in TB incidence,12 as recently demonstrated by a cluster-randomized trial of IPT in South African gold mines.13 The mechanism of IPT effect in the era of antiretroviral therapy (ART) is also uncertain, with a large trial in Botswana suggesting rapid waning of efficacy among PLHIV once IPT was discontinued.14 Whether this result reflects the high risk of TB reinfection in Botswana or a general inability of IPT to provide lasting protection against TB among PLHIV is unclear. If IPT is to be broadly recommended as a public health strategy, its epidemiological impact in this population must be better understood.
The potential for benefit of IPT is arguably greatest in moderate-burden settings, where TB incidence is sufficiently high for IPT to prevent substantial disease burden but rates of TB reinfection are relatively low. In a recent cluster-randomized trial in 29 public HIV clinics in Rio de Janeiro, IPT reduced the adjusted hazard of active TB by 27% among participants who contacted the study clinics.15 However, this trial was limited in duration and did not aim to evaluate the impact of IPT at a population level, including transmission effects among people who did not present to the study clinics (e.g., HIV-negative, unaware of HIV serostatus, or HIV-infected but poorly connected to care). This trial also provides an opportunity to evaluate potential relationships between the mechanism/duration of IPT effect in PLHIV and the expected epidemiological impact of IPT in a setting where TB reinfection is relatively uncommon. To explore these questions, we created a dynamic transmission model of the TB/HIV epidemic in Rio de Janeiro, Brazil.
METHODS
Study Design
The design of the TB/HIV in Rio de Janeiro (THRio) study has been described elsewhere.16,17 Briefly, 29 public primary HIV clinics in Rio de Janeiro were assigned, in stepped-wedge fashion18,19 to a random date of implementing TB screening and IPT over a 28-month period, then followed for an additional 20 months. During the time of this trial, Brazilian policy was to offer antiretroviral therapy (ART) to all HIV-infected adults with CD4+ T-cell counts ≤350 cells/mm3; it is estimated that ART coverage among such individuals was 80%.20 Clinics aimed to provide IPT (six months of isoniazid) to all HIV-infected adults with a positive tuberculin skin test (TST) result in whom active TB had been excluded. The primary outcomes were incidence of active TB and a composite of incident TB and all-cause mortality. The National Health Information System in Brazil captures both incident TB and death and was linked to study data to ensure complete ascertainment of TB cases and death.
Modeled Intervention
Our primary aim was to estimate the potential epidemiological impact of a feasible intervention of training clinics to perform TST screening and provide IPT in a moderate-burden setting, using the THRio intervention as a paradigm. We modeled this intervention as continuous delivery of TST screening and IPT to a proportion of the eligible population (HIV-infected adults with no prior history of active TB or IPT) every year: the “IPT delivery rate.” To estimate this rate in the THRio study, we fit an exponential decay curve to the probability of an eligible individual not receiving IPT on each day after the randomization date of that individual’s clinic (or the individual’s first visit date for any reason, among people whose first clinic visit occurred after clinic randomization). In making this calculation, we included all people eligible for TB screening on each day regardless of whether they actually contacted the clinic, assuming that rates of TST positivity were similar in patients who never received TST as in those who did. This analysis therefore differs from the primary THRio analysis, in which people became eligible for the intervention only after contacting a study clinic following implementation of the intervention.
Model Structure and Calibration
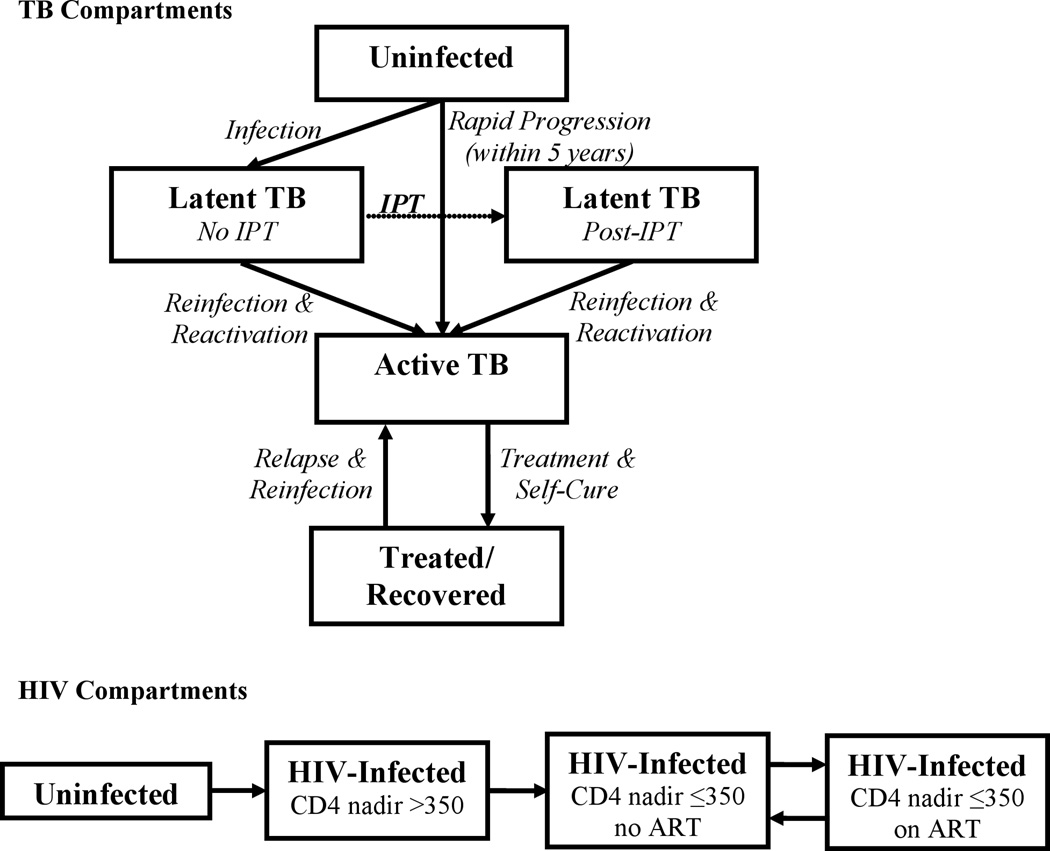
We used the estimated IPT delivery rate from the trial to populate a compartmental transmission model of TB in the adult (age 15–59) population of Rio de Janeiro (Figure 1). We then used literature estimates of the individual-level effect of IPT to project the epidemiological impact on TB incidence over five years under different assumptions about IPT duration of effect (persistent versus lasting only for the six-month duration of IPT itself). In model development, we strove for simplicity in order to maximize transparency and interpretability, and to reduce reliance on excessive assumptions regarding parameter values. This model uses ordinary differential equations to describe rates of flow between population states defined by HIV and TB status (as shown in Figure 1), and among those infected with TB, TB drug resistance status (susceptible and multidrug-resistant, MDR). Complete details are provided in the Supplemental Digital Content 1, and model code is available in a public repository (https://github.com/ddowdy/THRio-model).
Figure 1. Structure of Compartmental Transmission Model.
Rates of flow between each compartment are governed by differential equations, as described in the Appendix. Each compartment is further sub-divided by HIV status and drug-resistance status (multidrug-resistant or not multidrug-resistant). IPT (dotted line) is modeled as a one-time intervention, delivered only to people living with HIV, providing immediate partial protection (among those who will complete therapy) against reactivation TB, no protection against reinfection, and no benefit for those infected with multidrug-resistant TB. After completing IPT, individuals remain in the post-IPT state for the remainder of the analysis. Per contemporary Brazilian policy, ART was assumed to be available only to those with CD4 counts ≤350, and to provide immediate benefit (equivalent to having a CD4 count >350) upon initiation.
This model conceptualizes IPT as a one-time intervention that provides immediate, partial (67%) protection against reactivation of latent, drug-susceptible TB. We assumed in the base case that this benefit would last at least five years, based on data from the THRio study suggesting that TB incidence did not increase during this time frame after IPT completion. We compared this assumption against a pessimistic scenario in which IPT provides only six months of protection, and we also considered an alternative model structure in which IPT provided protection from both reactivation and reinfection. Given high rates of IPT completion in the trial, we did not model partial courses of IPT. Differential equations were solved using the deSolve package in R (R Project for Statistical Computing), in time steps of 0.1 year.
We fit our model simultaneously to 11 key epidemiological data points, estimated from surveillance systems in Rio de Janeiro where available (year 2008) and from Brazilian country-wide estimates otherwise (Table 1). We accomplished this model fit by matching each of 11 model parameters to the best corresponding epidemiological data point in one-to-one (identifiable) fashion to create an equilibrium (steady-state) model. For example, the dynamic model requires a parameter for the HIV transmission rate, but since no estimates for HIV incidence in Rio de Janeiro exist, we varied the HIV transmission rate parameter until a steady-state condition was achieved that matched the estimated HIV prevalence (see Table 1). Such variation was done in iterative fashion for all 11 model parameters (using a manual variation of the downhill simplex algorithm) until we achieved a good-fit equilibrium, defined a priori as a state in which none of the 11 model outputs varied by more than 1% over a 5 year period, and in which each model output matched its corresponding epidemiological estimate (from surveillance data or country-wide estimates) to within 1%. Seven model parameters (at the bottom of Table 1) had no corresponding epidemiological data point; their initial values were drawn from the literature. In Rio de Janeiro, both HIV and TB incidence have remained within a +/−10% range (in relative terms) over the last four years for which data are available, supporting the concept of near-equilibrium.20 Our primary outcomes were the projected declines in TB incidence and mortality among PLHIV at the end of the fifth year following rollout of the intervention.
Table 1.
Model Parameters
| Epidemiological Data Point |
Value, Rio de Janeiro (age 15–59) |
Model Output |
Matched Model Parameter | Parameter Value (Fit to Data) |
Sensitivity Analysis Range |
Reference |
|---|---|---|---|---|---|---|
| TB incidence, per 100,000/yr |
122 | 122 | Number of TB transmissions per infectious person-year |
6.54 | 5.13–7.50 | City of Rio de Janeiro27 |
| TB prevalence, per 100,000 |
132 | 132 | Rate of TB diagnosis and treatment |
0.87/year | 0.78–1.00 | World Health Organization28 |
| TB mortality, per 100,000/yr |
6.5 | 6.5 | Pre-diagnosis TB mortality rate (HIV-negative) |
0.038/year | 0.023–0.054 | City of Rio de Janeiro20 |
| TB incidence in PLHIV, per 100,000 pop/yr |
15.7 | 15.7 | Proportion of new TB infections resulting in rapid progression (HIV-positive) |
1.0a | 0.09–1.0 | City of Rio de Janeiro20 |
| Rate of slow TB progression after remote infection (HIV-positive) |
0.10/yeara | 0.04–0.31 | Gilks et al29 Horsburgh et al30 |
|||
| TB mortality in PLHIV, per 100,000/yr |
1.54 | 1.54 | Pre-diagnosis TB mortality rate (HIV, CD4 ≤350) |
0.087/yearb | 0.048–0.130 | City of Rio de Janeiro20 |
| ART coverage, CD4 ≤350 |
80% | 80.0% | Rate of ART initiation | 1.04/year | 0.77–1.47 | UNAIDS31 |
| Rate of ART discontinuation | 0.2/year | 0.12–0.29 | Assumption | |||
| HIV prevalence, per 100,000 |
600 | 600 | HIV incidence | 3.8x10−4/year | 1.7–5.9x10−4 | City of Rio de Janeiro20 |
| HIV mortality, per 100,000/yr |
24.6 | 24.6 | HIV mortality rate (CD4 ≤350, non-TB) |
0.061/yearb | 0.030–0.100 | City of Rio de Janeiro20 |
| Proportion of retreatment cases |
0.274 | 0.274 | TB relapse rate | 0.013/year | 0.006–0.030 | City of Rio de Janeiro20 |
| Proportion of TB that is MDR |
0.019 | 0.019 | Relative infectiousness of MDR-TB |
0.65 | 0.33–0.85 | World Health Organization28 |
| Proportion of MDR-TB that is retreatment |
0.52 | 0.52 | Proportion of recoveries ending in new MDR-TB |
0.005 | 0.002–0.007 | World Health Organization28 |
|
Literature-Based Model Parameter |
Value (from Literature) |
Sensitivity Range |
Reference | |||
| Relative infectiousness of TB in PLHIV, per person-day |
0.75 | 0.4–1.0 | Golub et al32 | |||
| Partial immunity to TB re- infection if latently infected |
0.56 | 0.0–1.0 | Cohen et al23 Sutherland et al33 Vynnycky et al34 |
|||
| Proportion of infections that progress rapidly to active TB |
0.087 | 0.066–0.100 | Vynnycky et al34 | |||
| Rate of slow progression from remote TB infection (HIV-negative) |
0.0005/year | 0.0002–0.0011 | Horsburgh et al30 | |||
| Protective efficacy of IPT against reactivation TBc |
0.67 | 0.5–1.0 | Ferebee3 Akolo et al5 |
|||
| Rate of CD4 progression to ≤350 cells/mm3 |
0.2 | 0.0–0.4 | Sanders et al35 | |||
| Non-HIV, non-TB mortality | 0.004/year | 0.0–0.008 | City of Rio de Janeiro20 |
|||
Initial estimates were that 25% of TB infections would result in rapid progression, and the rate of slow progression after remote infection was 0.026/year.30 However, these estimates resulted in substantial underestimates of the burden of HIV/TB. Reasoning that this was more likely due to underestimation of recent, rather than remote, infection (e.g., clustering of recent infection), we allowed the ratio between these two parameters to remain constant, even though this ultimately resulted in an estimated proportion of rapid TB progression equal to 1.0. This provides a maximally conservative estimate of the impact of IPT, which has no benefit against reinfection TB in the model. We also performed sensitivity analysis around a protective effect of antiretroviral therapy (ART) against TB infection of 0.0–1.0.
We assumed that HIV-infected populations with either CD4 nadir >350 cells/mm3 or use of ART would experience mortality rates at the mean of the rates listed for HIV-infected (CD4 ≤350) and -uninfected populations in the Table.
IPT was assumed to have a duration of effect of at least five years (the duration of the analysis); in a sensitivity analysis, this duration of effect was reduced to six months, as described in the text.
Sensitivity and Uncertainty Analysis
We conducted one-way sensitivity analyses on all model parameters, taking the range of values for each parameter that changed the matched epidemiological data point (e.g., HIV prevalence for the HIV transmission parameter) by 25% over a five-year model simulation in which no IPT was delivered. When variation over a parameter’s possible range (e.g., 0 to 1 for a proportion) did not result in a 25% change, then we varied the parameter over its full possible range. Thus, parameters for which small variations caused dramatic deviations from measured epidemiological data (e.g., HIV prevalence in Rio de Janeiro) were varied over a tighter range, whereas parameters for which many values were consistent with the epidemiological data were varied over a broader range. We performed multi-way uncertainty analysis by simultaneously (using Latin hypercube sampling) varying all parameters over a beta distribution (alpha = 2) defined by the range in Table 1, taking the most likely value as the mode. For this analysis, we took the 95% confidence intervals on the time-to-IPT from the trial as the boundaries for this parameter in the model. We report the 95% uncertainty range as the 2.5th and 97.5th percentile of results from 2,000 such simulations.
Of note, in performing our fitting procedure, we were forced to set the corresponding parameters governing rates of TB rapid progression and reactivation among HIV-coinfected individuals near the extremes of their reasonable ranges to match the high TB incidence among PLHIV in Rio de Janeiro. This phenomenon may represent clustering (non-homogeneity) among individuals with TB and HIV and has also been observed in other compartmental models of these two diseases.21 In this case, we adopted a conservative strategy of minimizing the contribution of reactivation TB relative to recent infection in PLHIV, thereby reducing the potential effect of IPT. As a result of this conservative approach, our base-case values of IPT impact often fall near the low end, if not outside, our 95% uncertainty ranges, which reflect results from model simulations in which reactivation contributes a larger proportion of TB among PLHIV.
RESULTS
Population-Level Rate of IPT Delivery
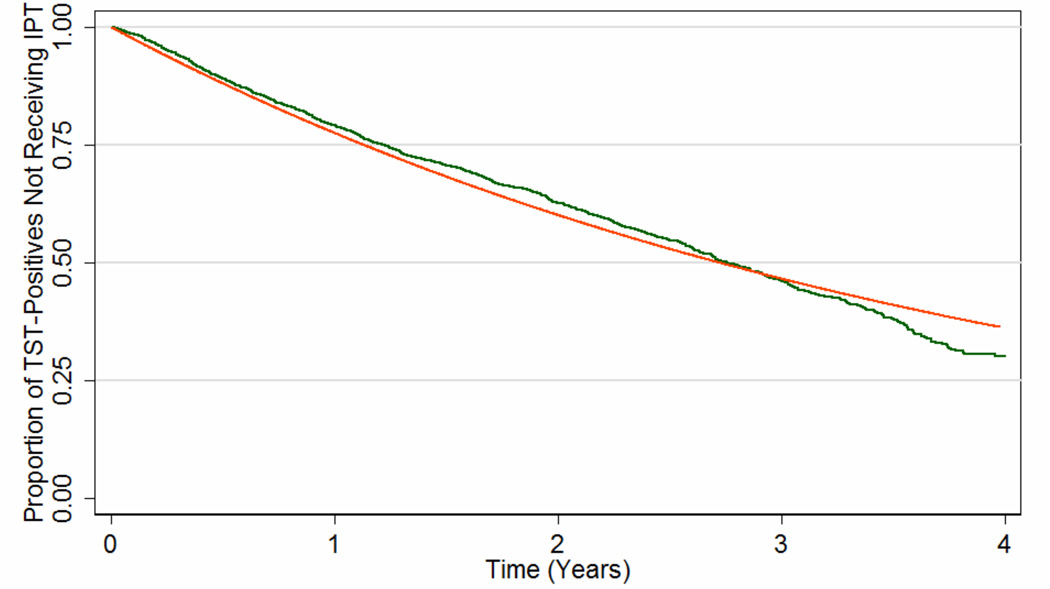
A total of 13,283 individuals were eligible for TB screening and/or IPT at one of the 29 study clinics and thus contributed data to the estimation of the IPT delivery rate. This number included 467 people who were not eligible for the primary trial because they did not visit study clinics within a window of eligibility. Data on time to IPT fit an exponential curve well (Figure 2), suggesting that IPT was delivered at a rate covering 20% (95% confidence interval, CI: 16%, 24%) of the eligible population each year (i.e., mean time from eligibility to IPT initiation = 3.93 years, 95%CI: 3.73, 4.13). The model estimated that 16% of all HIV-infected individuals not previously treated for TB were infected with TB at baseline, compared to 15% of initial TST results positive in the primary study.17
Figure 2. Estimated Rate of Isoniazid Preventive Therapy (IPT) Delivery in the THRio Trial.
The green line shows the Kaplan-Meier survival function of time from clinic randomization date to receipt of IPT in the THRio study, among all HIV-infected individuals without a history of TB treatment or IPT and having a positive tuberculin skin test (TST). This analysis assumes that the probability of a positive TST was the same in those who were never screened as in those who were. The orange line shows the predicted function after fitting these data to an exponential decay curve.
Projected Epidemiological Impact of IPT
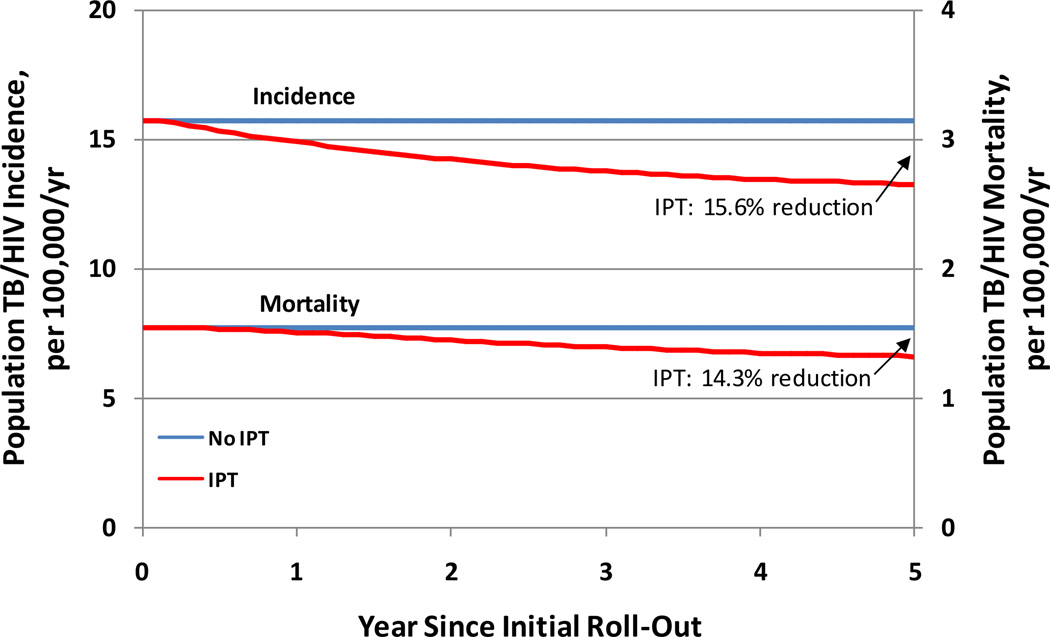
In our model of the adult population of Rio de Janeiro (population 4.1 million in 201022), we projected a total of 25,500 incident TB cases, of which 3,300 occurred in HIV-coinfected individuals. Annually delivering IPT to 20% of HIV-infected individuals with latent TB infection and no prior IPT averted a projected 438 TB cases and 27 TB deaths over 5 years (1.8% of cumulative TB cases over five years, 2.1% of cumulative TB deaths), including 325 cases and 24 deaths in HIV-infected individuals (10.1% reduction in TB incidence among PLHIV, 7.6% reduction in mortality). The impact of IPT was magnified over time as cumulative uptake improved and transmission effects accrued (Figure 3). By the end of year 5 after program roll-out, IPT had reduced population TB incidence by 3.0% (95% uncertainty range, UR: 1.6%, 7.2%) and TB incidence among PLHIV by 15.6% (95%UR: 15.5%, 36.5%). Corresponding reductions in TB mortality were 4.0% (95%UR: 2.2%, 10.3%) for all TB and 14.3% (14.6%, 33.7%) for TB among PLHIV.
Figure 3. Five-Year Impact of Isoniazid Preventive Therapy on HIV-Associated TB in Rio de Janeiro, Brazil.
Blue lines show the baseline (steady-state) scenario, while red lines show the projected TB incidence and mortality as a function of time after rolling out a program for isoniazid preventive therapy (IPT) capable of providing IPT to 20% of the eligible HIV-infected population per year. The upper set of lines and leftward axis display TB incidence, whereas the lower set of lines and the rightward axis display TB mortality.
Increasing the rate of IPT delivery to HIV-infected individuals with latent TB from 20% to 37%/year augmented the impact on TB incidence by 1.6-fold (i.e., 15.6% vs. 25.5% reduction in TB incidence among PLHIV at end of year 5). By contrast, assuming that IPT provided protection for only six months reduced the estimated impact of IPT by 70% (i.e., 4.6% reduction in TB incidence and mortality among PLHIV at end of year 5).
Sensitivity and Scenario Analyses
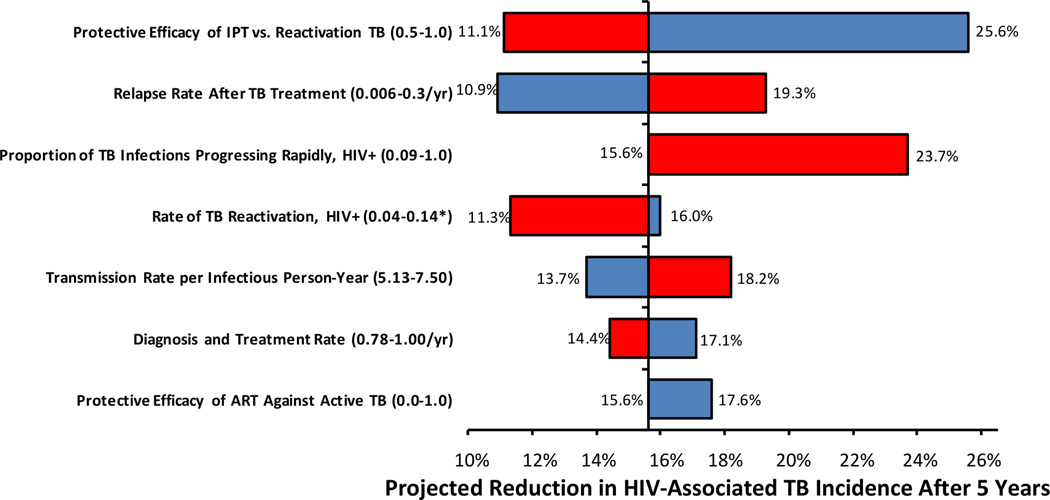
Variation of all parameters across the ranges shown in Table 1 demonstrated that model projections were most sensitive to the protective efficacy of IPT against reactivation TB, the relapse rate after successful TB treatment, and the relative proportion of HIV-associated TB due to primary progression of recent infection versus endogenous reactivation of remote infection. No one-way parameter variation – other than the sensitivity analysis described above in which the duration of IPT effect was reduced to six months – reduced the relative projected impact of IPT among PLHIV by more than 30%, whereas increasing IPT protective efficacy or decreasing the proportion of TB in PLHIV due to recent infection both augmented IPT’s projected impact by over 50%.
Assuming 100% efficacy of IPT against TB reinfection (in addition to 67% efficacy against reactivation) for the entire on- and post-IPT period augmented the projected impact of IPT on TB incidence among PLHIV at the end of five years from a 15.6% reduction to a 16.0% reduction. Increasing the transmission rate by a factor of 1.6 (thus giving a steady-state TB incidence of 1014 per 100,000/year) diminished this impact from a 15.6% reduction in incidence to an 8.1% reduction. Regarding generation of MDR-TB, even under “worst-case” assumptions, IPT delivery generated no more than 1.2 MDR-TB cases over the entire five-year period (see Supplemental Digital Content 1 for more details).
DISCUSSION
This transmission model of HIV and TB in Rio de Janeiro suggests that feasible treatment of no more than 500 PLHIV per year with isoniazid preventive therapy in a city of 6 million people and over 20,000 HIV-infected adults can prevent one in seven TB deaths among PLHIV (including those not linked to care) within a five-year timeframe. Although these projections assume persistent benefit of IPT up to five years among PLHIV (most of whom are also taking ART), comparison with trial results and simulation over reasonable parameter ranges suggests that these projections may in fact underestimate the impact of IPT. In moderate-burden settings such as urban Brazil, feasible programs of TB screening and IPT delivery are likely to produce important reductions in TB mortality and incidence among PLHIV.
Our model highlights IPT as a life-saving intervention for PLHIV and provides some insight as to the potential differences between moderate- and high-burden settings in terms of the role of IPT. A number of trials and modeling analyses performed in sub-Saharan Africa have suggested that IPT may have limited population-level impact in that setting.13,14,23,24 Our model suggests that the larger relative impact in moderate-burden settings such as urban Brazil may in part reflect differential risks of infection. In our model, the measured TB incidence rate among adults in Rio de Janeiro suggests an annual risk of TB infection (ARTI) of 0.8% – a risk that may be five times greater in hyperendemic settings in southern Africa.25,26 When we increased the TB transmission rate to generate a TB incidence reflective of South Africa (approximately 1,000 per 100,000/year), the impact of IPT on TB incidence in PLHIV was cut nearly in half. Importantly, assumptions regarding the effectiveness of IPT against reinfection TB (e.g., while actively taking IPT) had almost no bearing on projections of IPT impact in Brazil, whereas assumptions regarding the duration and magnitude of protection against reactivation TB were critical. In the end, the epidemiological impact of IPT is likely to be context-dependent and may be maximized, in relative terms, where reactivation is more important than reinfection as a cause of TB in PLHIV (i.e., low-to-moderate burden settings) and where the duration and magnitude of protection against reactivation may be highest (i.e., settings with high ART coverage).
Although the epidemiological benefits of IPT were substantial relative to the intensity of the intervention (treatment of no more than 500 individuals per year), they remained largely confined to the population of PLHIV. After 5 years of IPT implementation, while TB mortality among PLHIV had fallen by 14%, TB incidence among HIV-uninfected adults had fallen by only 1%. Thus, to achieve control of the TB epidemic in this setting where less than 15% of all TB cases are HIV-coinfected, a combined strategy of improved case-finding, diagnosis, treatment, and prevention is essential. IPT for PLHIV can serve as one useful component of such a strategy but is unlikely to reverse the TB epidemic on its own.
As with any modeling analysis, our study has limitations. Our approach of fitting parameters in an already-parsimonious modeling framework to data from epidemiological surveillance and an implementation trial minimized the number of assumptions required to populate this model. Nevertheless, our model does rely on certain standard simplifying assumptions (e.g., homogeneous mixing, division of HIV and TB status into discrete states) that are unlikely to be fully met in any population. Specifically, we were forced to assume a high risk of progression to active TB among HIV-positives in order to fit the high rates of HIV-associated TB seen in Rio de Janeiro. We may therefore have underestimated the true population-level impact of IPT in this model. This underestimation is compounded by the fact that IPT is best envisioned as a package that includes TB screening, which can further reduce TB transmission through detection of active cases – a benefit we did not include here. As this model was not designed to evaluate effects on HIV epidemiology, we modeled HIV transmission very simply as a uniform incidence rate over time. Finally, the current model does not answer certain questions of relevance to IPT scale-up, including generalizability to different operational settings and cost/cost-effectiveness.
Despite these limitations, the present analysis provides important guidance for both policy and future research. Our findings suggest that TB screening and IPT delivery for PLHIV is likely to be a valuable public-health intervention in moderate-burden urban settings, even if only 20% of the eligible population can be screened each year. Further model-based and empirical studies could better identify those settings (e.g., according to HIV prevalence, annual risk of TB infection, and MDR-TB prevalence) in which IPT is likely to have maximal population-level effectiveness.
In conclusion, we have used a simple TB/HIV transmission model, fit to surveillance and trial data, to demonstrate that TB screening and IPT, delivered at feasible levels, can reduce TB mortality and incidence among PLHIV by 15% or more over five years in an urban, moderate-burden setting with good ART coverage. This estimate could be refined through better knowledge of the duration of IPT protection among PLHIV taking ART and is unlikely to be equaled in settings where the risk of TB reinfection is higher. Nevertheless, in moderate-burden settings, IPT can serve as an important component of a multi-faceted strategy to feasibly reduce the population burden of HIV-associated TB.
Supplementary Material
Figure 4. Sensitivity Analysis: Five-Year Impact of IPT on TB Incidence.
Each model parameter was varied across a range (shown in Table 1) capable of changing the corresponding epidemiological data point (e.g., TB incidence) by 25% over 5 years; only those parameters that changed the projected impact of IPT on HIV-associated TB incidence at 5 years by 10% or more (i.e., outside the range of 14.1%–17.1% reduction) are shown. Red bars indicate a reduction in the specified parameter; blue bars indicate an increase. All parameter values were simultaneously varied (including those not shown here) across beta distributions in probabilistic uncertainty analysis. * = projected impact of IPT reached a maximum at a rate of 0.14/year; increases or decreases in parameter value from this estimate lowered the projected impact of IPT, with the minimum value shown.
Acknowledgments
DWD, JEG, LHM, REC, and BD developed the study concept. DWD wrote the model code and performed all analyses. VS, SCC, and SC collected the corresponding field data. JEG, LHM, and AGP provided statistical support. DWD wrote the first draft, which was revised by JEG, VS, and LHM. All authors saw and approved the final manuscript version. This work was funded by the Bill and Melinda Gates Foundation, grant 19790.01, as well as the National Institutes of Health, AI066994 and AI001637, and the Johns Hopkins University Center for AIDS Research (1P30AI094189).
Source of Funding: This work was funded by the Bill and Melinda Gates Foundation, grant 19790.01, as well as the National Institutes of Health, AI1066994 and AI001637.
Footnotes
Conflicts of Interest
None of the authors has any other conflicts to declare.
Presented in part at the 43rd World Lung Conference, Kuala Lumpur, Nov. 2012
List of Supplemental Digital Content
Supplemental Digital Content 1.pdf
References
- 1.World Health Organization, Stop TB Department. Guidelines for Intensified Tuberculosis Case-Finding and Isoniazid Preventive Therapy for People Living with HIV in Resource-Constrained Settings. Geneva: World Health Organization; 2011. [Google Scholar]
- 2.Comstock GW, Ferebee SH, Hammes LM. A controlled trial of community-wide isoniazid prophylaxis in Alaska. Am Rev Respir Dis. 1967;95:935–943. doi: 10.1164/arrd.1967.95.6.935. [DOI] [PubMed] [Google Scholar]
- 3.Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis. A general review. Bibl Tuberc. 1970;26:28–106. [PubMed] [Google Scholar]
- 4.Pape JW, Jean SS, Ho JL, Hafner A, Johnson WD., Jr Effect of isoniazid prophylaxis on incidence of active tuberculosis and progression of HIV infection. Lancet. 1993;342:268–272. doi: 10.1016/0140-6736(93)91817-6. [DOI] [PubMed] [Google Scholar]
- 5.Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2010;1:CD000171. doi: 10.1002/14651858.CD000171.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bucher HC, Griffith LE, Guyatt GH, Sudre P, Naef M, Sendi P, et al. Isoniazid prophylaxis for tuberculosis in HIV infection: A meta-analysis of randomized controlled trials. AIDS. 1999;13:501–507. doi: 10.1097/00002030-199903110-00009. [DOI] [PubMed] [Google Scholar]
- 7.Whalen CC, Johnson JL, Okwera A, Ho DL, Hubner R, Mugyenyi P, et al. A trial of three regimens to prevent tuberculosis in Ugandan adults infected with the human immunodeficiency virus. N Engl J Med. 1997;337:801–808. doi: 10.1056/NEJM199709183371201. [DOI] [PubMed] [Google Scholar]
- 8.Comstock GW, Baum C, Snider DE., Jr Isoniazid prophylaxis among Alaskan Eskimos: A final report of the bethel isoniazid studies. Am Rev Respir Dis. 1979;119:827–830. doi: 10.1164/arrd.1979.119.5.827. [DOI] [PubMed] [Google Scholar]
- 9.Horwitz O. Long-term results of the chemoprophylactic trial in Greenland. Bull Int Union Tuberc. 1968;41:167–168. [PubMed] [Google Scholar]
- 10.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 11.Chaisson RE, Martinson NA. Tuberculosis in Africa--combating an HIV-driven crisis. N Engl J Med. 2008;358:1089–1092. doi: 10.1056/NEJMp0800809. [DOI] [PubMed] [Google Scholar]
- 12.Mills HL, Cohen T, Colijn C. Modelling the performance of isoniazid preventive therapy for reducing tuberculosis in HIV endemic settings: The effects of network structure. J R Soc Interface. 2011;8:1510–1520. doi: 10.1098/rsif.2011.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Churchyard GJ, Fielding KL, Lewis JJ, Coetzee L, Corbett EL, Godfrey-Faussett P, et al. A trial of mass isoniazid preventive therapy for tuberculosis control. N Engl J Med. 2014;370:301–310. doi: 10.1056/NEJMoa1214289. [DOI] [PubMed] [Google Scholar]
- 14.Samandari T, Agizew TB, Nyirenda S, Tedia Z, Sibanda T, Shang N, et al. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: A randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:1588–1598. doi: 10.1016/S0140-6736(11)60204-3. [DOI] [PubMed] [Google Scholar]
- 15.Durovni B, Saraceni V, Moulton LH, Pacheco AG, Cavalcante SC, King BS, et al. Effect of improved tuberculosis screening and isoniazid preventive therapy on incidence of tuberculosis and death in the TB/HIV in patients with HIV in clinics in Rio de Janeiro, Brazil: a stepped wedge, cluster randomised trial. Lancet Infect Dis. 2013;13(10):852–858. doi: 10.1016/S1473-3099(13)70187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moulton LH, Golub JE, Durovni B, Cavalcante SC, Pacheco AG, Saraceni V, et al. Statistical design of THRio: A phased implementation clinic-randomized study of a tuberculosis preventive therapy intervention. Clin Trials. 2007;4:190–199. doi: 10.1177/1740774507076937. [DOI] [PubMed] [Google Scholar]
- 17.Durovni B, Cavalcante SC, Saraceni V, Vellozo V, Israel G, King BS, et al. The implementation of isoniazid preventive therapy in HIV clinics: The experience from the TB/HIV in Rio (THRio) study. AIDS. 2010;24(Suppl 5):S49–S56. doi: 10.1097/01.aids.0000391022.95412.a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhoda DA, Murray DM, Andridge RR, Pennell ML, Hade EM. Studies with staggered starts: Multiple baseline designs and group-randomized trials. Am J Public Health. 2011;101:2164–2169. doi: 10.2105/AJPH.2011.300264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown CA, Lilford RJ. The stepped wedge trial design: A systematic review. BMC Med Res Methodol. 2006;6:54. doi: 10.1186/1471-2288-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Municipal Secretary of Health and Civil Defense. Epidemiological Bulletin (Coordination of Infectious Diseases: AIDS, Tuberculosis, Leprosy) Rio de Janeiro: City of Rio de Janeiro; 2008. [Google Scholar]
- 21.Sanchez MS, Lloyd-Smith JO, Williams BG, Porco TC, Ryan SJ, Borgdorff MW, et al. Incongruent HIV and tuberculosis co-dynamics in Kenya: Interacting epidemics monitor each other. Epidemics. 2009;1:14–20. doi: 10.1016/j.epidem.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. [Accessed January 2, 2014];Municipal Secretary of Health, Rio de Janeiro. Vital statistics. http://www.rio.rj.gov.br/web/sms/analise-situacoes-saude.
- 23.Cohen T, Lipsitch M, Walensky RP, Murray M. Beneficial and perverse effects of isoniazid preventive therapy for latent tuberculosis infection in HIV–tuberculosis coinfected populations. Proc Natl Acad Sci U S A. 2006;103:7042–7047. doi: 10.1073/pnas.0600349103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guwatudde D, Debanne SM, Diaz M, King C, Whalen CC. A re-examination of the potential impact of preventive therapy on the public health problem of tuberculosis in contemporary sub-Saharan Africa. Prev Med. 2004;39:1036–1046. doi: 10.1016/j.ypmed.2004.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bekker LG, Wood R. The changing natural history of tuberculosis and HIV coinfection in an urban area of hyperendemicity. Clin Infect Dis. 2010;50(Suppl 3):S208–S214. doi: 10.1086/651493. [DOI] [PubMed] [Google Scholar]
- 26.Kritzinger FE, den Boon S, Verver S, Enarson DA, Lombard CJ, Borgdorff MW, et al. No decrease in annual risk of tuberculosis infection in endemic area in Cape Town, South Africa. Trop Med Int Health. 2009;14:136–142. doi: 10.1111/j.1365-3156.2008.02213.x. [DOI] [PubMed] [Google Scholar]
- 27. [Accessed November 29, 2011];Municipal Secretary of Health and Civil Defense, Rio de Janeiro. Infectious diseases: Information and epidemiological data on AIDS and tuberculosis. http://www.rio.rj.gov.br/web/smsdc/exibeconteudo?article-id=126082.
- 28.World Health Organization. Global Tuberculosis Report 2012. Geneva: WHO Press; 2012. [Google Scholar]
- 29.Gilks CF, Godfrey-Faussett P, Batchelor BIF, Ojoo JC, Ojoo SJ, Brindle RJ, et al. Recent transmission of tuberculosis in a cohort of HIV-1-infected female sex workers in Nairobi, Kenya. AIDS. 1997;11:911–918. doi: 10.1097/00002030-199707000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Horsburgh CR, Jr, O'Donnell M, Chamblee S, Moreland JL, Johnson J, Marsh BJ, et al. Revisiting rates of reactivation tuberculosis: A population-based approach. Am J Respir Crit Care Med. 2010;182:420–425. doi: 10.1164/rccm.200909-1355OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joint United Nations Programme on HIV/AIDS (UNAIDS) UNAIDS Report on the Global AIDS Epidemic 2012. Geneva: UNAIDS; 2012. [Google Scholar]
- 32.Golub JE, Saraceni V, Cavalcante SC, Pacheco AG, Moulton LH, King BS, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2007;21:1441–1448. doi: 10.1097/QAD.0b013e328216f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutherland I, Svandová E, Radhakrishna S. The development of clinical tuberculosis following infection with tubercle bacilli: 1. A theoretical model for the development of clinical tuberculosis following infection, linking from data on the risk of tuberculous infection and the incidence of clinical tuberculosis in the Netherlands. Tubercle. 1982;63:255–268. doi: 10.1016/s0041-3879(82)80013-5. [DOI] [PubMed] [Google Scholar]
- 34.Vynnycky E, Fine PEM. The natural history of tuberculosis: The implications of age-dependent risks of disease and the role of reinfection. Epidemiol Infect. 1997;119:183–201. doi: 10.1017/s0950268897007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanders GD, Bayoumi AM, Sundaram V, Bilir SP, Neukermans CP, Rydzak CE, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med. 2005;352:570–585. doi: 10.1056/NEJMsa042657. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.